Abstract
Root development in plants is affected by light and phytohormones. Different ranges of light wavelength influence root patterning in a particular manner. Red and white light promote overall root development, whereas blue light has both positive as well as negative role in these processes. Light-mediated root development primarily occurs through modulation of synthesis, signaling and transport of the phytohormone auxin. Auxin has been shown to play a critical role in root development. It is being well-understood that components of light and auxin signaling cross-talk with each other. However, the signaling network that can modulate the root development is an intense area of research. Currently, limited information is available about the interaction of these two signaling pathways. This review not only summarizes the current findings on how different quality and quantity of light affect various aspects of root development but also present the role of auxin in these developmental aspects starting from lower to higher plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plant development comprises of shoot and root development, which depend on various factors such as light, water, nutrients, temperature, hormones, pathogens, etc. (Lahti et al. 2005; Giuliani et al. 2005; Simonetta et al. 2007). Light and phytohormones act as major external and internal factors respectively and they cross-talk with each other to control different aspects of plant growth in a coordinated manner (Kurepin et al. 2012). This cross-talk plays a crucial role in cotyledon development, seedling etiolation, hypocotyl elongation, root development, etc., throughout the plant’s life (Nakazawa et al. 2001; Takase et al. 2004; Sibout et al. 2006). However, the mechanisms behind this cross-talk have not been studied in depth and remains obscure.
The current review is focused to understand how light-auxin interaction regulates root development in plants. Root is an essential organ in plant and it helps in anchorage to soil, absorption of water, oxygen, nutrients and minerals, and it acts as storage organ for water and carbohydrates. Generally, roots growing beneath the soil are negatively phototropic and thus never experience high light but still get influenced by light quality and quantity (Correll and Kiss 2005; Lee et al. 2016). Root development is affected by various extrinsic as well as intrinsic factors; for example, light as an extrinsic factor modulates root architecture and development, and similarly phytohormones act as intrinsic factor (Fu and Harberd 2003). Plants perceive light via various photoreceptors such as phytochromes (PHYs), cryptochromes (CRYs), phototropins (PHOTs), UV-B Resistance 8 (UVR8), etc. (Briggs and Olney 2001; Tilbrook et al. 2013). These photoreceptors are involved directly or indirectly in root development. They alter the root architecture individually as well as in a collective manner (Kelly and Leopold 1992; Van Gelderen et al. 2018). PHYs are the red (R) and far-red (FR) light photoconvertible photoreceptors in plants. In Arabidopsis thaliana, the PHY family consists of five members: Phytochrome A (PHYA), Phytochrome B (PHYB), Phytochrome C (PHYC), Phytochrome D (PHYD) and Phytochrome E (PHYE). Among these, PHYA is the major FR light (λmax 720 nm) and other PHYs (PHYB-E) are mainly R light (λmax 660 nm) photoreceptors. They exist in two photo interconvertible forms: Pr, (R light absorbing form) is the inactive form of PHYs which is synthesized in dark and the Pfr (FR light absorbing form), active form that performs most of the light regulated functions in plants. PHYA is photolabile with a half-life of about 30 min whereas other PHYs (PHYB-PHYE) are photostable in nature (Li et al. 2011). PHYs regulate several functions such as seed germination, de-etiolation, gravitropic response of root and hypocotyl, shade avoidance, flowering time, etc. in plants. PHYA, PHYB and PHYE have major contributions in seed germination as PHYA and PHYE promote it under continuous FR light but PHYB regulates the seed germination under R light in low-fluence response mode. At higher temperature (22–28°C), PHYB has the major role in seed germination whereas at low temperature (7–10°C), it is predominantly regulated by PHYE (Heschel et al. 2007; Li et al. 2011). De-etiolation is the change in gene expression as well as change in morphology of seedlings upon light irradiation. PHYA and PHYB are primarily involved in the regulation of de-etiolation of seedlings under FR and R light respectively, PHYC, PHYD and PHYE have minor or non-significant role in this phenomenon (Heschel et al. 2007). PHYs also control the phototropism and gravitropism of root and hypocotyl. PHYA and PHYB suppress hypocotyl negative gravitropism by inhibiting Phytochrome Interacting Factors, PIFs (PIF1, PIF3, PIF4, PIF5). PIFs are the transcription factors having basic helix-loop-helix (bHLH) domain, which physically interact with PHYs and antagonize their functions. PHYs control the hypocotyl negative gravitropism by mediating the conversion of gravity sensing endodermal amyloplast to etioplastic or chloroplastic plastids. This conversion is inhibited by PIFs and hence PIFs act antagonist to PHYs in this process (Kim et al. 2011). PIF3 functions downstream to PHYB signaling and its overexpression causes reduction in root growth in presence of nitric oxide. It has been shown that upon nitric oxide treatment, PIF3 mediated root growth inhibition involves PHYB signaling (Bai et al. 2014). On the other hand, PIF4 has been shown to promote Aluminum-dependent primary root inhibition. PIF4 inhibits root growth through regulating the auxin level by upregulating the expression of YUC5, YUC8 and YUC9 genes (Liu et al. 2016).
