Abstract
An efficient method for the synthesis of pyrazolo\([1{,}5\hbox {-}a]\)pyrimidines by the tandem reactions of dienones with pyrazole-3-amine through an aza-Michael addition/nucleophilic addition/1,3-hydrogen transfer process in the presence of potassium hydroxide is described. This protocol offers access to 7-arylethyl-5-arylpyrazolo\([1{,}5\hbox {-}a]\)pyrimidines in good to excellent yield. Meanwhile for 4-substituted dienones, different products, 7-arylethylene-5-arylpyrazolo\([1{,}5\hbox {-}a]\)pyrimidines, are given through an aza-Michael addition/nucleophilic addition/oxidation process. A gram-scale reaction has been performed to demonstrate the potency of optimized procedure for the scale-up process.
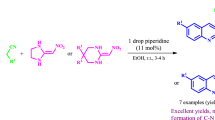
Graphical Abstract
SYNOPSIS7-Arylethyl-5-arylpyrazolo\([1{,}5\hbox {-}a]\)pyrimidines were efficiently synthesized by the reactions of 4-unsubstituted dienones with pyrazole-3-amine. In contrast, 7-arylethylene-5-arylpyrazolo\([1{,}5\hbox {-}a]\)pyrimidines could be afforded by the reactions of 4-substituted dienones with pyrazole-3-amine.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
During the past decade, the synthesis of pyrazolo\([1{,}5\hbox {-}a]\)pyrimidine derivatives and the investigation of their chemical and biological behavior have gained more importance due to pharmaceutical reasons.[1, 2] For example, the hypnotic drug Zaleplon (I),[3] the anticancer agent Dinaciclib (II),[4] the fungicide Pyrazophos (III),[5] the hypnotic sedative Indiplon (IV)[6] and the anxiolytic drug Ocinaplon (V)[7] all have this structural motif of pyrazolo\([1{,}5\hbox {-}a]\)pyrimidine (Figure 1). In parallel to medicinal chemistry, discoveries in material sciences have proved that pyrazolo\([1{,}5\hbox {-}a]\)pyrimidines containing an arylazo or heteroarylazo group are useful synthetic intermediates in the dyestuff industry.[8,9,10,11] Consequently, synthetic methodologies for novel pyrazolo\([1,5\hbox {-}a]\)pyrimidine derivatives are of particular interest to organic and medicinal chemists.
The recently reported methods for the synthesis of pyrazolo\([1{,}5\hbox {-}a]\)pyrimidines include i) gold-acid-co-catalyzed reactions of propargylic hydroperoxides with 3-aminopyrazole;[12] ii) the base induced reactions of 1,3,5-trisubstituted pentane-1,5-diones with substituted pyrazoles;[13] iii) the base catalyzed reactions of chalcones with 3-aminopyrazoles;[14] iv) the reactions of 1,2-allenic ketones with 3-aminopyrazoles;[15] v) the concentrated hydrochloric acid catalyzed condensation of 1,3-diketones with substituted aminopyrazoles;[16] vi) the hydrotalcite catalyzed reactions of enaminones with aminopyrazole derivatives;[17] and vii) the condensation of 2-pyrone with 3-amino-5-arylpyrazoles.[18] However, commercially unavailable reagents, harsh reaction conditions, and regioselectivity concerns restricted their use in regular processes. Therefore, straightforward and more efficient approaches which enable to access these structures in a more efficient manner by means of readily available and highly economical precursors and reagents are quitedesirable.
In this paper, we report an efficient method for the synthesis of 7-arylethyl-5-arylpyrazolo\([1{,}5\hbox {-}a]\)pyrimidines by one-step tandem reactions of commercially available and easily prepared dienones with pyrazole-3-amine through a aza-Michael addition/nucleophilic addition/1,3-hydrogen transfer process in the presence of potassium hydroxide.
