Abstract
This study was conducted to determine whether exposure to particulate matter 2.5 (PM2.5) affects the immune tolerance of neonatal mice via the regulation of PD-L1 expression. One-week-old BALB/c mice were exposed to PM2.5 for 8 days. From day 8 to day 18, the mice were treated with 5 μg house dust mite (HDM) (i. n.) every two days. Adenovirus-carried PD-L1 overexpression vectors were infected into mice via nasal inhalation 6 days after exposure to PM2.5. Airway hyper-responsiveness (AHR) was examined in mice 19 days after exposure to PM2.5, and the related parameters of airway inflammation were studied on day 22. Co-exposure to PM2.5 and HDM reduced PD-L1 expression but greatly increased infiltration of inflammatory cells, which was reversed by PD-L1 overexpression. Co-exposure to PM2.5 and HDM also elevated serum IL-4, IL-5 and IL-13 levels and reduced TGF-β level. Exposure to PM2.5 alone slightly increased the numbers of dendritic cells (DCs) but reduced the numbers of antigen-presenting cells expressing PD-L1 and Treg cells. Therefore, early exposure to PM2.5 reduced PD-L1 expression in the lungs of neonatal mice, which interfered with immune tolerance establishment and subsequently resulted in allergic airway inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Particulate matter 2.5 (PM2.5) refers to airborne particles with an aerodynamic diameter less than or equal to 2.5 μm which threaten human health, causing conditions including cardiovascular and respiratory morbidity and mortality (Madrigano et al. 2013; Habre et al. 2014; Neophytou et al. 2014). PM2.5 is a serious air pollutant that consists of sulfate, nitrate, nitrogen salt and heavy metals. Due to its small size, PM2.5 can reach the deep respiratory tract and affect blood circulation by penetrating lung cells (Perrone et al. 2013; Bono et al. 2015). Several research studies have demonstrated that exposure to PM2.5 may lead to acute and chronic respiratory disease including chronic bronchitis (Abbey et al. 1995), asthma in children (Mar et al. 2005; Nikasinovic et al. 2006; Tecer et al. 2008), and lung cancer (Loomis et al. 2013). Exposure to PM2.5 also increases the risk of developing cardiovascular disease (Dockery 2001; Brunekreef et al. 2005) and even liver cancer (Zhang et al. 2017). With the rapid economic development in China, air pollution due to PM2.5 is growing more and more serious and is becoming one of the leading causes of death. It is urgent to understand how and by which mechanism PM2.5 affects human health, especially the respiratory system.
Previous study showed that allergic airway inflammation was aggravated upon four-time co-exposure to ovalbumin and Asian sand dust, while eight-time co-exposure attenuates inflammation with significantly increased transforming growth factor-beta 1 (TGF-β) in bronchoalveolar lavage fluid (BALF) (He et al. 2013), suggesting that the responses are correlated with TGF-β-related immune tolerance. We hypothesized that long-term exposure to PM2.5 affects immune tolerance. The underlying mechanism needs to be further elucidated. TGF-β is a key regulator of immune regulation and has been shown to regulate T-cell differentiation and function. Loss of TGF-β results in systemic inflammation (Shull et al. 1992). TGF-β plays fundamentally important roles in maintaining immune tolerance and is a well-known promoter of CD4+ regulatory T (Treg) cell differentiation (Fantini et al. 2004; Mucida et al. 2007). Treg cells play a vital role in the maintenance of immune tolerance to self and innocuous non-self. Treg cells develop as an independent T-cell lineage from common T-cell precursors in the thymus, in which Treg cell differentiation requires signals from the T-cell receptor (TCR) for antigen, from co-stimulatory molecules, and from cytokine receptors (Apert et al. 2018). Treg can be divided into two subpopulations: naturally occurring (nTreg) and adaptive (iTreg) which develop in the thymus and the periphery from CD4+FoxP3− naive T cells, respectively. In the context of IL-2 stimulation, the Foxp3 gene was induced to express TGF-β in mouse peripheral CD4+CD25− naive T cells, converting them into Foxp3+ Treg cells (iTreg cells) capable of suppressing T-cell proliferation (Chen et al. 2003). The surface receptor programmed death-1 (PD-1) is critical for regulating T-cell function during immunity and tolerance. The interaction of PD-1 with its ligands PD-L1 and PD-L2 inhibits the functioning of the T-cell effector in an antigen-specific manner (Fife and Pauken 2011). It is noteworthy that PD-1 and PD-L1 were detected on Treg and were linked to Treg development and activation (Francisco et al. 2010). It was demonstrated that TGF-β upregulated the expression of PD-L1 on dendritic cells (DCs) with the induction of immune tolerance (Song et al. 2014). The binding of PD-L1 with PD-1 expressed on T cells is responsible for T-cell immune activity suppression. TGF-β is required for Treg cell production and co-regulation of Foxp3+iTregs development with PD-L1, indicating that PM may regulate immune tolerance through PD-L1.