PHYA and PHYB are also involved in red light-mediated positive phototropism of roots whereas other PHYs have shown non-significant role (Kiss 2003). Plants trick to avoid shade and compete for light when grown under dense canopy and become taller. Shade avoidance syndrome is the phenomenon by which plants sense the neighboring plants and low R:FR ratio of light, that result in elongated hypocotyl, reduced amount of chlorophyll, smaller leaf area, earlier flowering etc. PHYB has significant role while PHYD and PHYE act redundantly with PHYB in regulating shade avoidance responses. PHYA plays minor role in this phenomenon which is antagonistic to PHYB (Martínez-García et al. 2014). Shading also causes earlier flowering in plants. In general, flowering is delayed under continuous light of high R:FR ratio whereas promoted by light of low R:FR ratio. PHYA has been shown to promote flowering and it is independent of photoperiodism. PHYB, PHYD and PHYE delay flowering in a redundant manner and PHYB affects the flowering predominantly under short days (SDs). PHYB alongwith PHYE delay flowering under SDs but, PHYB and PHYD together delay the flowering both under Long Days (LDs) and SDs (Lin 2000; Franklin and Quail 2010). During root development, PHYA and PHYB are shown to inhibit the root elongation while PHYA, PHYB and PHYE are known to promote lateral root generation (Correll and Kiss 2005; Salisbury et al. 2007).
Blue light signaling is regulated by CRYs and PHOTs photoreceptors. In Arabidopsis, CRYs are mainly of three types, CRY1, CRY2 and CRY3 and PHOTs are of two types, PHOT1 and PHOT2 (Liu et al. 2012; Zhao et al. 2013). CRYs are unique kind of photoreceptors which are conserved in both animals and plants. They are primarily involved in de-etiolation, flowering, circadian clock regulation, root development etc. CRY3 is a distinct member of CRY family which acts as single stranded DNA repair enzyme in mitochondria and chloroplast and it has non-significant role in light signaling. Blue light-mediated de-etiolation is controlled by both CRY1 and CRY2 and it involves interaction with various downstream signaling genes such as CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1), SUPPRESSOR OF PHYA 1 (SPA1), ELONGATED HYPOCOTYL 5 (HY5), HY5 HOMOLOGUE (HYH) (Yu et al. 2010). COP1 is an E3 ubiquitin ligase and it suppresses photomorphogenesis. SPA1 inhibits photomorphogenesis by modulating the enzyme activity of COP1 and specifically involved in degradation of PHYA protein. HY5 and HYH are the enhancer of photomorphogenesis and they interact with photoreceptors for promoting the downstream light signaling processes. CRY1 has a minor role in flowering time control whereas CRY2 has predominant effect which positively regulates photoperiodic flowering time. CRY2 acts antagonistic to PHYB, as PHYB negatively regulates timing to flower (El-Din El-Assal et al. 2003). CRY1 and CRY2 both act redundantly in regulation of circadian clock (Devlin 2000). In case of root development, it is reported that CRY2 acts opposite to CRY1 and inhibits root elongation (Canamero et al. 2006). It was further studied that cytoplasmic CRY1 promotes root elongation whereas the nuclear one suppresses this process (Wu and Spalding 2007). PHOTs also regulate various plant developmental aspects such as hypocotyl phototropism, stomatal opening, root growth, root phototropism, etc. PHOT1 acts under both low and high fluences of blue light whereas PHOT2 functions only under high fluence of blue light. Blue light causes hypocotyl phototropism by inducing cytosolic Ca2+ and this is mediated via PHOT1 and PHOT2 (Zhao et al. 2013). Blue light also causes stomatal opening, where both PHOT1 and PHOT2 are involved (Kinoshita et al. 2001). In PHOT-dependent root growth, it has been reported that PHOT1 negatively regulates lateral root growth, lateral root number and density and these are mediated through inhibition of lateral root epidermal cell elongation (Moni et al. 2015). PHOT1 and NON-PHOTOTROPIC HYPOCOTYL 3 (NPH3) both control the blue light-mediated negative root phototropism. NPH3 is a blue light signal transducer acting alongwith with PHOTs. PHOT1 and NPH3 induce positive root geotropism, mediated through PIN2 localization and also control auxin efflux rate (Wan et al. 2012).