2 Experimental
2.1 General information
\(^{1}\hbox {H}\) NMR and \(^{13}\hbox {C}\) NMR spectra were obtained with Mercury–600BB instrument using \(\hbox {CDCl}_{3}\) as solvent and \(\hbox {Me}_{4}\hbox {Si}\) as the internal standard. Elemental analyses were performed on a Vario El Elemental Analysis instrument. Melting points were observed in an electrothermal melting point apparatus. Dienones were synthesized according to literature method.[19]
2.2 General procedure for synthesis of 7-arylethyl-5-arylpyrazolo[1,5-a]pyrimidines \({{\varvec{(2a--u)}}}\) and 7-arylethylene-5-arylpyrazolo[1,5-a]pyrimidines \({{\varvec{(3a-e)}}}\)
The mixture of dienone (0.5 mmol), pyrazol-3-amine (0.6 mmol) and potassium hydroxide (0.5 mmol) in n-propanol (5 mL) was stirred under air at \(100{^{\circ }}\hbox {C}\) for 1 h. The reaction was monitored by TLC. After the completion of the reaction, the mixture was extracted with ethyl acetate \((3 \times 10\, \hbox {mL}\)), and the combined liquid was washed with saturated brine \((3 \times 10 \,\hbox {mL}\)). The resulting organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was isolated by column chromatography using petroleum ether and ethyl acetate (v/v 10:1) as eluent to give pure product. The analytical data for products are given in Supporting Information section.
3 Results and Discussion
Initially, (2E, 4E)-1,5-diphenylpenta-2,4-dien-1-one (1a) was selected as a model substrate to react with pyrazol-3-amine using n-propanol as solvent and potassium hydroxide as a base (Scheme 1). The reaction might take place through 1,4-aza-Michael addition and nucleophilic addition to afford A as a final product, or A continue to oxidize to B as a final product. However, the expected product was not observed. Instead, an unexpected product, 7-phenethyl-5-phenylpyrazolo\([1{,}5\hbox {-}a]\)pyrimidine (2a), was isolated, which was confirmed by \(^{1}\hbox {H}\) NMR and \(^{13}\hbox {C}\) NMR spectra. This result indicated that 1,3-hydrogen transfer also occurred for the reaction of 1a and pyrazol-3-amine in addition to 1,4-aza-Michael addition and nucleophilic addition. The possible reason is that compared to structure A, the product 2a has more stable aromatic fused heterocycle. This implied that the driving force for 1,3-hydrogen transfer is to form more stable structure.

In order to optimize the reaction conditions, the different solvents and bases were screened (Table 1). When various solvents were tested, it was found that the reaction could not proceed in toluene and xylene (Table 1, entries 1, 2). Moderate yields of 2a were obtained in DMF, DMSO, 1,4-dioxane, EtOH and n-BuOH (Table 1, entries 3–7). However, the best yield was obtained by using n-PrOH as solvent (Table 1, entry 8).
In addition, bases also played an important role in the reaction. Some tested inorganic bases, such as NaOH, \(\hbox {K}_{2}\hbox {CO}_{3}\), \(\hbox {Cs}_{2}\hbox {CO}_{3}\) and \(\hbox {NaHCO}_{3}\), had a certain effect on the reaction (Table 1, entries 9–12). Organic bases, such as DBU, DABCO, DMAP and \(\hbox {Et}_{3}\hbox {N}\) could not promote the reaction (Table 1, entries 13–16). The highest yield of 2a was obtained by using KOH as a base (Table 1, entry 8).
With the optimal conditions in hand, we turned our focus to unearth the generality of the method to access various 7-arylethyl-5-arylpyrazolo\([1{,}5\hbox {-}a]\)pyrimidines, and the results are summarized in Table 2. It was found that the reactions could tolerate a wide range of functional groups, and produce the corresponding products in good to high yield. Dienones bearing electron-donating groups (Me, MeO and \(\hbox {NMe}_{2})\) on aromatic rings of \(\hbox {Ar}_{1}\) and \(\hbox {Ar}_{2}\) gave the corresponding products in higher yield (Table 2, 2b–2e, 2r–2t). In contrast, dienones bearing electron-withdrawing groups (F, Cl and Br) on aromatic rings of \(\hbox {Ar}_{2}\) gave slightly lower yield (Table 2, 2f–2k). In addition, dienones containing heterocyclyls, such as thienyl, furanyl, pyrrolyl, pyridinyl and pyrazinyl, could transform into the desired products in good yield (Table 2, 2l–2p,2t). Unfortunately, the reactions for dienones bearing nitro and amino groups on aromatic rings of \(\hbox {Ar}_{2}\) were not successful. The tested aliphatic dienones did not give the corresponding product. The reactions of dienone with substituted pyrazol-3-amine, such as 4-cyanopyrazol-3-amine, could also give the corresponding heterocycle in high yield (Table 2, 2u).
