Abstract
A ternary mixed ligand Cu(II) complex, [Cu(L)(Phen)], was prepared from the reaction of (E)-N′-((2-hydroxynaphthalen-1-yl)methylene)acetohydrazide [HL], Cu(NO3)2.3H2O and 1,10-Phenanthroline in 1:1:1 molar ratio. This complex was fully characterized using spectroscopic and physicochemical methods. The structure of the complex was determined by single crystal X-ray diffraction. The Cu(II) center is coordinated by two oxygen and one nitrogen donors of L2− and nitrogen atoms of the heterocyclic group. Electrochemical studies of the Cu(II) complex showed shifts in the ligand peaks as well as the appearance of new peaks after complexation. The electrochemical behavior of the Cu(II) complex was also studied using cyclic voltammetry. According to biochemical investigation (MCF-7 cells viability), anticancer activity of [Cu(L)(Phen)] was higher than those of Cu(NO3)2.3H2O, HL and 1,10-Phenanthroline.

A new ternary Cu(II) complex, [Cu(L)(Phen)], containing a hydrazone ligand has been synthesized and fully characterized. Furthermore, anti-cancer activities of Cu(II) complex, the ligand (HL), Cu(NO3)2.3H2O and 1,10-Phenanthroline were investigated against human breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent decades, hydrazone Schiff base ligands and their transition metal complexes have attracted much attention.[1,2] To mention the reasons, we can point out their structural similarities with the biological units, facile synthesis and applications in several areas. A wide range of biological properties such as antibacterial, anti-fungal,[3] anti-malarial,[4] anti-cancer[5] and other biochemical process[6] have been reported for hydrazone derivatives. Among these, different applications of Cu(II) complexes are documented in various fields.[7,8]
Copper exists in the biological system as asymmetric multi-dentate chelates.[9] The role of copper in both the etiology and growth of tumors has been extensively studied.[10] Also many of the Cu(II) Schiff base complexes can be good models for simulating and representing their function and providing models for the metal-containing sites in copper-containing proteins and enzymes such as ascorbic oxidase.[11]
In spite of the rapid development of novel anticancer drugs, outbreak of drug resistance and undesirable side effects have created many problems in cancer therapy. Thus, it is necessary to identify new compounds with improved properties in this regard.[12]
One of the characteristics of metal ions is their potential to undergo redox processes, as determined by their redox potentials. Especially, transition metal ions are usually able to switch between several oxidation states. Due to the redox activity of metals and, therefore, a possible disturbance of the sensitive cellular redox homeostasis, a tight regulation of the metal and redox balance is crucial for health and survival.[10,13,14]
Copper has a crucial role in redox reactions and triggers generation of reactive oxygen species (ROS) in human cells. Cu(II) complexes are well known for their redox activity, which seems to be, at least in part, involved in the most of their defined biological activities.[15–18]
Herein, we describe the synthesis, characterization of a new mixed-ligand Cu(II) complex. Furthermore, the effects of the synthesized compounds on the anti-cancer viability of breast cancer cells are investigated and described.
2 Experimental
2.1 Materials and instrumentation
All chemicals and solvents were of analytical reagent grade and used as received. Ligand HL was prepared according to previous report.[5] Melting points were determined with the help of an Electrothermal Apparatus-9200. Elemental analyses were carried out using a Thermo Finnigan Flash Elemental Analyzer 1112EA. FT-IR spectra were recorded at a Bruker-Tensor 27 by embedding the material in KBr discs in the range of 400–4000 cm −1. Molar Conductance measurements were made by means of a Metrohm 712 Conductometer in DMSO. Electronic spectra were recorded at 2 ∘C using a Cary 50 UV–Vis spectrophotometer. 1H-NMR spectrum was recorded at 25 ∘C on an Avance BRX 400 MHz spectrometer. All electrochemical experiments were performed on a Sama 500 electrochemical analyzer (Iran) at room temperature in DMSO solution with 0.1 M tetrabutylammonium bromide as the supporting electrolyte. An Ag/AgCl (saturated KCl)/3 M KCl reference electrode, a Pt wire as counter electrode and a glassy carbon electrode as working electrode were employed for the electrochemical studies. Cell culture reagents, penicillin-streptomycin solution, trypsin EDTA, and fetal bovine serum (FBS) were obtained from Biosera CO. (East Sussex, UK). Culture flasks and dishes were acquired from SPL lifesciences Inc. (Gyeonggi-Do, South Korea). 3-[4,5]-Dimethyl-2-thiazolyl-2,5-diphenyl-2-tetrazolium bromide (MTT) was purchased from Sigma (St. Louis, MI, USA).