Plants also resist UV light perceived through UVR8 photoreceptor. This photoreceptor also plays many roles in plant development such as inhibition of hypocotyl, altered flowering and root growth. Low dose of UV-B irradiation induces photomorphogenesis and hence causes inhibition of hypocotyl elongation (Li et al. 2013). UV-B mediated flowering is regulated by UVR8 and it induces flowering under noninductive photoperiod through the regulation of CO (CONSTANS) and FT (FLOWERING LOCUS T) genes. CO is a B-box zinc finger protein which promotes flowering and is influenced by different quality of light. FT is the florigen and the integrator that plays downstream to CO in the process of flower induction (Arongaus et al. 2018). In root development on exposure of low fluence of UV-B light, overexpression of UVR8 causes reduction in primary root growth, lateral root density and delayed emergence of lateral roots. This altered root phenotype is most likely due to UV-B dependent change in auxin transport (Fasano et al. 2014).
In plants, various hormones are present including auxin, cytokinin, gibberellins, and abscisic acid, etc. Among them, auxin has multiple roles in plant development such as apical dominance, organogenesis, tropic responses, lateral root branching, etc. (Fahad et al. 2015). Apical dominance means the primary shoot growth dominates over lateral branching, arising from the main stem and auxin promotes the apical dominance phenomenon (Mller and Ottoline 2011). Auxin also regulates the organogenesis through generation of local auxin gradient and its polar transport (Bohn-Courseau 2010). Similarly, auxin has been shown to control phototropism and gravitropism and these processes are dependent on the regulation of auxin accumulation and transport (Muday 2001). It has a major role in root growth, as it promotes lateral root branching by promoting cell elongation and also promotes root hair elongation (Pitts et al. 1998; Fukaki et al. 2007). Light has been shown to affect auxin at several level such as its synthesis, transport, signaling by modulating the genes involved in these processes. Active form of PHYs reduce auxin level in plants by regulating its biosynthetic pathway genes, including SUPEROOT2 (SUR2) and TRYPTOPHAN AMINOTRANSFERASE ARABIDOPSIS 1 (TAA1) which act as suppressor and enhancer of auxin synthesis respectively. PHYA and PHYB also control auxin concentration by regulating the expression of GH3 family genes. SUR2 gene encodes CYP83B1 protein which is a member of P450-dependent monooxygenase and is involved in auxin biosynthesis. TAA1 also plays an important role in auxin biosynthesis as it controls the production of Indole-3-pyruvate (IPA), one of the precursors of auxin. PHYs and CRYs regulate the polar auxin transport (PAT) from shoot to root and affect root and shoot development. Light affects the auxin re-distribution by controlling the localisation of auxin efflux proteins such as Phosophoglycoproteins (PGPs) and PINs. It has been reported that, light controls the auxin flux by regulating the function of PIN1, PIN2, PIN3 and PIN7 proteins. Pfr form of PHYB negatively regulates PIN3 expression, on the other hand PHYA, PHYB, CRY1 and CRY2 have been shown to collectively regulate PGP19 protein expression. Blue light has specifically been shown to regulate PIN2 expression through HY5. HY5 also regulates the expression of AUXIN RESISTANT 2 (AXR2/IAA7) and SOLITARY ROOT/ INDOLE-3-ACETIC ACID INDUCIBLE 14 (SRL/IAA14) genes under shade condition (low R:FR). Under low R:FR ratio, PHYTOCHROME RAPIDLY REGULATED 1 (PAR1) and PAR2 negatively regulate the expression of auxin inducible genes such as SMALL AUXIN UP RNA 15 (SAUR15) and SAUR68 alongwith this, LONG HYPOCOTYL IN FAR-RED1 (HFR1) downregulates the IAA29 expression (Halliday et al. 2009). PAR1 and PAR2 are the bHLH transcriptional factors which act as negative regulators of shade avoidance response. HFR1 is also a bHLH transcription factor and it controls the photomorphogenesis through regulating the PHY and CRY signaling. In shade condition, PIF7 influences YUC gene expression, which encodes an enzyme involved in tryptophan-dependent auxin biosynthesis (Yang and Lin 2017). These findings explained a link between light and auxin signaling elucidating the role of their cross-talk in plant development (figure 1). Auxin is the most important phytohormone specifically regulating the growth and development of root.
