Abstract
Nanoparticles (NPs) synthesized by laser ablation in distilled water were used to study their biological effect on normal and cancer cells. Parameters such as cell morphology, cell proliferation and viability were examined for treated cell lines, and the effect was represented in terms of cells cytotoxicity using standard procedures. The study reveals the higher cytotoxic effect of nanoparticles on cancerous cells of breast, melanoma and colon origin compared to normal fibroblast cells NIH-3T3. Furthermore, DNA fragmentation assay results demonstrated the apoptosis mediated cell death in nanoparticle-treated cancer cells. The distinct role of nanoparticles in normal and cancer cells of different origin showed that nanoparticles were specific to cause cytotoxicity in particular cancer cells type. NPs exhibit cytotoxic effects in cancer cells by inducing apoptosis. These studies provide fundamental evidence for the easy, simple and safe mode of nanoparticles synthesis and their application in cancer cells death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Studying nanoparticles and using them in various applications is quite common among the material scientists around the globe. It is very crucial to control the size of such nanoparticles, in whatever route is employed for their synthesis. We have attempted here in studying the controlled growth of nanoparticles, with a familiarly known physical technique called laser ablation (Aye et al. 2010), which gives insight on ablation and the preparation of nanoparticles by laser ablation on copper target. Recently, pulsed laser ablation was employed for preparation of several metals and semiconductor materials in different media such as vacuum, reactive gas and liquid. Using liquid as a media, both direct ablation of a bulk target such as gold, silver, palladium, and some materials like metal oxide and magnetic nanoparticles were synthesized (Tilaki and Mahdavi 2007).
Cancer is one of the leading causes of death and is considered a disease of global burden. (Siegel et al. 2014). Despite tremendous progress in the management of cancer, much advancement is needed to combat this disease. Development of effective therapy with zero side-effects to normal tissue is a major challenge. Nanomedicine-based therapy has with enormous potential because of its safe nature (Hubbell and Chilkoti 2012). Nanoparticles are nanometer-size particles and considered superior to their parent larger-sized material because they possess large surface area for better attachment to target for accomplishing cytotoxicity and other functions (Chithrani et al. 2006) such NP-based drug delivery system, which are very specific to the target cancer cells causing minimal or no damage to normal cells (Tang et al. 2014). Metal-based nanoparticles have been reported to exhibit cytotoxic effect in cancer cells (Dreaden et al. 2012); however, no studies had been performed using CuNPs synthesized by laser ablation. The objective of this study was to examine the cytotoxicity of NPs in different cancer cell and compare them with normal cells.
2 Materials and methods
2.1 Preparation and characterization of nanoparticles
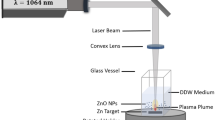
The commercially available copper sheet was purchased in a local metal shop and used as target for laser ablation. The laser beam is focused on this sheet which was placed in a petri dish containing 25 ml of pure water. The Nd-YAG (Quanta Ray INDI-40-10) laser beam was used to a target was of wavelength 1064 nm, with energy 250 mJ. This beam was made to vertically focus on the metal target using appropriate mirrors and lens to get sharp intense spot (Moniri et al. 2007). The pictorial representation of ablation setup and solutions containing nanoparticles are shown in figure 1. Elemental analysis was done using EDAX equipped SEM instrument HITACHI S3400N, USA. The particle size distribution of the nanoparticles was analyzed using Microtrac DLS instrument.
2.2 Cell culture and maintenance
Human ovarian cancer cell lines SK-OV3; murine Melanoma-B16F10; human colon Cancer- HT-29 and Murine fibroblast cells- NIH3T3 were procured from Cell Repository, National Centre for Cell Science, Pune, India. All cell lines were grown in DMEM (Himedia, Mumbai India) medium supplemented with heat inactivated 10% Fetal Bovine Serum (FBS) (Himedia, Mumbai India) and maintained at 37°C in an atmosphere of 5% CO2 in 95% humidified air.
2.3 Cytotoxicity assay (MTT assay)
The antiproliferative potentiality of NPs was performed using MTT reagent (Himedia, Mumbai, India) as described earlier (Yashaswini et al. 2017). Cells were seeded in 96-well plate at 10,000 cells per well and incubated for 24 h at 37°C to allow them to adhere to the bottom of the plate. After 24 h, media was removed and replaced with copper nanoparticles at different concentration (5–50% volume/volume) in a total of 150 µl volume per well in triplicates and incubated for 48 h. After the incubation, 15 µl of MTT reagent (5 mg/ml in 1X phosphate buffer saline) was added in each well and incubated in dark at 37°C for 3–4 h. Subsequently, the MTT reagent containing culture media was aspirated and the formazan precipitate was formed was dissolved in 100 µl dimethyl sulphoxide. The dissolved formazan precipitate was measured using 96-well plate reader at 570 nm. Cell viability was expressed as a percentage of the untreated control (100% cell viability). The effect of the NPs on the viability of the cells was measured in triplicate, and the experiments were repeated at least twice.