2.2 Synthesis of (E)-N’-((2-hydroxynaphthalen-1-yl) methylene)acetohydrazide [HL]
A 5 mL ethanolic solution of 2-hydroxy naphtaldehyde (2 mmol, 0.2 g) was added to an ethanolic solution (4 mL) of acetohydrazide (2 mmol, 0.30 g). The mixture was stirred for 10 min at 50 ∘C. The resulting precipitate was filtered off, washed with cold ethanol and dried in desiccator over silica gel. Yield: 0.51 g, 86%. M.p.: 211 ∘C. FT-IR (KBr), cm −1: ν(OH) 3185, ν(NH) 3012, ν(C =O) 1678, ν(C =N) 1642, ν(C =C ring) 1467, ν(C-O) 1286, ν(NN) 1126, δ oopb(OH) 720. 1H-NMR (400 MHz, DMSO- d 6, 25 ∘C, ppm): δ=12.61 (s, 1H, -OH), 11.74 (s, 1H, NH), 9.14 (s, 1H, -CH =N), 8.21-7.22 (m, 6H, ArH), 2.02 (s, 3H,-CH3). UV/Vis (DMSO) \(\lambda _{{\max }}\), nm (ε, L mol −1 cm −1): 315 (12022), 323 (14454), 360 (12882), 371 (12303), 417 (1513).
2.3 Preparation of (1,10-phenanthroline)(E)-1-((2- acetylhydrazono)methyl)naphthalen-2-olate copper(II) [Cu(L)(Phen)]
Cu(NO3)2⋅2H2O (0.1 mmol, 0.0223 g) was added to a solution of HL (0.1 mmol, 0.0228 g) in 5 mL of boiling ethanol and the mixture was refluxed in a water bath for 10 min. 1,10-Phenanthroline (0.1 mmol, 0.0180 g) was added to the resulting dark-green colored solution which was further refluxed for ca. 1 h. After cooling, the precipitate was separated and then recrystallized from methanol to give green single crystal and were dried in a vacuum desiccator over CaCl2. Yield: 0.030g, 64%. M.p.:260 ∘C. Anal. Calc. (%) for C25 H 18CuN4 O 2 (469.98 g mol −1): C, 63.89; H, 3.86; N, 11.92. Found (%): C, 63.82; H, 3.79; N, 11.94%. Molar conductance (10 −3 M, DMSO) 7.1 ohm −1 cm 2 mol −1. FT-IR (KBr), cm −1: ν(C =N) 1621, ν(C =C ring) 1465, ν(C-O) 1241, ν(N-N) 1141. UV/Vis (DMSO, λ m a x , nm (ε, L mol −1 cm −1): 265(43651), 335(9332), 395(14125), 415(17782), 435(13490), 648(158).
2.4 Crystal structure determination
X-ray data for [Cu(L)(Phen)] was collected at room temperature with a Bruker APEX II CCD area-detector diffractometer using Mo K α radiation (λ=0.71073 Å). Data collection, cell refinement, data reduction and absorption correction were performed using multiscan methods with Bruker software.[19] The structures were solved by direct methods using SIR2004.[20] The non-hydrogen atoms were refined anisotropically by the full matrix least squares method on F 2 using SHELXL.[21] All the hydrogen (H) atoms were placed at calculated positions and constrained to ride on their parent atoms. Details concerning collection and analysis are reported in table 1.