Root development involves many aspects such as primary root growth, lateral branching, root hair formation, adventitious root growth, root gravitropism etc. In plant kingdom, different forms of root are present. For instance, in lower plants, true roots are absent; instead of root, rhizoids are present. Rhizoids are similar to root hairs in structure and function, they can be unicellular or multicellular in nature. Rhizoids help in anchorage to the substratum along with absorption of water and minerals (Goffinet et al. 2009). In lower model plant like Physcomitrella patens (P. patens), rhizoids are predominant (figure 2A). In monocots such as rice, two different types of roots are majorly present such as seminal and crown roots (figure 4A). Seminal roots are the first to emerge from the radicle after seed germination and are functional for very short period. They are important for healthy growth of rice seedlings as they help in the absorption of water and nutrients during seedling stage. On the other hand, crown roots emerge from nodal region, they are aerial in nature and are major part of fibrous root system, they also help in anchorage and absorption of water and nutrients at adult stage (Lynch and Brown 2012).
Cryptochrome mediated rhizoid development in P. patens. (A) Rhizoid formation in P. patens (image source: Sakakibara et al. 2003). (B) Cryptochrome mediated rhizoid development through auxin inducible genes in P. patens.
In this review, we have highlighted various types of root present in the plants under study and few related aspects of root development; such as rhizoid (root-hair like structures) development in mosses, U-turn formation at root apex in maize, seminal and crown root development in rice, primary and lateral root development as well as root greening phenomenon in Arabidopsis, primary and secondary lateral root development in tobacco and root elongation, root area, total root biomass, etc., in grapes. Light signaling is involved in the regulation of above-mentioned aspects of root growth directly or indirectly through interaction with auxin signaling. Auxin-dependent root development has been well studied in plants whereas the study demonstrating light-dependent root architecture is far from being systematically investigated. The connecting nodal elements among light signaling, auxin signaling and root development have not been well explored. Hence, light and auxin together modulating the root architecture or its development is an active area under investigation. Here, we elucidate some of the facts about how root development is affected by cross talk between light and auxin signaling pathways using a lower non-flowering plant P. patens and few higher flowering plants.
2 Rhizoid development in moss Physcomitrella patens
P. patens is a bryophyte with multicellular rhizoids (figure 2A). In P. patens, the role of interaction between light and auxin in the development of rhizoids has been studied. It has been reported that, in Physcomitrella rhizoid development is promoted by auxin through positive regulation of P. patens ROOTHAIR DEFECTIVE SIX-LIKE 1 (PpRSL1) and PpRSL2 genes (Jang and Dolan 2011). RSL are bHLH transcription factors which are implicated in auxin signaling for rhizoid development. On the other hand, it has been shown that the disturbance in CRY signaling influences the expression of auxin-inducible genes such as P. patens GH3-like 1 (PpGH3L1) and P. patens Indole-3 Acetic Acid 1 (PpIAA1). Under blue light, CRYs negatively regulate the auxin induced gene expression (Imaizumi et al. 2002). The promotion of rhizoid development in mosses by auxin and negative regulation of auxin induced genes by CRY, support the fact regarding the control of CRY in the rhizoid development by modulating the auxin signaling (figure 2B) (Sakakibara et al. 2003).