2.4 Detection of apoptosis by DNA fragmentation assay
The apoptotic effect of NPs on B16F10 cells was studied using DNA fragmentation assay. 2 × 105 cells were seeded to each 60 mm dish containing 4 mL of media and incubated for 24 h. 5% NPs sysnthezed was added along with fresh media and incubated for 48 h. Cells were harvested by scraping the adherent cells, which were then combined with floating cells present in the culture medium. The cells were washed with PBS followed by centrifugation at 1000 rpm for 2 min. 500 µL of lysis buffer was added to the pellet, mixed well until the pellet dissolved and incubated overnight at 37°C. The top layer containing dissolved DNA was carefully removed after centrifugation at 11,000 rpm for 10 min. The supernatant was treated with RNase (60 µg/mL) for 1 h at 50°C. The DNA was precipitated with 0.6 volume of isopropanol. After centrifugation, the pellet was dried completely and dissolved in TE buffer and kept at 65°C for 10 min to redissolve the DNA (Gansukh et al. 2019). The extracted DNA was run on 1% agarose gel at 80 V for 2 h, stained with ethidium bromide and the image was documented using Bio-Rad Gel Documentation system.
3 Results
3.1 Characterization of nanoparticles
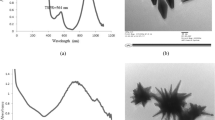
Elemental analysis using EDAX results were carried out to confirm the presence of nanoparticles in water and also for the copper sheet used to prepare these nanoparticles. Figure 2A shows the EDAX of copper used in this experiment and it is evident that the sheet is 99% copper. These recordings also quantify the percentage presence of the nanoparticles. This was done using EDAX equipped SEM instrument HITACHI S3400N, USA. The particle size distribution of the nanoparticles was analyzed using Microtrac DLS instrument
The particle size distribution of solutions ablated for various time intervals (shown in figure 2B) indicates the intended results of size variations. The solutions of 30 min and 120 min possessed particles ranging between 30 and 100 nm; ablation duration does influence the size of nanoparticles and thus one can tailor the required size of a particle by fixing the energy and controlling the time duration (figure 3). The results are given in tables 1 and 2.
3.2 Nanoparticles inhibit the cancer cell proliferation
We determined whether nanoparticles synthesized by laser ablation had any effect on the cell proliferation of normal cells (NIH3T3) and cancer cells (SKOV3, B16F10 and HT-29). Nanoparticles at the concentrations of 0.5 to 5% inhibit the cell proliferation in normal as well as in cancer cells in a dose-dependent manner; however, a level of inhibition in cell proliferation was minimal in normal cells in comparison to cancerous (figure 4).
We have also compared the effect of nanoparticles synthesized at variable time points of laser focusing (30, 60, 90 and 120 min) on cell proliferation. Carbon and Silica were the present in majority of NPs synthesized at 30 min (figure 3), the effects of these NPs vary from cells to cells, and our result showed that the ovarian cancers were maximum effected compared to other cells at lower concentration (figure 5A and 5B).
Carbon and sulphur nanoparticles were synthesized at 90 min (figure 7). These NPs showed the higher anti-proliferative effect at low dose in ovarian cancer (figure 5). Carbon NPs synthesized at 2 h (figure 2A) were efficient in inhibiting the rate of cell proliferation in ovarian cancer cells at a lower dose (figure 5) and similar effect were observed in colon and skin cancer cells at increasing concentration of NPs (figures 5, 6, 7). Overall, our cell lines studies signify the distinct roles of NPs synthesized at different points in tested normal and cancer cells of different origin, indicating that specific NPs were effective in controlling particular cancer.
3.3 NPs induce apoptosis in cancer cells
Since the anti-proliferative effect of anticancer agents is tightly linked to their ability to induce apoptosis, we investigated whether NPs could induce apoptosis in cancer cells. Loss of cell membrane integrity, chromatin condensation and DNA fragmentation are the hallmark of apoptosis (Kaufmann and Hengartner 2001). DNA fragmentation assay was performed in B16F10 cells to identify the mode of cell death, i.e. apoptosis/necrosis (figure 8). The result showed the DNA fragments detected as ladder/bands in B16F10 cells induced by treatment of 5% NPs, indicating that NPs induces apoptosis selectively in cancer cells. DNA bands appear as a result of nuclear damage and is a classical hall marks of apoptosis in cells, which is observed in B16F10 cells. The above findings provide the mechanistic aspect of cytotoxicity in cancer cells which was mediated through programmed cell death.