It was noteworthy to mention that when 4-substituted dienone, such as 4-Me substituted dienone 1v, was used as a substrate, the reaction could not give the similar 7-phenylethyl product, instead a 7-phenylethylene product 3a was efficiently afforded in high yield under the standard conditions, and no other by-products were observed (Scheme 2). This result indicated that the reaction might undergo a different air oxidation process in addition to 1,4-aza-Michael addition and nucleophilic addition compared with analogous reaction of 1a. The possible reason is that the existence of 4-Me in dienone 1v hindered the 1,3-hydrogen transfer. And another conjugate fused heterocycle structure was produced by dehydrogenation oxidation in the presence of air.
This reaction could also extend to other similar 4-Me substituted dienones bearing different substituents (such as Me, Br) on aromatic rings (Table 3, 3b–d) and heteroaryl (such as thienyl) (Table 3, 3e) to give the corresponding products in high yield.
The synthesis of 2a could also be conducted through a one-pot three-component reaction of cinnamaldehyde, acetophenone and pyrazol-3-amine in 63% yield by the standard conditions (Scheme 3).
With the success over generality of the protocol, the reaction of dienone 1a with pyrazol-3-amine was also performed on gram scale. The reaction of 1.20 g of 1a with 0.50 g of pyrazol-3-amine in the presence of 0.28 g of potassium hydroxide in n-propanol (15 mL) was performed under the optimized condition to give 1.02 g of 2a in 68% isolated yield. This success of gram scale reaction further showed the potency of optimized condition for the bulk processes (Scheme 4).
A plausible mechanism is proposed for the synthesis of 2a and 3a (Scheme 5). Pyrazol-3-amine first tautomerizes to pyrazol-5-amine, which attacks the dienone 1 through 1,4-aza-Michael addition to give intermediate A. Then the amino group of A nucleophilically attacks the intramolecular carbonyl group, followed by dehydration to afford intermediate B. The intermediate B (R=H) with assistance of two moles of water can afford intermediate C, which can easily undergo loss of two moles of water to achieve 1,3-hydrogen transfer to give 2a as a final product. Meanwhile intermediate B (R=Me) can also undergo dehydrogenation oxidation in the presence of air by loss of water to afford 3a as a final product.
4 Conclusions
In conclusion, we have developed a simple and effective method for the synthesis of 7-arylethyl-5-arylpyrazolo\([1{,}5\hbox {-}a]\)pyrimidines through aza-Michael addition/nucleophilic addition/1,3-hydrogen transfer tandem reactions of dienones with pyrazol-3-amine in the presence of potassium hydroxide. For the 4-substituted dienones, different products, 7-arylethylene-5-arylpyrazolo\([1{,}5\hbox {-}a]\)pyrimidines, were obtained through aza-Michael addition/nucleophilic addition/oxidation process. Mild reaction conditions, shorter reaction time, high yield, good functional group tolerance and simple work-up procedure are some of the salient features of this protocol. A gram-scale reaction has been attempted to illustrate the potency of reported procedure towards the bulk synthesis. This method will be an important alternative to multifunctional intermediates including pyrazolo\([1{,}5\hbox {-}a]\)pyrimidine, aryls and alkenyl, and will possibly find potential applications in medicinal chemistry and material chemistry.