3 U-turn formation at root apex in Zea mays L. cv
In monocots like Zea mays, U-turn formation (figure 3A) at the root apex has been shown to be dependent on thigmotropism and gravitropism. These two phenomena are mediated through light irradiation and need an intact root apex. Root cap is responsible for sensing light and gravity and U-turn formation depends on the gravity and light sensing ability of the root cap. When root is irradiated with white light, U-turn formation is induced. It has also been shown that, light enhances the concentration of Indole 3-acetic acid (IAA), a naturally occurring auxin, in the root transition zone by regulating YUC-mediated IAA synthesis pathway. By the tryptophan-dependent auxin synthetic pathway and YUC-mediated IAA synthetic pathway, first tryptophan is converted to Indole 3 pyruvic acid (IPA) by tryptophan aminotransferase and then oxidation takes place, forming IAA by YUCCA (Dai et al. 2013). Hence, the U-turn formation at root apex is positively regulated by light and it occurs through increment in IAA accumulation and its partitioning in root (figure 3B) (Suzuki et al. 2016).
Light regulated and auxin dependant root phenotype in maize. (A) U-turn formation at root apex in maize (image source: Suzuki et al. 2016). (B) White light irradiation promotes YUC-mediated IAA synthesis and leads to U-turn formation at root cap in Zea Mays.
4 Seminal and crown root development in Oryza sativa
In Oryza sativa, continuous white light inhibits the seminal root growth that is required for the healthy survival of young seedlings whereas promotes crown roots which emerge at the adult stage (figure 4D). In seminal root development, PHYA and PHYB control very low-fluence responses (VLFR) and low-fluence responses (LFR) respectively but their effects are not significant. Therefore, it can be concluded that light other than red and far-red, for instances blue light may play major role in regulation of seminal root development (Shimizu et al. 2009). Light-induced root morphology vary among different varieties of rice such as, Taichung Native 1 (TCN1) and Tainung 67 (TNG67), indica and japonica varieties respectively. Both of them respond differently to auxin during the root development. In Zea mays, constitutive expression of Oryza sativa root architecture associated 1 (OsRAA1) gene under the control of ubiquitin promoter causes reduced primary root growth, large number of adventitious and primary root helix; in rice seedlings it causes retardation in gravitropic response of roots. OsRAA1 is an auxin-inducible gene in rice involved in root development. Studies have demonstrated that light induces the expression of OsRAA1 gene in seminal root of TCN1. Continuous white light causes shorter and wavy seminal roots in TCN1 but not in TNG67 (figure 4B and C) which is dependent on light-mediated increment in auxin concentration and its polar transport (figure 4E) (Wang et al. 2011).
White light regulated root development in rice. (A) Seminal and crown root growth in rice seedling (image source: Shimizu et al. 2009). (B) Wave formation in seminal root of rice (image source: Wang et al. 2011). (C) Light and NAA induced seminal root development in two different varieties of rice, TCN1 and TNG67 (image source: Wang et al. 2011). (D) Differential effect of white light irradiation over growth of seminal and crown root in Oryza sativa. (E) Seminal root development under white light through regulation of auxin concentration and its transport in Oryza sativa.
5 Primary and lateral root development, root phototropism and greening in Arabidopsis thaliana
Arabidopsis thaliana is the extensively studied model plant, where various aspects of root architecture have been studied such as primary, secondary root growth, etc. (figure 5A). HY5, is one of the positive regulators of photomorphogenesis, acting downstream to PHYs and encodes a basic leucine zipper (bZIP) type of transcription factor (Oyama et al. 1997; Chattopadhyay et al. 1998). HYH also belongs to a bZIP family of transcription factors and is involved in PHYB signaling (Holm et al. 2002). Under constant white light, hy5 mutant of Arabidopsis shows more lateral root branching and downregulation of negative regulators of auxin signaling such as AXR2 and SLR genes (figure 5C). Hence, it can be correlated that large number of lateral root formation in hy5 mutant is due to enhanced auxin signaling (Cluis et al. 2004). In hy5 mutant, emergence of lateral root and its growth are enhanced, which suggest inhibitory effect of HY5 on root growth, similarly HYH also suppresses the root growth. But surprisingly, it has been observed that in hy5hyh double mutant, root growth is reduced as compared to hy5 mutant as well as wild type. hy5hyh mutant has fused cotyledons and impaired vasculature along with impaired root growth. hy5 and hy5hyh mutants show altered expression of auxin responsive and signaling genes. HY5 and HYH act as the negative regulators of auxin signaling from an early stage (embryogenesis) till further stages of seedling development. The unusual root growth phenotype in the double mutant can be explained as: increased auxin signaling in single mutant (hy5) enhances the root growth but further enhancement in auxin signaling in case of double mutant (hy5hyh) cause suppression of root growth (figure 5D). Hence, this abrupt enhancement in auxin signaling beyond the threshold in double mutant causes reduced root development (Sibout et al. 2006). DWARF IN LIGHT 1 (DFL1) is an auxin-responsive GH3 gene analogue. dfl1-D is a dominant mutant having shorter hypocotyl under continuous blue, R and FR light conditions. It has been shown that it is involved in auxin signal transduction and causes inhibition of the lateral root growth but has no effect on primary root growth under white light (figure 5E) (Nakazawa et al. 2001). This can be a candidate gene playing role in auxin and light signaling cross-talk in regulation of root growth.