2.5 Cell culture
MCF-7 (breast cancer) cells were obtained from National Cell Bank of Iran (NCBI)–Pasteur Institute of Iran (Tehran, Iran). Cells were grown with Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, penicillin (100 U/mL) and streptomycin (100 g/mL). They were maintained at 37 ∘C in a 5% CO2 atmosphere. Growth medium was changed three times a week. Cells were plated at the density of 5000 per well in a 96 micro plate well for the MTT assay. The cells were incubated with different doses of compounds. MCF-7 cells are useful for in vitro breast cancer studies because they have retained several characteristics of mammary epithelium. MCF-7 cell line is an estrogen receptor (ER) positive cell line.[22]
2.6 MTT assay
Cellular viability was assessed by the reduction of 2-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to Formazan. The reduction of tetrazolium salts is now widely accepted as a reliable way to examine cell proliferation. The yellow tetrazolium MTT (3-(4, 5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) is reduced by metabolically active cells, in part by the action of dehydrogenase enzymes, to generate reducing equivalents such as NADH and NADPH. The resulting intracellular purple Formazan can be solubilized and quantified by spectrophotometric means.[23] MTT was dissolved in PBS, and added to the culture at final concentration of 0.5 mg/ml. After additional 2 h incubation at 37 ∘C, the media were carefully removed and 100 μL DMSO was added to each well, and the absorbance (OD) values were determined at 490 nm with microplate reader (BioTek ELX808). Results were expressed as percentages of control.[5]
2.7 Statistical analysis
The results are expressed as mean ±SEM. The differences in cell viability (mean MTT assay) between groups were determined by one-way ANOVA, followed by the Tukey test. P < 0.05 was considered significant.
3 Result and Discussion
A ternary Cu(II) complex containing ONO hydrazine ligand (HL), Cu(NO3)2.2H2O and 1,10-Phenanthroline in molar ratio 1:1:1 has been prepared. The complex is stable in air and soluble in most organic solvent except n-hexane and diethyl ether.
3.1 FT-IR study
In the FT-IR spectrum of HL, the bands observed at 3012 and 720 cm −1 are respectively associated to stretching and out of plane bending vibrations of OH[5] and NH stretching vibration in HL is located at 3012 cm−1.[24] Disappearance of these bands in the spectrum of the complex is strong evidence for complexation. Coordination of nitrogen of the azomethine group results in a red shift of CN vibration from 1642 cm−1 to 1621 cm−1.[25] A 45 cm−1 red shift is observed in the stretching vibration of CO of the complex relative to that of the free ligand, consistent with the coordination of the Schiff base ligand with the Cu(II) central ion through the phenolic oxygen.[26] The IR spectrum of the complex reveals stretching vibrations of Cu-O and Cu-N at 650 and 525 cm −1, respectively.[3]
3.2 Electronic spectra
Electronic spectra of compounds were recorded in DMSO. In the UV–Vis spectrum of the Schiff base ligand [HL], π→π* transitions of the aromatic rings and π→π* and n →π* transitions of the carbonyl moiety and imine bonds were appeared as five absorption bands in the range of 315–417 nm (figure 1).[5] Similar features were observed in the spectrum of [Cu(L)(Phen)]. Appearance of two peaks at 415 and 435 nm with epsilon values of log ε (M −1 cm −1)=4.25 and 4.16, respectively, confirmed coordination of the ligand to the metal center. These high epsilon values indicate that the corresponding peaks were originated from MLCT (metal to ligand) and LMCT (ligand to metal) charge transfer transitions. In five-coordinated Cu(II) complexes, the electronic spectrum in visible region can be used for predicting the preferred geometry (SP or TBP). It has been demonstrated that presence of a peak in 550–660 nm range which is related to dxz, d\(_{\text {yz}}{\to } \textit {d}_{\mathrm {x}}^{~2}-_{\mathrm {y}}^{~2}\) shows SP or distorted SP geometries.[27,28] For example, Cu(TMCPMP-TS)(Phen)] complex synthesized by Vyas and coworkers showed a peak in the 670–690 nm range resulted in SP geometry which was confirmed by other characterization techniques including X-ray crystal structure.[29] On the other hand, TBP structures exhibit a characteristic peak around >800 nm that is assigned to dxz, d\(_{\mathrm {x}}^{~2}-_{\mathrm {y}}^{~2}{\to } \mathrm {d}_{\mathrm {z}}^{~2}\).[30] In the visible spectrum of [Cu(L)(Phen)], a band was observed at 648 nm (figure 1). Accordingly, the geometry of the Cu(II) complex synthesized here is closer to SP.[3]
3.3 X-ray Crystal Structure
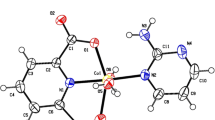
The title complex, [Cu(L)(Phen)] consists of a Cu(II) ion coordinated by an deprotonated dianionic tridentate ONO schiff base ligand (E)-1-((2-acetylhydrazono) methyl)naphthalen-2-olate (L 2−) and one bidentate 1,10 phenanthroline ligand. An ORTEP view of the asymmetric unit of [Cu(L)(Phen)] is shown in figure 2. Selected bond lengths and angles for the Cu(II) complex are set out in table 2.