This study demonstrated that exposure to PM2.5 caused defects in the establishment of immune tolerance and led to allergic lung disease. We found that exposure to PM changed PD-L1 expression levels in newborn mice. There is a correlation between PD-L1 and airway sensitivity in mice. Exposure to PM affects the acquired immune tolerance of neonatal mice.
2 Materials and methods
2.1 Preparation of aPM2.5
Mesoporous silica nanoparticles (MSNs) were synthesized according to the published protocol (Wu et al. 2013) using tetraethyl orthosilicate (J&K Chemical Ltd., China) as a silicon source and cetyltrimethylammonium bromide (J&K Chemical Ltd., China) as the template. The following steps were adopted according to the protocol published by Yan (Yan et al. 2017). Three chemical species, H2SO4, NH4NO3, and K2Cr2O7, were loaded into the pores of MSNs using the impregnation method. MSN (100 g each) was added to 1 L of ethanol solution containing 0.04 mg/mL of H2SO4, 0.04 mg/mL of NH4NO3, and 1.6 × 10−6 mg/mL of K2Cr2O7. Magnetic stirring was done at room temperature for 24 h to maximize the loading efficiency. The chemical species-loaded MSNs (aPM2.5) were collected via centrifugation, washed twice with ethanol and dried under vacuum. To determine the size of the aPM2.5, a particle powder (2.0 mg) was resuspended and ultrasonicated in 10 mL of water and then measured with a NICOMP 380 ZLS Particle Sizer (USA).
2.2 Mice and treatment
BALB/c mice were maintained in specific pathogen-free conditions. One-week-old BALB/c mice were exposed to PM2.5 for 8 days (1.13 g/m3, 60 min each time, 1× per day). From day 8 to day 18, the mice were treated with 5 μg house dust mite (HDM) (i. n.) once every two days. Airway hyper-responsiveness (AHR) was examined in mice 19 days after exposure to PM2.5, and the related parameters of airway inflammation were studied on day 22. aPM2.5 was administrated using a nose-only inhalation system with a particle flow of 1 mL/min and an internal flow of 0.8 m3/h. The animals were fixed on the aerosol generator to ensure the balance and consistency of the inhaled PM aerosol. The exposure procedure lasted 60 min/day, 7 days/week using a Hope-med 8052 automatic inhalation device (Hope-med Industry and Trade Co., Ltd., Tianjin, China). This study was approved by the ethics committee of our hospital (approval No. KJ1600206, 2016).
