Abstract
Two new kinds of diamines, 3-[bis-(3-aminophenyl)-phosphinoyl)-phenyl]-3-(triphenyl-phosphoranylidene)-pyrrolidene-2,5-dione, (DAP) with phosphorus moiety and bis-(5-amino-naphthalene-1-yl) dimethyl silane (DAS) with silicon moiety are synthesized. A series of novel aromatic polyamide-imides (PAIs) are prepared from three dicarboxylic acids and synthesized diamines. The phosphorus and silicon containing diamines and all polymers are characterized by FT-IR, NMR spectroscopic techniques and elemental analysis. The polymers obtained have good thermal stability and glass transition temperature (Tg) in the range of 254–315∘C. All these novel polyamide-imides (PAIs) contain 10% weight loss at the temperature above 506∘C and more than 59% residue at 600∘C in nitrogen atmosphere. The resulting polymeric films exhibit high optical transparency and inherent viscosity in the range of 0.68 to 0.79 dL/g. These polymers are found to be soluble in aprotic polar solvents such as NMP, DMSO, DMF and DMAc. Wide angle X-ray diffraction revealed that these polymers are predominantly amorphous in nature.

TGA, DSC, curves, UV-Vis spectra and WAXD diffractogram of polymers are studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polyimides are an essential class of engineering thermoplastic that possess advantageous physical properties such as high thermo oxidative property, solvent resistance, toughness, high modulus, chemical resistance and relatively low dielectric constant.[1–6] These polymers are used in a variety of applications such as coatings, composites, adhesives, aerospace, optoelectronics and microelectronics.[7–13] However, commercial use of these polymers is often limited due to their poor solubility in most organic solvents and their high softening or melting temperature. In order to overcome these limitations, various approaches to processable aromatic polyimides have focused on chemical modifications, mainly by preparing new monomers that provide less molecular order, better torsional mobility and low inter-molecular interactions and improvement in solubility of polymers in organic solvents without sacrificing the above excellent properties. Some general approaches that have been commonly implemented, are incorporation of flexible linkages, attachment of bulky pendant substituents, use of asymmetric monomers, non-coplanar biphenyl moiety and heteroaromatic rings into polyimide chains.[14–22] Among various strategies adopted so far to improve processability of polyimides, insertion of an amide group in imide backbone has proven satisfactory. PAIs contain amide and cyclic imide units along the chain and hence inherit desirable characteristic balanced between those of polyimides and this class of polymers offer a good compromise between solubility, high thermal properties and processability.[23–25]
According to the phosphorylation technique first described by Yamazaki et al., a series of high-molecular-weight PAIs are synthesized from the imide ring bearing dicarboxylic acids with phosphorus and silicon containing new aromatic diamines.[26] Introduction of silicon and its groups into polymeric chain is attractive for its significant improvement on the electrical properties, thermal stability and flame retardancy of polymers.[27–29] Some other properties, such as high resistance to thermal oxidation and low toxicity are also observed for silicon containing compounds and polymers.[30] The excellent thermal stability of silicon based polymers satisfies the requirements for application in advanced electronic industries. Silicon is therefore considered as one of the ‘environment friendly’ flame-retardant element, including phosphorus and nitrogen.[31,32] Literature survey revealed that introduction of phosphorus into polymer backbone can largely improve the flame retardancy and also decrease the contamination of environment on pyrolysis.[33] It has been proposed that phosphorus moiety decomposes at lower temperature relative to polymer matrix and forms a protective char layer over it and this char layer plays an important role in improving the flame retardancy of polymers.[34,35]