The Cu(II) ion is situated in a distorted square pyramidal environment (N3 O 2 chromophore) with trigonality index τ=0.21 (τ=(β−α)/60 where β and α are the two largest bond angles around the metal center in five coordinated environment. for ideal square pyramid, τ is 0 but this value for ideal trigonal-bipyramidal structure is equal to 1).[31] The square plane is made up of enolate oxygen atom (O2), the phenolate oxygen atom (O1), and the imine nitrogen atom (N3) of dianionic tridentate ligand. The fourth site is occupied by nitrogen atom of the hetrocyclic ligand (N2). The other nitrogen atom of 1,10 phenantroline (N1) occupies the axial position.
The Cu-O1, Cu-O2, Cu-N2 and Cu-N3 bond distances are 1.9161(13), 1.9466(14), 1.9095(15) and 2.0280(16) Å, respectively which are similar to those values in similar reported structures. The unit cell and Crystal packing representation for title complex are shown in figure 3. As shown in this figure, the short contact (van der Walls interactions) stabilizes the crystal structure of this compound.
3.4 Electrochemical studies
The electrochemical behavior of the ligand and its Cu(II) complex (10 −3 M) were evaluated using cyclic voltammetry in DMSO containing 0.1 M TBAB as supporting electrolyte. Cyclic voltammograms of the ligand and its Cu(II) complex are depicted in figure 4. The free ligand exhibits a quasi-reversible electrochemical behavior (oxidation wave at ∼0.58 V and reduction wave at ∼0.33 V). With increasing the potential scan rate, the oxidation peak shifted slightly towards more positive potentials while the reduction one shifted towards more negative potentials, as expected for a quasi-reversible electron transfer process.
In the voltammogram of the complex, a redox couple at 0.10 V and 0.065 V with the peak currents of 1.56 and 2.58 μA, respectively were observed which can be assigned to Cu II/I.[32,33] Once again, increasing the potential scan rate shifted the first peak (oxidation) towards more positive potentials and shifted the second one (reduction) towards more negative potentials.
3.5 Anticancer activity
Breast cancer cells (MCF-7 cell line) were selected to investigate the anticancer activity of the synthesized compounds. The cancer cells were treated with different concentrations of Cu(NO3)2.3H2O, [HL], 1,10-phenanthroline and [Cu(L)(Phen)] (0, 1, 5, 10 and 20 μM) for 24 h. The obtained results are depicted in figure 5. As expected, the cell viability decreases with increasing the concentration of each compound in a dose-dependent manner.
Effects of different doses of Cu(NO3)2.3H2O, [HL], 1,10-Phenanthroline and [Cu(L)(Phen)] on MCF-7 cancer cells viability determined by MTT assay. The components have dose dependent toxic effect on cancer cells. Data are expressed as mean ± SEM; n =6 well for each group; *p < 0.05,**P < 0.01 and ***p < 0.001 versus control (non-treated) cells; + p < 0.05, ++P < 0.01 and +++p < 0.001 versus Cu(NO3)2.3H2O -treated cells; #P < 0.05; ###P < 0.001 versus 1,10-Phenanthroline-treated cells and ===P < 0.001 versus [HL]-treated cells in same doses.
Generally, cytotoxic effect of the complex is more than those of the other tested compounds. It has been reported that 1,10-phenanthroline derivatives has potent cytotoxicity against various cancer cell lines and can induce apoptosis.[34] In addition, hydrazone derivatives elicit potent toxic effect on cancer cells.[35] It is clear that the anticancer activity of the synthesized Cu(II) complex here has been significantly improved compared with the parent Schiff base ligand [HL] and the hetrocyclic ligand 1,10-phenanthroline and also the metal salt.