2.3 Construction of PD-L1 overexpression vector and adenovirus transfection
The adenovirus-carried PD-L1 overexpression vector was constructed and produced using the AdEasy Adenoviral Vector System kit (Stratagene, USA) according to the manufacturer’s protocol. For the overexpression of PD-L1, the open reading frame (ORF) of the mouse PD-L1 gene was amplified and cloned into the backbone vector pShuttle-IRES-hrGFP. Adenoviruses carrying PD-L1 or Ad-PD-L1 were amplified in 293AD cells. Viral particles were injected into the mice. According to the previous reports (Peng et al. 2011), adenoviruses carrying PD-L1 (Ad-PD-L1) was constructed. Briefly, the desired replication-deficient adenovirus containing the PD-L1 ORF was generated by homologous recombination through co-transfection of plasmids pShuttle-IRES-hrGFP-PD-L1 and adenoviral skeletal plasmid pBHG1oXE1, 3Cre in AD-293 cells in the presence of DOTAP liposome (Roche, Germany). After plaque purification of several rounds, ad-PD-L1 was amplified and purified from cell lysates. Viral products were desalted and stored at 80°C in phosphate-buffered saline (PBS) containing 10% glycerol (v/v). A recombinant adenovirus carrying the LacZ gene under the control of CMV (Ad-LacZ) served as the control. Two days before the HDM treatment, the Ad-LacZ and Ad-PD-L1 viral particles were separately intravenously injected at 1×109 PFU (plaque-forming unit) per mouse per week for 2 weeks (Cheong et al. 2014).
2.4 Sample preparation
After mice were euthanized with methoxyflurane, en-bloc lungs were collected. Half of the right lobe was ground in a mortar with liquid nitrogen for total RNA extraction, and the other half was homogenized and lysed within RIPA buffer and used for Western blot analysis. The left lung lobe was fixed in 4% paraformaldehyde for PAS and HE staining.
2.5 RNA extraction and quantitative PCR
Total RNA extraction from lung tissue was performed using Trizol reagent (Invitrogen). The total RNA (1 μg) obtained was reversely transcribed into cDNA using M-MLV Reverse Transcriptase (Promega) following the manufacturer’s protocol. qPCR was performed on the ABI 7500 fast real-time PCR system (Applied Biosystems) using SYBR Premix Ex Taq (Takara). The endogenous control was GAPDH mRNA.
2.6 Western blot analysis
Protein samples from lung tissue were prepared using RIPA buffer (Beyotime, China) containing protease inhibitor cocktail (Sigma). After determination of protein concentration using bicinchoninic acid protein assay kit (Beyotime, Shanghai, China), SDS-PAGE electrophoresis was performed. The separated protein blots were then transfected onto a PVDF membrane (Millipore). After blocking with 5% (w/v) nonfat milk, the membrane was incubated with primary antibodies (against PD-L1 [Abcam, ab213480, 1:1000], PD-L2 [Abcam, ab187662, 1:2000], PD-1 [Abcam, ab187662, 1:1500] and β-actin [Abcam, ab8226, 1:2000]) overnight and secondary antibody for about 4 h. After being visualized using ClarityTM Western ECL substrate (Bio-Rad), the protein bands were quantified using Image J software.
2.7 Histological analysis
Tissues were fixed with 4% paraformaldehyde. Tissue sections were stained with hematoxylin and eosin for pathological evaluation. Periodic acid–Schiff (PAS) staining was performed to analyze changes in mucus secretion. The PAS staining procedure was the same as described in the previous literature (Cheong et al. 2014).
2.8 Cytokine measurements and inflammatory cell count in BALF
After the animals were killed, BALF was collected through lavage of the right lung using 0.6 mL of ice-cold PBS. After centrifugation at 400 g for 15 min, the supernatant was collected for IL-4, IL-5, IL-13 and TGF-β determination using a Milliplex MAP assay kit (Millipore, Billerica, MA). IL-33, thymic stromal lymphopoietin (TSLP), and IL-25 production were determined using commercial ELISA kits (R&D systems), according to the manufacturers’ protocol. However, the pellet was used for inflammatory cell counts and classification using a hematology analyzer (Nihon Kohden, Tokyo, Japan) after resuspension in PBS.
2.9 Serum immunoglobulins assay
The serum concentration of IgE and HDM-specific IgG1 was determined using ELISA, as described elsewhere (Trompette et al. 2014).