Different aspects of root development in Arabidopsis. (A) Primary and secondary root formation in Arabidopsis. (B) Root Phototropism in Arabidopsis thaliana (image source: Boccalandro et al. 2008). (C) HY5-mediated lateral root branching through AXR2 and SLR genes in Arabidopsis thaliana under white light. (D) HY5 and HYH negatively regulate auxin signal transduction and hence reduce lateral root emergence and root growth in Arabidopsis thaliana. (E) Under white light, DLF1 negatively regulates auxin signaling and reduces the lateral root no. in Arabidopsis thaliana. (F) Auxin transport regulating primary root growth by controlling cryptochrome translocation in Arabidopsis thaliana. (G) Blue light-mediated lateral root development through PIN distribution and auxin accumulation in Arabidopsis thaliana. (H) Blue light-mediated negative root phototropism in Arabidopsis thaliana via PINs. (I) Red light, blue light and auxin signaling affecting root greening through HY5 in Arabidopsis thaliana.
Under blue light, CRY1 positively regulates the primary root elongation whereas CRY2 has an antagonistic role. In cry1cry2 double mutant, the primary root is shorter than the wild type and which is possibly due to the inhibitory effects of CRY2. CRY signal is perceived at the shoot region and further translocate from shoot to root to modulate the root architecture. Hence, in spite of CRY located in the root, the CRYs present in shoot are involved in root development. It has been shown in N-1-naphthylphthalamic acid (NPA) treatment experiment that, CRY translocation from shoot to root is associated with inhibition of PAT, resulting in reduction of root growth (figure 5F). This indicates that components of auxin and blue light signaling cross-talk in regulating the root development (Canamero et al. 2006). Further it has been shown that, CRY1 negatively regulates lateral root development under higher intensity of blue light as number of lateral roots is reduced in case of CRY overexpressing plants (CRYox) whereas cry1 and cry1cry2 mutants have enhanced number of lateral roots in comparison to wild type. CRY2 has no significant role in lateral root development as the lateral root growth is similar in case of CRY2ox and cry2 mutant plants. CRY1 controls the development of lateral root by modulating the IAA level in plants. It negatively regulates the lateral root growth by inhibiting auxin transport as it downregulates the PIN1 gene expression whereas PIN2 expression remains unaffected (figure 5G) (Zeng et al. 2010). It was observed that PIN1 expression gets reduced to half in CRY1ox as compared to wildtype whereas in case of cry1 and cry1cry2 mutant, PIN1 expression is upregulated. PIN1 and PIN2 are PIN-FORMED proteins which act as efflux transporters of auxin through the membrane and hence regulate the asymmetric distribution of auxin (Zhang et al. 2014). It has also been shown that under blue light, the endogenous level of IAA in CRY1ox root is almost half in comparison to cry1 mutant and wildtype. The reduction of IAA content is due to suppression in PAT from shoots to roots. Flavonoid content has been shown to be more in CRY1ox seedlings which suggests the suppression in polar auxin transport, as flavonoids are known to inhibit auxin transport. Under red light or in dark condition, CRY1 doesn’t affect lateral root growth. It has also been reported that low fluence of UV-B suppresses the primary root, density of lateral root as well as lateral root emergence. In this altered root development, since the cell count remains constant then reduction in cell elongation could be the possible cause for the reduction in root growth. In UVR8 overexpressing plants, roots show higher accumulation of flavonoids and UVR8-mediated modified root phenotype is most likely due to alteration in auxin transport as flavonoids act as internal regulator of auxin transport (Fasano et al. 2014).