It has been documented that copper complexes of 1,10-phenanthroline can intercalate into the DNA minor grooves. This interaction enables redox reactions of the Cu(II) core with DNA and even RNA.[36,37]
Metal complexes have been proposed to trigger apoptosis pathway leading to cytochrome c release and caspase-3 activation.[38] Apoptosis is a multi-step and multi-pathway programmed cell death that is inherent in all cells. Cancer treatment by chemotherapy and irradiation primarily kills target cells through apoptosis.
IC50 value for the complex against MCF-7 cell line was calculated to be 2.4 μM which was significantly smaller than that reported for the well-known anticancer drug, cis-platin.[39,40] IC50 value for HL was found to be 17.4 μM. So, the [Cu(L)(Phen)] synthesized here can be proposed as an efficient anti-cancer agent alternative for cis-platin. However, further studies are needed to investigate all aspects of treatment by this drug.
4 Conclusions
A new ternary mixed-ligand copper(II) complex, [Cu (L)(Phen)] was synthesized and structurally characterized. Single crystal X-ray diffraction analysis revealed that this complex has square pyramidal geometry around Cu(II) center and positions around it are occupied with the oxygen and nitrogen atoms of [HL] and nitrogen atoms of 1,10-Phenanthroline. Anti-cancer activities of [HL], Cu(NO3)2.3H2O, 1,10-phenanthroline and [Cu(L) (Phen)] against human breast cancer cells (MCF-7) were investigated and compared with each other. It was found that [Cu(L)(Phen)] complex showed higher anticancer activity than the others.
References
Ebrahimipour S Y, Khabazadeh H, Castro J, Sheikhshoaie I, Crochet A and Fromm K M 2015 Inorg. Chim. Acta 427 52
Chang H Q, Jia L, Xu J, Xu Z Q, Chen R H, Wu W N, Bie H Y, Zhu T F, Ma T L and Wang Y 2015 Inorg. Chem. Commun. 57 8
Ebrahimipour S Y, Sheikhshoaie I, Crochet A, Khaleghi M and Fromm K M 2014 J. Mol. Struct. 1072 267
Verma G, Marella A, Shaquiquzzaman M, Akhtar M, Ali M R and Alam M M 2014 J. Pharm. Bioallied Sci. 6 69
Ebrahimipour S Y, Sheikhshoaie I, Castro J, Haase W, Mohamadi M, Foro S, Sheikhshoaie M and Esmaeili-Mahani S 2015 Inorg. Chim. Acta 430 245
Ebrahimipour S Y, Sheikhshoaie I, Mohamadi M, Suarez S, Baggio R, Khaleghi M, Torkzadeh-Mahani M and Mostafavi A 2015 Spectrochim. Acta A 142 410
Collman J P, Zhong M, Zhang C and Costanzo S 2001 J. Org. Chem. 66 7892
Narani A, Marella R K, Ramudu P, Rao K S R and Burri D R 2014 RSC Adv. 4 3774
Crichton R 2007 In Biological Inorganic Chemistry: An Introduction (Amsterdam: Elsevier)
Gupte A and Mumper R J 2009 Cancer Treat. Rev. 35 32
Moradi-Shoeili Z, Amini Z, Boghaei D M and Notash B 2013 Polyhedron 53 76
Vogel H G 2008 In Drug Discovery and Evaluation: Pharmacological Assays (Berlin: Springer)
Arredondo M and Núñez M T 2005 Mol. Aspects Med. 26 313
Balamurugan K and Schaffner W 2006 Biochim. Biophys. Acta 1763 737
Antholine W E and Taketa F 1984 J. Inorg. Biochem. 20 69
Byrnes R W, Mohan M, Antholine W E, Xu R X and Petering D H 1990 Biochemistry 29 7046
Chikira M, Tomizawa Y, Fukita D, Sugizaki T, Sugawara N, Yamazaki T, Sasano A, Shindo H, Palaniandavar M and Antholine W E 2002 J. Inorg. Biochem. 89 163
Narasimhan J, Antholine W E, Chitambar C R and Petering D H 1991 Arch. Biochem. Biophys. 289 393
(a) COSMO, Version 1.60 2005 (Madison WI: Bruker AXS Inc.); (b) SAINT, Version 7.06A 2005 (Madison WI: Bruker AXS Inc.); (c) SADABS, Version 2.10 2005 (Madison WI: Bruker AXS Inc.)