2.10 Flow cytometry
To analyze antigen-presenting cell (APC) subsets, lung tissue was digested by collagenase IV (Sigma, USA) in Iscove’s modified Dulbecco’s medium at 37 °C for 30 min. A 70-μm cell strainer was used to filter samples that were then washed in PBS containing 0.2% bovine serum albumin and counted with a Coulter counter. Cells were then stained with PerCP/Cy5.5 anti-mouse CD103 antibody (clone: 2E7, 121416, 1:400), Pacific Blue™ anti-mouse/human CD11b antibody (clone: M1/70, 101224, 1:600), APC/Cy7 anti-mouse CD11c antibody (clone: N418, 117324, 1:600), Alexa Fluor® 700 anti-mouse I-A/I-E antibody (clone: M5/114.15.2, 107622, 1:800), and Alexa Fluor® 647 anti-mouse F4/80 antibody (clone: BM8, 123122, 1:500). The percentage of regulatory T cells of the whole CD4+ T-cell compartment in the lung was determined by staining with anti-CD4-PerCP, anti-CD3, anti-CD25-PE and intracellularly with anti-FoxP3. All antibodies were purchased from Biolegend (San Diego, USA). Cells were measured on LSRII (BD Biosciences, USA). Results were analyzed using FlowJo 9.4.11 software (Tree Star Inc.).
2.11 AHR measurement
One day after the last challenge, mice were anesthetized using i.p. injection of ketamine (10 mg/mL) and xylazine (1 mg/mL). Measurements of airway resistance and dynamic compliance were conducted with the Fine Pointe RC System (Buxco Research Systems, Wilmington, NC), in which mice were mechanically ventilated as described previously in detail (Lombardi et al. 2010; Galle-Treger et al. 2016). Mice were sequentially challenged with aerosolized PBS (baseline), followed by increasing doses of acetylcholine. Maximum lung resistance (RL) and minimum compliance (Cdyn) values were recorded during a 3-min period after each methacholine challenge.
2.12 Statistical analysis
Results were analyzed using SPSS 18.0 software (SPSS, Chicago, IL) and presented as mean ± SD values. One-way ANOVA and Student’s t-test were employed to analyze the difference in data among various groups and between two groups, respectively. A p-value less than 0.05 was considered statistically significant.
3 Results
3.1 Exposure to PM2.5 reduced PD-L1 expression in the lung of neonatal mice
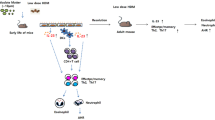
To study the effect of PM2.5 on PD-L1 expression, we constructed artificial PM2.5 (aPM2.5) particles and exposed the mice for 15 days (1.13 g/m3, 60 min each time, 1× per day), followed by HDM treatment (5 μg, i.n.) every two days. The schematic working model is shown in figure 1A. We detected PD-L1/L2 and PD-1 expression after exposure to PM2.5. The results showed that HDM exposure had little influence on PD-L1/L2 and PD-1 expression, while simultaneous exposure to HDM and PM2.5 significantly reduced PD-L1 expression in lung (figure 1B and C). The reduced expression of PD-L1 can be rescued by PD-L1 overexpression vector (figure 1B and C). PD-L1 mRNA level was also attenuated upon exposure to PM2.5 and HDM, as detected by qRT-PCR (figure 1D). Importantly, HDM+PM2.5 only affected PD-L1 expression but had no effect on PD-L2 and PD-1 (figure 1B–D).
Exposure to PM2.5 reduced PD-L1 expression in the lung of neonatal mice. (A) The schematic working model of the experimental design. (B) PD-1, PD-L1, and PD-L2 protein expression measured using Western blotting. (C) Protein quantification results of PD-L1. (D) PD-1, PD-L1, and PD-L2 mRNA expression determined by RT-qPCR assay. Error bars indicate SD. **P<0.01 vs PM2.5; ##P<0.01 vs PM2.5 plus Ad-LacZ.