Very low fluence (10 mol m−2 s−1) of blue light affects root phototropism (figure 5B). This blue light-mediated root phototropism defect has been shown in phototropin mutant 1 (phot1) but non-significant difference has been observed in case of cry1 and phot2 mutants. The root negative phototropism occurs via asymmetric auxin redistribution. When root is irradiated with unilateral blue light, PIN3 accumulates towards outer lateral membrane of columella cells, as a result auxin accumulates on irradiated side of root. The increased auxin concentration promotes growth on illuminated side of root and also causes bending of root away from light. The PIN3 polarization results in the asymmetric distribution of auxin and root negative phototropic response in Arabidopsis (Zhang et al. 2013). PHOT1-mediated root negative phototropism occurs through interaction with the proteins such as PHYTOCHROME KINASE SUBSTRATE 1 (PKS1), (Lariguet et al. 2006; Boccalandro et al. 2008), ROOT PHOTOTROPISM 2 (RPT2), (Inada et al. 2004) and NPH3 (Wan et al. 2012). PKS1 binds to PHYA or PHYB and it negatively regulates PHYB signaling. RPT2 is a light inducible signal transducer involved in phototropism. NPH3 is blue light transducer and involved in blue light signaling pathway. Blue light dependent root phototropism in Arabidopsis is also mediated through PIN5. In dark, PIN1 is localized in intracellular compartments of root, and upon blue light irradiation; it gets translocated to root stele cells in basal plasma membranes. The blue-light mediated distribution of PIN1 is responsible for the asymmetric auxin distribution and negative phototropism of root. PHOT1 is the major blue light photoreceptor which regulates PIN1 redistribution in roots. CRY1 and PHOT2 don’t have significant effects in this phenomenon. This PIN1 localization and negative root phototropism are regulated by Brefeldin A (BFA)-sensitive vesicle trafficking pathway and protein kinase PINOID/protein phosphatase 2A (PID/PP2A) activity (Zhang et al. 2014). BFA is a vesicle trafficking inhibitor (Satiat-Jeunemaitre and Hawes 1992), PID is involved in regulating auxin signaling and PP2A is a protein phosphatase involved in PIN localization. PID and PP2A act antagonistically in the regulation of PIN localization (Michniewicz et al. 2007). Hence, the blue light-mediated root negative phototropism occurs through PIN1, PIN3 and PIN5 localisation by PHOT1 (figure 5H).
Promotion of root greening by blue light has been described long back. PHYA, PHYB and CRYs regulate root greening individually or collectively. Root greening takes place due to chlorophyll synthesis in root which is controlled by auxin and cytokinin signaling. In blue light-mediated root greening process, the CRY1 and PHYs play major role while CRY2 has less significant role, on the other hand in red light-mediated root greening PHYB has the predominant role (Usami et al. 2004). HY5 controls the expression level of important genes involved in chlorophyll biosynthesis in roots. In hy2slr-1 double mutant, the chlorophyll amount has been shown to be comparable to the wildtype whereas in hy5slr-2 mutant, chlorophyll content is very less. SLR genes belongs to AUX/IAA family and are involved in lateral root development. Hence, it can be concluded that root greening by SLR is dependent on HY5. It has also been shown that HY5 along with GOLDEN 2-LIKE (GLK) induce greening of roots by promoting the genes involved in chlorophyll biosynthesis (Usami et al. 2004). GLK is a root greening transcription factor involved in chloroplast biogenesis and chlorophyll biosynthesis (Waters et al. 2008; Waters et al. 2009). It has been further shown in an IAA treated root experiment that, root greening is affected by auxin transport. The chlorophyll content in intact root after auxin treatment don’t change but drastically increases in case of detached roots. It has also been observed that the chlorophyll content is enhanced in case of auxin influx transport defective mutant, axr4-2 aux1-7 (Yamamoto and Yamamoto 1999). Auxin signaling negatively affects root greening through changing the expression of IAA14, AUXIN RESPONSE FACTOR 7 (ARF7) and ARF19 genes. Auxin and cytokinin signaling cross-talk with each other and affect root greening via HY5 (Kobayashi et al. 2012). GLK and HY5 promote root greening in a coordinated manner downstream to the hormone and light signaling pathways. Hence, the root greening phenomenon in plants is the outcome of light and hormone interactions (figure 5I).