2 Experimental
2.1 Materials
5-Amino-naphthalene-1-ol (AN) and 2-(triphenyl phosphoranylidene) succinic anhydride (TPSA) all are purchased from Sigma Aldrich and used as received. Dichloro-dimethyl-silane (DDS) and glacial acetic acid are purchased from Merck and used without any purification. Pyridine (CDH) is purified by distillation under reduced pressure over calcium hydride. Triphenyl phosphite (TPP, Merck) is used as received. Anhydrous calcium chloride is dried under reduced pressure at 150∘C for 6 h prior to use. N-methyl-2-pyrrolidone (NMP, Sigma Aldrich) is dried over phosphorus pentoxide for at least 15 h and distilled under reduced pressure. Methanol (Fisher Scientific) is received as HPLC grade solvent and used without purification. Tris (3-aminophenyl) phosphine oxide (TAPO) and all diimide-diacids are synthesized in our laboratory in the previous study.[36] Structures of all dicarboxylic acids are shown below:

2.2 Measurements
FT-IR spectra are recorded on Perkin Elmer RXI spectrophotometer in the range 4000–400 cm−1. Elemental analysis is carried out by using GmbH VarioEL CHNS elemental analyser. NMR spectra are recorded on a BRUKER TOP-SPIN 300 MHz spectrophotometer using DMSO- d 6 as a solvent and tetramethyl silane as an internal reference at room temperature. The inherent viscosity of the polymers is measured with an Ubbelohde viscometer at 30∘C. UV-visible spectra of the polymers in dilute N,N-dimethylformamide solution are recorded on Shimadzu UV-1601 UV-visible spectrophotometer. Differential scanning calorimetry (DSC) of the polymers is performed on TA 2100 thermal analyser having DSC 910 module with the heating rate of 10∘C/min. in nitrogen atmosphere. Thermogravimetric data are obtained on a Perkin Elmer Diamond model at a heating rate of 10∘C/min. in nitrogen atmosphere. X-ray diffraction patterns of the polymers are obtained at room temperature on a Bruker Model D-8 Advance diffractometer with a scanning speed of 4∘/min, and recorded in the 2𝜃 range of 5–50∘.
2.3 Monomer synthesis
2.3.1 Synthesis of 3-[bis-(3-aminophenyl)-phosphinoyl)-phenyl]-3-(triphenyl-phosphoranylidene)-pyrrolidene-2,5-dione (DAP)
DAP is prepared according to scheme 1. TPSA (0.026 mol) is dissolved in glacial acetic acid (25 mL) and TAPO (0.026 mol) is added. The solution is refluxed for 12 h and the resulting mixture is precipitated in ice cold water (100 mL), to give brown coloured solid. The precipitate is initially washed with water and then with aqueous sodium bicarbonate solution to remove acid content. The synthesized solid is dried in vacuum at 7∘C for 5–6 h and recrystallized with chloroform to give pure compound. FT-IR (KBr, cm−1): 3353 (N-H stretching), 2930 (aromatic C-H stretching), 1558 (aromatic C-C stretching), 1195 (P =O stretching), 1418 (P-Ar stretching), 1682 (imide C =O stretching), 1375 (imide C-N stretching). 1H-NMR, δ (ppm, DMSO- d 6, 300 MHz): 2.29 (s, 1H, -CH2), 3.97 (s, 2H, -NH2), 6.15 (d, 1H, Ar-H), 6.28 (d, 1H, Ar-H), 6.81 (s, 1H, Ar-H), 7.12 (t, 1H, Ar-H), 7.33 (d, 1H, Ar-H), 7.42 (m, 1H, Ar-H).13C-NMR, δ (ppm, DMSO- d 6, 300 MHz): 162.6 and 155.6 (C of imide C =O), 139.2 (aromatic C-NH2), 138.9 (aromatic C attached to imide group), 132.6 and 131.3 (aromatic C-P =O), 128.8 (aromatic C meta to -NH2 or P =O), 127.9 (aromatic C meta to P =O or imide group), 127.4 (aromatic C of phosphoranylidene group), 126.6 (aromatic C ortho to P =O or imide group), 124.8 (aromatic C ortho to P =O or para to imide group), 122.6 (aromatic C ortho to P =O or para to -NH2), 121.1 (aromatic C ortho to imide group or para to P =O), 119.8 (aromatic C ortho to -NH2 or para to P =O), 117.3 (aromatic C ortho to -NH2 or P =O), 15.9 (C of –CH2-).