DNA fragmentation apoptosis assay: Measurement of apoptosis by DNA fragmentation upon treatment with Nanoparticle synthesized at 30, 60, 90 and 120 min at 5% concentration. B16F10 murine melonoma cell were treated with 5% NPs for 48 h, then cells were harvested for DNA fragmentation assay to estimate apoptosis. Lane 1, Media control cells; Lane 2, Vehicle control (water) cells; Lane 3, NPs of 30 min; Lane 4, NPs of 60 min; Lane 5, NPs of 90 min; Lane 6, NPs of 120 min and Lane 7, DNA marker.
4 Discussion
Overall, these results suggest that varying levels of cytotoxicity due to nanoparticles was observed in different cancer cell types. NPs exhibited a pronounced cell killing effect in cancer cells without causing damage to normal cells. NPs were capable of inhibiting the proliferation of tumour cells. Our results suggest that possible mechanisms of cytotoxicity in various types of cancer cells could be different. This study provides a new perspective on cell biology research in nanomedicine. NPs treatment should be able to effectively target and kill cancer cells. This study may pave the way for the simple and safe procedure of nanoparticle synthesis and their application in the management of cancer. Assessment of apoptosis by DNA fragmentation assay showed that DNA fragments appearing as ladder/bands in B16F10 cells induced by treatment of 5% NPs, indicating that NPs induce apoptosis selectively in cancer cells. DNA bands appear as a result of nuclear damage and is a classical hall mark of apoptosis in cells, which is observed in B16F10 cells. NPs effectively induces apoptosis in cancer cells. The anti-proliferative potentiality of NPs could be attributed to its induction of apoptosis. Further experiments can be performed to decipher the molecular mechanism of cytotoxicity and cell death for the better understanding of effects and successful treatment of cancers.
Here we would like to add the historical importance associated with miracle dip in river Ganges, which according to the results in this study, may be due to the presence of nanoparticles in the Ganges river. Of course, over the years the river has been polluted. Nanoparticles have a very large surface charge density and can very easily get into the body through the pores regions and these have an affinity to get attached to the cancerous or diseased cells because of surface charge density and lead to death of cancerous /diseased cells as per our study reported here. This needs further investigation, and at this stage we can only prophesy this aspect.
References
Aye HL, Choopun S and Chairuangsri T 2010 Preparation of nanoparticles by laser ablation on copper target in distilled water. Adv. Mat. Res. 93 83–86
Chithrani BD, Ghazani AA and Chan WC 2006 Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano. Lett. 6 662–6688
Dreaden EC, Alkilany AM, Huang X, Murphy CJ and El-Sayed MA 2012 The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 41 2740–2779
Gansukh E, Mya KK, Jung M, Keum YS, Kim DH and Saini RK 2019 Lutein derived from marigold (Tagetes erecta) petals triggers ROS generation and activates Bax and caspase-3 mediated apoptosis of human cervical carcinoma (HeLa) cells. Food Chem. Toxicol. https://doi.org/10.1016/j.fct.2019.02.037
Hubbell JA and Chilkoti A 2012 Nanomaterials for drug delivery. Science 337 303–305
Kaufmann SH and Hengartner MO 2001 Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 11 526–534
Moniri S, Ghoranneviss M, Hantehzadeh MR and Asadabad MA 2007 Synthesis and optical characterization of copper nanoparticles prepared by laser ablation. Bull. Mater. Sci. 40 37-43
Siegel R, Ma J, Zou Z and Jemal A 2014 Cancer statistics. Cancer J. Clin. 64 9–29
Tang W, Yuan Y, Liu C, Wu Y, Lu X and Qian J 2014 Differential cytotoxicity and particle action of hydroxyapatite nanoparticles in human cancer cells. Nanomedicine 9 397–412
Tilaki RM and Mahdavi SM 2007 Size, composition and optical properties of copper nanoparticles prepared by laser ablation in liquids. Appl. Phys. A. 88 415–419
Yashaswini PS, Kurrey NK and Singh SA 2017 Encapsulation of sesamol in phosphatidyl choline micelles: Enhanced bioavailability and anti-inflammatory activity. Food Chem. 228 330–337
Acknowledgements
The authors thank UGC, India, for their support to the University of Mysore through the UPE and CPEPA major projects. NKK is thankful to the Department of Science & Technology, India, for INSPIRE Faculty award.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B J Rao.
Corresponding editor: BJ Rao
Rights and permissions
About this article
Cite this article
Mahadevaiah, Kurrey, N.K., Gowtham, G.K. et al. Laser-ablation-synthesized nanoparticles and animal cell lines studies. J Biosci 44, 135 (2019). https://doi.org/10.1007/s12038-019-9956-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-019-9956-5