3.2 PD-L1 expression level was highly correlated with allergic airway inflammation
Since exposure to PM2.5 reduced PD-L1 expression, we further studied the role of PM2.5 in immune response. We exposed mice to HDM to induce allergic airway inflammation. We found that only when mice were co-exposed to PM2.5 and HDM, the numbers of inflammatory cells including lymphocyte, eosinophils, and neutrophil in BALF increased significantly (figure 2A). This infiltration of inflammatory cells was related to PD-L1 expression. PD-L1 overexpression reduced infiltrated inflammatory cell numbers (figure 2A). HE staining confirmed the infiltration of inflammatory cells in lung tissue treated with exposure to PM2.5 and HDM (figure 2B). Higher mucus production was also observed upon exposure to PM2.5 and HDM, indicated by PAS staining (figure 2B). Both the infiltration of inflammatory cells and mucus production were reduced when PD-L1 was overexpressed (figure 2B), indicating a PD-L1-mediated immune response. Exposure to PM2.5 together with HDM also increased IL-4, IL-5 and IL-13 levels in the BALF, which were downregulated upon PD-L1 overexpression (figure 2C). PM2.5 largely reduced HDM-induced TGF-β upregulation, which was blocked by PD-L1 (figure 2D). In addition, we found that PM2.5- and HDM-exposed mice developed severe AHR (figure 2E). PD-L1 overexpression reduced HDM-induced AHR development (figure 2E). Exposure to PM2.5 and HDM increased serum IgE and HDM-specific IgG1 levels (figure 2F). These results together proved that PM2.5-induced immune response was related to PD-L1. PD-L1 expression level was highly correlated with the infiltration of inflammatory cells in the BALF of HDM-allergic mice.
PD-L1 expression level was highly correlated with allergic airway inflammation. (A) Frequencies and total numbers of lymphocytes, eosinophils, and neutrophils infiltrating the BALF. (B) HE and PAS staining of histological sections. (C) Concentrations of interleukin-4 (IL-4), IL-5 and IL-13 in BALF. (D) Concentrations of TGF-β and IFN-γ in BALF. (E) AHR assessment by measuring lung resistance (RL) and dynamic compliance (Cdyn). (F) Concentrations of total IgE and HDM-specific IgG1 in serum. Error bars indicate SD. **P<0.01 vs HDM; ##P<0.01 vs PM2.5 plus HDM plus Ad-LacZ.
3.3 Exposure to PM2.5 induced downregulation of PD-L1 altered inflammatory cells numbers in the lung tissue
We sorted and quantified the inflammatory cells in the lung tissue. There were no significant changes in macrophages and mDCs, but exposure to HDM+PM2.5 significantly decreased the numbers of pDCs and APCs expressing PD-L1 and Treg cells, which returned to normal levels by PD-L1 overexpression (figure 3A and B). These data showed that PM2.5 played a role in the formation of APC and Treg cells. Meanwhile, co-exposure to PM2.5 and HDM largely increased activated CD8+ and CD4+ cells and Th2 effector cells (figure 3C). PD-L1 overexpression abolished HDM+PM2.5-induced immune cell activation (figure 3C).
Exposure to PM2.5-induced downregulation of PD-L1 altered inflammatory cells numbers in the lung tissue. (A) Relative numbers of macrophages, DCs, and APCs expressing PD-L1. (B) Relative numbers of Treg cells. (C) Relative numbers of activated CD4+, CD8+, and Th2 effector cells. Error bars indicate SD. **P<0.01 vs control in A and B or HDM in C; ##P<0.01 vs PM2.5 plus Ad-LacZ in A and B or PM PM2.5 plus HDM plus Ad-LacZ in C.
3.4 Exposure to PM2.5 had little influence on the increased production of IL-25, IL-33 and TSLP induced by HDM in the lung tissue
We then determined the effects of HDM and/or PM2.5 treatment on the epithelial cell-derived cytokines such as IL-25, IL-33 and TSLP in lung tissue. As shown in figure 4, HDM alone induced upregulation of IL-25, IL-33 and TSLP. Neither PM2.5 nor PD-L1 had any effect on these cytokines.