2.3.2 Synthesis of bis-(5-amino-naphthalene-1-yl) dimethyl silane (DAS)
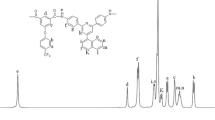
AN (0.05 mol) is dissolved in 1,4-dioxane (40 mL) and introduced into 250 mL three necked flask equipped with mechanical stirrer, refluxed condenser and dry nitrogen inlet. The mixture was heated at 80∘C under nitrogen atmosphere and stirred until the AN dissolved completely. DDS (0.025 mol) is added dropwise over a period of 1 h into the stirred solution and the mixture is refluxed for 24 h at 100∘C. A brown coloured precipitate is obtained which is filtered and washed with 1,4-dioxane. The crude is dried at 70∘C under vacuum for overnight. FT-IR (KBr, cm−1): 3378 (N-H stretching), 1610 (N-H flexion), 1540 (aromatic C-C stretching), 3018 (aromatic C-H stretching), 1250 (aromatic C-O stretching), 1310 (Si- C), 1050 (Si-O).1H-NMR, δ (ppm, DMSO- d 6, 300 MHz): 1.39 (s, 3H, Si-CH3), 3.98 (s, -NH2), 6.34 (t, 1H, Ar-H), 6.45 (d, 1H, Ar-H), 6.82 (d, 1H, Ar-H), 7.12 (d, 1H, Ar-H), 7.88 (t, 1H, Ar-H), 9.32 (d, 1H, Ar-H). 13C-NMR, δ (ppm, DMSO- d 6, 300 MHz): −1.8 (Si-CH3), 118.2 (aromatic C ortho to –NH2), 119.6 (aromatic C linked to –O-Si), 121.4 (aromatic C para to –NH2), 128.2 & 130.1 (aromatic C connecting benzene ring), 136.2 (aromatic C meta to –NH2), 148.4 (aromatic C-NH2), 157.3 ((aromatic C–O-Si).
2.4 Polymer synthesis
A typical example of TPP-activated polycondensation of polymer synthesis is shown in scheme 2 and described as follows. A mixture of DIDA (1mmol) and aromatic amine (1mmol), anhy. CaCl2 (0.30 g), TPP (2.3 mL) and pyridine (2.3 mL) in NMP (16 mL) is stirred at 110∘C for 4 h in a 25 mL flask. The viscosity of the reaction solution is increased after 1 h; therefore an additional volume of NMP is added to carry out the reaction in homogeneous medium. At the end of the reaction, the polymer solution is poured slowly into methanol (250 mL) with stirring, to give a fibre-like precipitate which is washed thoroughly with hot water and methanol, filtered and dried in vacuum at 120∘C.
All the polyamide-imides are synthesized by similar procedure.
3 Results and Discussion
3.1 Monomer synthesis and characterization
Silicon and phosphorus containing diamines are synthesized according to scheme 1 and their structures are confirmed by spectroscopic techniques and elemental analysis. The physical characterization and elemental data are shown in table 1.
Figure 1 represents the FT-IR spectrum of diamine, DAS. This spectrum shows the characteristic absorption bands at 3378 cm−1 (N-H stretching of –NH2 group), 1540 cm−1 (aromatic C-C stretching), 1310 cm−1 (Si-C stretching) and 1050 cm−1 due to Si-O stretching. The absorption band at 1250 cm−1 appears due to aromatic C-O stretching of ether group. As shown in figure 2, the1H-NMR spectrum of diamine, DAS presented signals in downfield region around 6.34–9.32 ppm due to Ar-H protons. A singlet is obtained at 3.98 ppm due to –NH2 protons. In addition, a chemical shift is observed at 1.39 ppm, and this is attributed to the -CH3 protons linked to Si atom. Figure 3 shows the13C-NMR spectrum of diamine, DAS. In this spectrum, carbon of methyl group is observed at −1.8 ppm. All aromatic carbons appeared in the range of 118.2–157.3 ppm and the carbon attached to –NH2 group appeared at 148.4 ppm. Similar spectra are obtained for diamine, DAP.
3.2 Polymer synthesis and characterization
The imide ring containing diimide-diacids, phosphorus and silicon containing diamines with phenyl moieties are established as potential monomers for the synthesis of polyamide-imides, capable of imparting high thermal stability and good processability. The synthesized polymers are insoluble in either chloroform or tetrahydrofuran (THF), even on heating. Hence their molecular weight distribution (MWD) cannot be determined by GPC.[37] The inherent viscosities of these polymers are recorded in the range of 0.68 to 0.79 dL/g and all data are summarized in table 2. The higher value of inherent viscosity indicated the formation of high molecular weight polymers, which may be due to the high reactivity of diamines used to synthesize polymers.