Burla M C, Caliandro R, Carrozzini B, Cascarano G L, Cuocci C, Giacovazzo C, Mallamo M, Mazzone A and Polidori G 2015 J. Appl. Crystallogr. 48 306
Sheldrick G M, SHELXL 97 1997 (Göttingen: University of Göttingen)
Levenson A S and Jordan V C 1997 Cancer Res. 57 3071
Denizot F and Lang R 1986 J. Immunol. Methods 89 271
Ebrahimipour S Y, Sheikhshoaie I, Kautz A C, Ameri M, Pasban-Aliabadi H, Amiri Rudbari H, Bruno G and Janiak C 2015 Polyhedron 93 99
Ebrahimipour S Y, Mohamadi M, Castro J, Mollania N, Amiri Rudbari H and Saccá A 2015 J. Coord. Chem. 68 632
Ebrahimipour S Y, Ranjabr Z R, Kermani E T, Amiri B P, Rudbari H A, Saccá A and Hoseinzade F 2015 Transition Met. Chem. 40 39
Hathaway B J 1987 In Comprehensive Coordination Chemistry, Vol. 5 (eds.) G Ilkinson, R D Gillard and J A McCleverty (Oxford, UK: Pergamon Press)
Hathaway J 1972 J. Chem. Soc., Dalton Trans. 1196
Vyas K M, Jadeja R N, Patel D, Devkar R V and Gupta V K 2013 Polyhedron 65 262
Massoud S S, Mautner F Z, Abu-Youssef M A M and Shuaib N M 1999 Polyhedron 18 2061
Addison A W, Rao T N, Reedijk J, van Rijn J and Verschoor G C 1984 J. Chem. Soc, Dalton Trans. 7 1349
Vo N H, Xia Z, Hanko J, Yun T, Bloom S, Shen J, Koya K, Sun L and Chen S 2014 J. Inorg. Biochem. 130 69
Özdemir U O, Aktan E, İlbiz F, Gündüzalp A B, Özbek N, Sarı M, Çelik O and Saydam S 2014 Inorg. Chim. Acta 423 194
Lin J C, Yang S C, Hong T M, Yu S L, Shi Q, Wei L, Chen H Y, Yang P C and Lee K H 2009 J. Med. Chem. 52 1903
Bak Y, Kim H, Kang J W, Lee D H, Kim M S, Park Y S, Kim J H, Jung K Y, Lim Y and Hong J 2011 J. Agric. Food Chem. 59 10286
Sigman D, Graham D, D’Aurora V and Stern A 1979 J. Biol. Chem. 254 12269
Ramírez-Ramírez N, Mendoza-Díaz G and Pedraza-Reyes M 2003 Bioinorg. Chem. Appl. 1 25
Mukherjee S, Chowdhury S, Chattapadhyay A P and Bhattacharya A 2011 Inorg. Chim. Acta. 373 40
Chen Y, Qin M Y, Wu J H, Wang L, Chao H, Ji L N and Xu A L 2013 Eur. J. Med. Chem. 70 120
Desbois N, Pertuit D, Moretto J, Cachia C, Chauffert B and Bouyer F 2013 Eur. J. Med. Chem. 69 719
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
FT-IR spectra of [HL] and [Cu(L)(Phen)], 1H NMR spectrum of ligand, atomic coordinates and equivalent isotropic displacement parameters, bond lengths and angles, and anisotropic displacement parameters for [Cu(L)(Phen)] are available at www.ias.ac.in/chemsci. CCDC 1043607 contains the supplementary crystallographic data for [Cu(L)(phen)]. A copy of this information may be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge, Cb2 1EZ, UK (fax: + 44 1223 336 033); web page: http://www.ccdc.cam.ac.uk/cgibin/catreq.cgi
Electronic supplementary material
Rights and permissions
About this article
Cite this article
SHEIKHSHOAIE, I., EBRAHIMIPOUR, S.Y., SHEIKHSHOAIE, M. et al. Synthesis, characterization, X-ray crystal structure, electrochemical evaluation and anti-cancer studies of a mixed ligand Cu(II) complex of (E)-N′-((2-hydroxynaphthalen-1-yl)methylene)acetohydrazide. J Chem Sci 127, 2193–2200 (2015). https://doi.org/10.1007/s12039-015-0978-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0978-8