4 Discussion
PM2.5 has become the major air pollutant in China over the past years and due to its possible adverse effects on human health, more and more researchers have begun to study the contribution of PM2.5 to various diseases (Wang and Hao 2012; Han et al. 2014). The aim of this study was to determine the role of PM2.5 in immune tolerance regulation and it was found that exposure to PM2.5 affects immune tolerance establishment of neonatal mice by reducing PD-L1 expression. We used artificial PM2.5 particles to study its influence on immune response in lung tissue. Previous study demonstrated that in HDM-induced eosinophilia in the airways of newborn mice model, defects in the establishment of immune tolerance may lead to the occurrence of allergic lung disease. Many epidemiological studies demonstrated that exposure to PM2.5 may lead to acute and chronic respiratory disease. Because the lungs are the main respiratory organs and also the major target of ambient air contaminants, several studies have shown that PM2.5 may cause lung disease (Hamra et al. 2014; Kim et al. 2015).
Interestingly, we found co-exposure to PM2.5 and HDM specifically reduced the expression of PD-L1 but not PD-L2 or PD-1. Co-exposure to PM2.5 and HDM highly increased infiltration of inflammatory cells, which was reversed by PD-L1 overexpression. PD-L1 and PD-L2 are expressed in many tissues and are highly expressed in the lung, heart and liver. PD-L1 is expressed in lymphoid and non-lymphoid tissues, suggesting that PD-1–PD-L1 interaction may modulate immune responses in secondary lymphoid organs and target organs. It has been documented that PD-1 and its ligand PD-L1 are involved in central and peripheral tolerance modulation, and loss of PD-1 or PD-L1 induced the generation of CD4+CD8+ thymocytes (Keir et al. 2007). We found that exposure to PM2.5 increased inflammatory cell numbers in BALF, which was caused by PD-L1 downregulation. Co-exposure to PM2.5 and HDM increased serum IgE and HDM-specific IgG1 levels. Importantly, exposure to PM2.5 alone slightly increased DC numbers while it reduced numbers of APCs expressing PD-L1 and Treg cells. PD-L1 overexpression upregulated APC and Treg cell numbers. Taken together, our results showed that exposure to PM2.5 reduced PD-L1 expression and increased infiltration of inflammatory cells, which further affects immune tolerance.
PM2.5 largely reduced HDM-induced TGF-β upregulation, which was blocked by PD-L1 overexpression. TGF-β is well known as a repair and profibrotic cytokine (Bochner et al. 1994). Hyperplasia of bronchial structural cells is a typical feature of airway remodeling; hence, TGF-β plays a key role in the progress of airway remodeling (Halwani et al. 2011). TGF-β is also known to be a key immunosuppressive cytokine that induced Foxp3+Treg cell production, which plays an important role in immunological tolerance (Hammad and Lambrecht 2008; Wing et al. 2008). We demonstrated that exposure to PM2.5 induced the alteration of both PD-L1 and TGF-β, which together affect immune tolerance.