All the synthesized polymers are characterized by FT-IR, NMR spectroscopic techniques and elemental analysis. The elemental data of resulting polyamide-imides agreed well with the calculated values, given in table 2. Figure 4 shows the FT-IR spectrum of polymer, DAP/B. This spectrum shows N-H stretching frequency due to the amide group as a broad band at 3377 cm−1. The amide band associated with the stretching vibration of the carbonyl group, appeared around 1726 cm−1 (symmetrical stretching), 1780 cm−1 (asymmetrical stretching) and imide ring deformation around 763 cm−1, together with some absorption bands around 1410 and 1209 cm−1 due to P-Ar and P-O stretching respectively. A representative1H-NMR spectrum of polymer, DAP/B is shown in figure 5 which is in good agreement with the polymer structure. This spectrum shows characteristic resonance signals of aromatic protons in the region of 6.72–9.14 ppm and a signal at 10.2 ppm due to –NH of pyrrole ring. In addition, a characteristic signal is also obtained at 8.17 ppm due to –CONH proton.
3.3 Polymer properties
3.3.1 Solubility of polymers in organic solvents
The solubility of all the polymers is investigated qualitatively in various organic solvents and the results are tabulated in table 3. All the PAIs show higher solubility in polar aprotic solvents like DMF, DMSO, DMAc, NMP, pyridine and m-cresol. These polymers are still not soluble in common organic solvents such as THF, chloroform and toluene even at a high temperature due to low value of dielectric constant of solvents. These results show that the synthesized PAIs have characteristic solubility. However, the incorporation of oxyphenyl linkage, benzophenone group, naphthalene moiety and laterally attached bulky (Ph)3P group as well as Si-O-Ph linkage reduce intermolecular interaction between the polymer chain and rigidity. The solubility of polymers is greatly enhanced by the presence of non-coplaner and twist heterocyclic moiety in polymer backbone and also due to incorporation of phosphine oxide moiety.[38]
3.3.2 DSC measurements
Differential scanning calorimetry (DSC) is used to determine the Tg of all polymers at a heating rate of 10∘C min−1 in nitrogen atmosphere. DSC curves of polymers DAP/N and DAS/N are represented in figure 6 and all data are given in table 4. The Tg values are read at the middle of the first breakdown observed in DSC curves, and found in the range 254–315∘C. The glass transition temperature of polymers depends on the structure of diamine and dianhydride component of diimide-diacids, and decreases with increasing flexibility of polymeric backbone. It is observed that the Tg value of silicon containing polymers is lower than the polymers having phosphorus containing phenyl moiety which may be due to the flexible linkage of Si-O-Ph backbone in polymer. Incorporation of Si would result in polymers with bulk pendants and these bulk pendants may simultaneously increase the free volume and reduce the cross-linking density of the polymers, to lower the Tg.[39] The Tg value of DAP based polymers is also low due to the bulky and non-coplaner triphenyl phosphoranylidene group which increase the intermolecular distance that triphenyl phosphine oxide group is not rigid conjugate structure but rather a flexible structure which is convenient to molecular motion and formation of Tg. Therefore, triphenyl phosphoranylidene and
triphenyl phosphine oxide moiety in polymeric chain can provide motion space to groups and make the Tg of polymers decrease.[40]
Consequently, higher Tg value of polymer, DAP/N and DAS/N can also be observed due to incorporation of rigid and kinked naphthalene units in polymeric backbone. However, in addition to diimide-diacids, the values of Tg shows the order N >B >O as illustrated in table 4.
3.3.3 Thermal stability
The thermal properties of the resulting polyamide-imides are investigated by TGA at the heating rate of 10∘C min−1 in nitrogen atmosphere. The TG curves of polymers DAP/N and DAS/N are shown in figure 7. All data of initial decomposition temperature (IDT), temperature for 10% weight loss (Td10%) and weight residue at 600∘C of all polymers are summarized in table 4. The IDT and temperature for 10% weight loss in nitrogen stay within the range of 456–522∘C and 506–575∘C respectively, which are the main criteria to determine thermal stability of polymers. The amount of carbonized residue (char yield) of these polymers is found in the range 59–67% at 600∘C. The IDT of DAP based polymer is slightly lower than DAS based polymers, owing to the less strength of phosphorus bonds in polymers, and hence decompose at low temperature. The char yield of phosphorus containing polymers is higher even if they have low IDT and thus, phosphorus in polymeric chain can enhance polymer charring property during combustion. Thus, it can be deduced that triphenyl phosphoranylidene and triphenyl phosphine oxide structure have a synergistic effect and can promote materials to form strong char layer. The char yield of silicon containing polymers is also high but slightly less than polymers with phosphorus moiety. The incorporation of silicon in polymeric chain also enhances the char formation and protects the char from thermal degradation.[41] The high char yields of these polymers can be ascribed to their chemical structure, which has high aromatic content.[42] Obviously, the data from thermal analysis shows that these polymers have fairly high thermal stability.