References
Ivachtchenko A V, Golovina E S, Kadieva M G, Kysil V M, Mitkin O D, Tkachenko S E and Okun I M 2011 Synthesis and structure-activity relationship (SAR) of (5,7-disubstituted 3-phenylsulfonyl-pyrazolo[1,5\(\text{-}a\)) antagonists J. Med. Chem. 54 8161
Damont A, Medran-Navarrete V, Cacheux F, Kuhnast B, Pottier G, Bernards N, Marguet F, Puech F, Boisgard R and Dolle F 2015 Novel pyrazolo\([1,5\text{- }a]\)]-labeling, and in Vivo neuroinflammation PET images J. Med. Chem. 58 7449
Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, Maciocco E and Biggio G 2002 Comparison of the effects of zaleplon, zolpidem, and triazolam at various \(\text{ GABA }_{{\rm A}}\) receptor subtypes Eur. J. Pharmacol. 451 103
Martin M P, Olesen S H, Georg G I and Schonbrunn E 2013 Cyclin-dependent kinase inhibitor dinaciclib interacts with the acetyl-lysine recognition site of bromodomains ACS Chem. Biol. 8 2360
Castillo-Sanchez J, Aguilera-del Real A, Rodriguez-Sanchez M and Valverde-Garcia A 2000 Residue levels, decline curves, and plantation distribution of procymidone in green beans grown in greenhouse J. Agric. Food Chem. 48 2991
Walsh J K, Moscovitch A, Burke J, Farber R and Roth T 2007 Efficacy and tolerability of indiplon in older adults with primary insomnia Sleep Med. 8 753
Vinkers C H, Olivier B, Hanania T, Min W, Schreiber R, Hopkins S C, Campbell U and Paterson N 2011 Discriminative stimulus properties of \(\text{ GABA }_{\rm A}\) receptor positive allosteric modulators TPA023, ocinaplon and NG2-73 in rats trained to discriminate chlordiazepoxide or zolpidem Eur. J. Pharmacol. 668 190
Tsai P C and Wang I J 2007 A facile synthesis of some new pyrazolo[1,5-\(a\)]pyrimidine heterocyclic disazo dyes and an evaluation of their solvatochromic behaviour Dyes Pigm. 74 578
Tsai P C and Wang I J 2008 Synthesis and solvatochromic properties of 3,6-bis-hetarylazo dyes derived from pyrazolo[1,5\(\text{- }a\)]pyrimidine Dyes Pigm. 76 575
Tsai P C and Wang I J 2005 Synthesis and solvatochromic properties of some disazo dyes derived from pyrazolo[1,5\(\text{- }a\)]pyrimidine derivatives Dyes Pigm. 64 259
Sayed A Z, Aboul-Fetouh M S and Nassar H S 2012 Synthesis, biological activity and dyeing performance of some novel azo disperse dyes incorporating pyrazolo[1,5\(\text{- }a\)]pyrimidines for dyeing of polyester fabrics J. Mol. Struct. 1010 146
Alcaide B, Almendros P and Quiros M T 2014 Gold/acid-co-catalyzed direct microwave-assisted synthesis of fused azaheterocycles from propargylic hydroperoxides Chem. Eur. J. 20 3384
Saikia P, Gogoi S and Boruah R C 2015 Carbon-carbon bond cleavage reaction: Synthesis of multisubstituted pyrazolo[1,5\(\text{- }a\)]pyrimidines J. Org. Chem. 80 6885
Kaswan P, Pericherla K, Purohit D and Kumar A 2015 Synthesis of 5,7-diarylpyrazolo[1,5\(\text{- }a\)-pyrazol-3-amines and chalcones Tetrahedron Lett. 56 549
Zhang X, Song Y, Gao L, Guo X and Fan X 2014 Highly facile and regio-selective synthesis of pyrazolo[1,5\(\text{- }a\)]pyrimidines via reactions of 1,2-allenic ketones with aminopyrazoles Org. Biomol. Chem. 12 2099
Yin L and Liebscher J 2004 Convenient synthesis of substituted 3-alkenylpyrazolo[1,5\(\text{- }a\)]pyrimidines via Heck cross-coupling reaction Synthesis 2329
Mokhtar M, Saleh T S and Basahel S N 2012 Mg-Al hydrotalcites as efficient catalysts for aza-Michael addition reaction: A green protocol J. Mol. Catal. A: Chem. 353–354 122
Bassoude I, Berteina-Raboin S, Leger J M, Jarry C, Essassi E M and Guillaumet G 2011 One-step reaction leading to new pyrazolo\([1,5\text{- }a]\)pyrimidines by condensation of 2-pyrone with 5(3)-amino-3(5)-arylpyrazoles Tetrahedron 67 2279
Xin Y, Zang Z H and Chen F L 2009 Ultrasound-promoted synthesis of 1,5-diarylpenta-2,4-dien-1-ones catalyzed by activated barium hydroxide Synth. Commun. 39 4062
Acknowledgements
The authors thank the National Natural Science Foundation of China (21462038, 21362034) and Key Laboratory of Eco-Environment-Related Polymer Materials of Ministry of Education for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Xie, D., He, J. et al. Synthesis of 7-arylethyl-5-arylpyrazolo\([1{,}5\hbox {-}a]\)pyrimidines through an aza-Michael addition/nucleophilic addition/1,3-hydrogen transfer cascade. J Chem Sci 129, 1579–1586 (2017). https://doi.org/10.1007/s12039-017-1365-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-017-1365-4