References
Abbey DE, Ostro BE, Petersen F and Burchette RJ 1995 Chronic respiratory symptoms associated with estimated long-term ambient concentrations of fine particulates less than 2.5 microns in aerodynamic diameter (PM2.5) and other air pollutants. J. Expo. Anal. Environ. Epidemiol. 5 137–159
Apert C, Romagnoli P and van Meerwijk JPM 2018 IL-2 and IL-15 dependent thymic development of Foxp3-expressing regulatory T lymphocytes. Protein Cell 9 322–332
Bochner BS, Undem BJ and Lichtenstein LM 1994 Immunological aspects of allergic asthma. Annu. Rev. Immunol. 12 295–335
Bono R, Tassinari R, Bellisario V, Gilli G, Pazzi M, Pirro V, Mengozzi G, Bugiani M and Piccioni P 2015 Urban air and tobacco smoke as conditions that increase the risk of oxidative stress and respiratory response in youth. Environ. Res. 137 141–146
Brunekreef B, Janssen NA, de Hartog JJ, Oldenwening M, Meliefste K, Hoek G, Lanki T, Timonen KL, Vallius M, Pekkanen J and Van Grieken R 2005 Personal, indoor, and outdoor exposures to PM2.5 and its components for groups of cardiovascular patients in Amsterdam and Helsinki. Res. Rep. Health Eff. Inst. 1–70; discussion 71–79. https://www.ncbi.nlm.nih.gov/pubmed/15916017
Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G and Wahl SM 2003 Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 198 1875–1886
Cheong H, Wu J, Gonzales LK, Guttentag SH, Thompson CB and Lindsten T 2014 Analysis of a lung defect in autophagy-deficient mouse strains. Autophagy 10 45–56
Dockery DW 2001 Epidemiologic evidence of cardiovascular effects of particulate air pollution. Environ. Health Perspect. 109 483–486
Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR and Neurath MF 2004 Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172 5149–5153
Fife BT and Pauken KE 2011 The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann. N. Y. Acad. Sci. 1217 45–59
Francisco LM, Sage PT and Sharpe AH 2010 The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 236 219–242
Galle-Treger L, Suzuki Y, Patel N, Sankaranarayanan I, Aron JL, Maazi H, Chen L and Akbari O 2016 Nicotinic acetylcholine receptor agonist attenuates ILC2-dependent airway hyperreactivity. Nat. Commun. 7 13202
Habre R, Moshier E, Castro W, Nath A, Grunin A, Rohr A, Godbold J, Schachter N, Kattan M, Coull B and Koutrakis P 2014 The effects of PM2.5 and its components from indoor and outdoor sources on cough and wheeze symptoms in asthmatic children. J. Expo. Sci. Environ. Epidemiol. 24 380–387
Halwani R, Al-Muhsen S, Al-Jahdali H and Hamid Q 2011 Role of transforming growth factor-beta in airway remodeling in asthma. Am. J. Respir. Cell Mol. Biol. 44 127–133
Hammad H and Lambrecht BN 2008 Dendritic cells and epithelial cells: Linking innate and adaptive immunity in asthma. Nat. Rev. Immunol. 8 193–204
Hamra GB, Guha N, Cohen A, Laden F, Raaschou-Nielsen O, Samet JM, Vineis P, Forastiere F, Saldiva P, Yorifuji T and Loomis D 2014 Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis. Environ. Health Perspect. 122 906–911
Han L, Zhou W, Li W and Li L 2014 Impact of urbanization level on urban air quality: A case of fine particles (PM(2.5)) in Chinese cities. Environ. Pollut. 194 163–170
He M, Ichinose T, Yoshida S, Takano H, Nishikawa M, Sun G and Shibamoto T 2013 Induction of immune tolerance and reduction of aggravated lung eosinophilia by co-exposure to Asian sand dust and ovalbumin for 14 weeks in mice. Allergy Asthma Clin. Immunol. 9 19
Keir ME, Freeman GJ and Sharpe AH 2007 PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J. Immunol. 179 5064–5070
Kim JY, Lee EY, Choi I, Kim J and Cho KH 2015 Effects of the particulate matter(2).(5) (PM(2).(5)) on lipoprotein metabolism, uptake and degradation, and embryo toxicity. Mol. Cells 38 1096–1104
Lombardi V, Stock P, Singh AK, Kerzerho J, Yang W, Sullivan BA, Li X, Shiratsuchi T, Hnatiuk NE, Howell AR, Yu KO, Porcelli SA, Tsuji M, Kronenberg M, Wilson SB and Akbari O 2010 A CD1d-dependent antagonist inhibits the activation of invariant NKT cells and prevents development of allergen-induced airway hyperreactivity. J. Immunol. 184 2107–2115
Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K and International Agency for Research on Cancer Monograph Working Group IARC 2013 The carcinogenicity of outdoor air pollution. Lancet Oncol. 14 1262–1263
Madrigano J, Kloog I, Goldberg R, Coull BA, Mittleman MA and Schwartz J 2013 Long-term exposure to PM2.5 and incidence of acute myocardial infarction. Environ. Health Perspect. 121 192–196
Mar TF, Jansen K, Shepherd K, Lumley T, Larson TV and Koenig JQ 2005 Exhaled nitric oxide in children with asthma and short-term PM2.5 exposure in Seattle. Environ. Health Perspect. 113 1791–1794
Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M and Cheroutre H 2007 Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317 256–260
Neophytou AM, Costello S, Brown DM, Picciotto S, Noth EM, Hammond SK, Cullen MR and Eisen EA 2014 Marginal structural models in occupational epidemiology: Application in a study of ischemic heart disease incidence and PM2.5 in the US aluminum industry. Am. J. Epidemiol. 180 608–615
Nikasinovic L, Just J, Sahraoui F, Seta N, Grimfeld A and Momas I 2006 Nasal inflammation and personal exposure to fine particles PM2.5 in asthmatic children. J. Allergy Clin. Immunol. 117 1382–1388
Peng W, Ran B, Ma Y, Huang X, Chang Q and Wang X 2011 Dendritic cells transfected with PD-L1 recombinant adenovirus induces T cell suppression and long-term acceptance of allograft transplantation. Cell Immunol. 271 73–77
Perrone MG, Gualtieri M, Consonni V, Ferrero L, Sangiorgi G, Longhin E, Ballabio D, Bolzacchini E and Camatini M 2013 Particle size, chemical composition, seasons of the year and urban, rural or remote site origins as determinants of biological effects of particulate matter on pulmonary cells. Environ. Pollut. 176 215–227
Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvint D, Annunziata N and Doetschman T 1992 Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359 693–699
Song S, Yuan P, Wu H, Chen J, Fu J, Li P, Lu J and Wei W 2014 Dendritic cells with an increased PD-L1 by TGF-beta induce T cell anergy for the cytotoxicity of hepatocellular carcinoma cells. Int. Immunopharmacol. 20 117–123
Tecer LH, Alagha O, Karaca F, Tuncel G and Eldes N 2008 Particulate matter (PM(2.5), PM(10-2.5), and PM(10)) and children’s hospital admissions for asthma and respiratory diseases: A bidirectional case-crossover study. J. Toxicol Environ. Health A. 71 512–520
Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL and Marsland BJ 2014 Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20 159–166
Wang S and Hao J 2012 Air quality management in China: Issues, challenges, and options. J. Environ. Sci. (China) 24 2–13
Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T and Sakaguchi S 2008 CTLA-4 control over Foxp3+ regulatory T cell function. Science 322 271–275
Wu SH, Mou CY and Lin HP 2013 Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 42 3862–3875
Yan XD, Wang QM, Tie C, Jin HT, Han YX, Zhang JL, Yu XM, Hou Q, Zhang PP, Wang AP, Zhang PC, Gao Z and Jiang JD 2017 Polydatin protects the respiratory system from PM2.5 exposure. Sci. Rep. 7 40030
Zhang Q, Luo Q, Yuan X, Chai L, Li D, Liu J and Lv Z 2017 Atmospheric particulate matter2.5 promotes the migration and invasion of hepatocellular carcinoma cells. Oncol. Lett. 13 3445–3450
Acknowledgements
This study was supported by the Scientific and Technical Research Project of Chongqing Education Commission (Grant No.: KJ1600206, 2016).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Satyajit Rath.
Corresponding editor: Satyajit Rath
Rights and permissions
About this article
Cite this article
Yan, L., Gong, C., Ying, L. et al. PM2.5 affects establishment of immune tolerance in newborn mice by reducing PD-L1 expression. J Biosci 44, 41 (2019). https://doi.org/10.1007/s12038-019-9858-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-019-9858-6