3.3.4 Optical behaviour
To investigate the optical behaviour of resulting polymers, their solutions with concentration of 10−5 mol/l in DMF are separately prepared and then exposed to UV-vis light. The absorption edge value (λ o) of all the polymers are determined in the range 367–380 nm and the maximum absorption wavelength (λ max) appeared in the range 275–302 nm. The absorption spectra of all these polymer solutions are nearly identical with each other. The results obtained clearly show that these polymeric solutions have low colour intensity and high level of optical transparency in UV-vis light region. An UV-vis absorption spectrum of polymer, DAP/O is shown in figure 8 and values of λ o obtained from the diluted solutions of polymers are given in table 5. The images of all polymeric films are shown in figure 9.
3.3.5 WAXD studies
The crystallinity of the PAIs was examined by WAXD analysis with graphite-monochromatized radiation, with 2 𝜃 ranging from 5∘ to 50∘ using the polymer films. Wide angle X-ray diffractogram of polymer, DAS/O is represented in figure 10. Broad peaks are observed in the wide angle X-ray diffraction curve, indicating the absence of crystallinity in resulting polymers. The unsymmetrical kinky Si-O-Ph or/ (Ph)3 P=C groups and bulky oxy phenyl or/ naphthalene or/ benzophenone flexible chains might inhibit the polymer chain packing and interaction forces, therefore, resulting in lack of crystalline morphology. The amorphous nature of the polymers would endow a good solubility.
4 Conclusions
Two novel diamines, 3-[bis-(3-aminophenyl)-phosphinoyl)-phenyl]-3-(triphenyl-phosphoranylidene)-pyrrolidene-2,5-dione (DAP) and bis-(5-amino-naphthalene-1-yl) dimethyl silane (DAS) are successfully synthesized. A series of aromatic polyamide-imides have been readily prepared from synthesized diamines and various diimide-diacids via direct phosphorylation polycondensation. The introduction of bulky silicon and phosphorus containing phenylene groups in polymeric chain can increase solubility in organic solvents and disrupt the co-planarity of aromatic units in packing of chain. These polymers demonstrate nice balance of properties comprising excellent thermal stability with moderate glass transition temperature. All the low coloured polymeric films are significantly flexible and show high optical transparency in UV-Vis region. The high char yield of these polymers may be due to incorporation of silicon and phosphorus containing phenyl groups. The incorporation of flexible groups prevented polymeric chain from close packing and is responsible for their amorphous nature.
References
Marinovic-Cincovic M, Babic D, Dzunuzovic E, Popov-Pergal K and Rancic M 2007 Polym. Degrad. Stabil. 92 1730
Leung C L, Ghaffarian R and Leung K C 1997 Polym. Degrad. Stabil. 58 11
urRehman S, Li P, Zhou H W, Zhao X G, Dang G D and Chen C H 2012 Polym. Degrad. Stabil. 97 1581
Babanzadeh S, Mahjoub A R and Mehdipour-Ataei S 2010 Polym. Degrad. Stabil. 95 2492
Musto P, Ragosta G, Scarinzi G and Mascia L 2004 Polymer 45 4265
Tao L, Yang H, Liu J, Fan L and Yang S 2009 Polymer 50 6009
Behniafar H and Sefid-girandehi N 2011 J. Fluor. Chem. 132 878
Hasegawa M and Nomura R 2011 React. Funct. Polym. 71 109
Liaw D-J, Wang K-L, Huang Y-C, Lee K-R, Lai J-Y and Ha C-S 2012 Prog. Polym. Sci. 37 907
Hong S P, Kim I-C, Tak T and Kwon Y-N 2013 Desalination 309 18
Huang Y-C, Lin J-H, Tseng I-H, Lo A-Y, Lo T-Y, Yu H-P, Tsai M-H, Whang W-T and Hsu K-Y 2013 Compos. Sci. Technol. 87 174
Mallakpour S and Dinari M 2013 Appl. Clay Sci. 75–76 67
Kovalev M K, Kalinina F, Androsov D A and Cho C 2013 Polymer 54 127
Kim S D, Lee S, Heo J, Kim S Y and Chung I S 2013 Polymer 54 5648
Lin C H, Chang S L, Peng L A, Peng S P and Chuang Y H 2010 Polymer 51 3899
Yang F, Li Y, Bu Q, Zhang S, Ma T and Zhao J 2010 Polym. Degrad. Stabil. 95 1950
Shockravi A, Abouzari-Lotf E, Javadi A and Atabaki F 2009 Eur. Polym. J. 45 1599
Liaw D-J and Chen W-H 2006 Polym. Degrad. Stabil. 91 1731
Behniafar H and Abedini-pozveh A 2011 Polym. Degrad. Stabil. 96 1327
Liu C, Wang J, Lin E, Zong L and Jian X 2012 Polym. Degrad. Stabil. 97 460
Comesana-Gandara B, Calle M, Jo H J, Hernandez A, Campa J G D, Abajo J D, Lozano A E and Lee Y M 2014 J. Memb. Sci. 450 369
Liu J, Zhang Q, Xia Q, Dong J and Xu Q 2012 Polym. Degrad. Stabil. 97 987
Behniafar H, Beit-Saeed A and Hadian A 2009 Polym. Degrad. Stabil. 94 1991
Liu Y-L and Tsai S-H 2002 Polymer 43 5757
Song G, Zhang Y, Wang D, Chen C, Zhou H, Zhao X and Dang G 2013 Polymer 54 2335
Higashi F, Ogata S-I and Aoki Y 1982 J. Polym. Sci. A: Polym. Chem. 20 2081
Tao Z, Yang S, Chen J and Fan L 2007 Eur. Polym. J. 43 1470
Hamciuc E, Hamciuc C, Bruma M and Schulz B 2005 Eur. Polym. J. 41 2989
Bazzar M, Ghaemy M and Alizadeh R 2012 Polym. Degrad. Stabil. 97 1690
Hamciuc E, Hamciuc C and Bruma M 2007 Eur. Polym. J. 43 4739
Sponton M, Mercado L A, Ronda J C, Galia M and Cadiz V 2008 Polym. Degrad. Stabil. 93 2025
Zhang W, Li X, Jiang Y and Yang R 2013 Polym. Degrad. Stabil. 98 246
Ren H, Sun J, Wu B and Zhou Q 2007 Polym. Degrad. Stabil. 92 956
Liu Y L, Hsiue G H, Lee R H and Chiu Y S 1997 J. Appl. Polym. Sci. 63 895
Lin C H 2004 Polymer 45 7911
Agrawal S and Narula A K 2013 Polym. Bull. 70 3241
Liu C, Li X, Xu J and Jian X 2011 Eur. Polym. J. 47 1852
Jeong K U, Kim J-J and Yoon T-H 2001 Polymer 42 6019
Wu C S, Liu Y L and Chiu Y S 2002 Polymer 43 4277
Qian L-J, Ye L-J, Xu G-Z, Liu J and Guo J-Q 2011 Polym. Degrad. Stabil. 96 1118
Wang X, Hu Y, Song L, Xing W and Lu H 2010 J. Polym. Sci.: Part B: Polym. Phys. 48 693
Behniafar H and Mohammadparast-delshaad S 2012 Polym. Degrad. Stabil. 97 228
Acknowledgements
The author (S. Agrawal) wishes to express the gratitude to Guru Gobind Singh Indraprastha University, New Delhi for providing financial support in the form of IPRF (Indraprastha Research Felloship).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
AGRAWAL, S., NARULA, A.K. Facile synthesis of new thermally stable and organosoluble polyamide-imides based on non-coplaner phosphorus and silicon containing amines. J Chem Sci 126, 1849–1859 (2014). https://doi.org/10.1007/s12039-014-0727-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0727-4