6 Primary and secondary root growth in Nicotiana tabacum L.
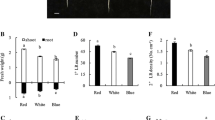
In Nicotiana tabacum, different quality of light has differential effects on root growth (figure 6A). Red light irradiation results in increased root fresh weight, primary lateral root number and secondary lateral root density but leads to shorter lateral root length compared to white and blue light. Conversely, blue light has more impact in promoting average primary and secondary root length with respect to other light quality. Endogenous auxin quantification in leaves and roots under red and blue light has shown that under red light, auxin content of leaves is less but more in case of roots but blue light irradiation caused the opposite effect. Red light irradiation accelerates PAT from leaves towards root as compared to other light qualities. Thus, different wavelengths of light control lateral root growth through auxin redistribution in plants. PIN expression has been shown to be affected by red and blue light. In shoot/root junction upon blue light irradiation, PIN1, PIN1b, PIN3, PIN4 genes are downregulated whereas PIN3b is upregulated in comparison to white light. On the other hand, PIN1, PIN1b, PIN3 and PIN3b are upregulated by red light as compared to white light. But in root, PIN1b and PIN3b genes are upregulated whereas expression of PIN1, PIN3, PIN4 and PIN9 is downregulated under blue light. On the other hand, in root, red light irradiation promotes the expression of PIN3, PIN3b and PIN9 genes. PIN3 gene is positively regulated by red light whereas PIN1, PIN3 and PIN4 genes are negatively controlled by blue light in root region as well as at the shoot/root junction. Hence, it has been concluded that in tobacco plants light dependent lateral root emergence and development are controlled by expression of PINs (figure 6B) (Meng et al. 2015).
Different spectrum of light regulates root growth in tobacco. (A) Root growth under different light conditions in tobacco. (B) Red and blue light modulating PINs leading to primary (1°), secondary (2°) root and lateral root development in Nicotiana tabacum. Image source: Meng et al. 2015.
7 Light quality affects root development in Vitis vinifera L.
The type of root present in Vitis vinifera has been shown (figure 7A) and it has been reported that blue light suppresses genes encoding auxin-repressed protein (Auxin-repressed 12.5 kDa protein like) leading to enhanced auxin accumulation. The higher accumulation of auxin than the required amount in roots exert negative effects on root development. Red and green light positively regulate the expression of auxin inhibitor protein gene and hence they maintain the optimal level of auxin concentration required for normal root growth. The impact of red and green light on overall root development is more pronounced as compared to white and blue light. Red and green light promote many aspects of root development such as total length of root, root area, root volume, root dry mass etc., whereas blue and white light have less significant effects over these growth parameters. On the other hand, root diameter is majorly increased by blue light irradiation (Li et al. 2017). Hence, it can be concluded that in Vitis light-mediated root development is dependent on light regulated auxin responsive gene expression and it also depends on the light quality (figure 7B).
Differential root growth under different quality of light. (A) Root development in grapes under different light quality (image sources: Ronseaux et al. 2013). (B) Root development in Vitis vinifera through light mediated auxin repressed protein activity.
8 Discussion
Despite limited data is available on the mechanisms of light-mediated root development through auxin, the current review has tried to present most of the signaling events and cross-talks documented in the plant kingdom into picture. Different wavelengths of light regulate different aspects of root growth and development. In lower plants such as in P. patens, the rhizoid development with respect to light and auxin has not been studied in detail. Previous studies showed that, CRYs regulate rhizoid growth by modulating the expression of auxin signaling genes. Since, CRYs have also being implicated in rhizoid development, PHOTs could be involved in this process as both of them perceive the same spectrum of light. In a recent report, PHOTs have shown to be involved in phototropism as well as chloroplast movement (Kimura et al. 2018). Further in this regard, the role of photoreceptors such as PHYs, PHOTs and their downstream genes could be explored. The detailed study in case of Physcomitrella will better help in understanding the evolution of photoreceptor’s interaction with phytohormones in rhizoid growth. In monocots, alongwith primary root, various types of adventitious roots also emerge, such as in Zea mays, seminal, crown, brace, prop roots are present. Seminal roots are the temporary, underground roots and they arise during embryogenesis. Crown roots also grow under the soil while brace and prop roots arise above the ground but in later stage, they come in contact with soil and grow similar like crown roots. In maize, root cap is the major site to sense the touch and gravity. It has been shown that, R and blue light mostly stimulate geotropism in roots whereas FR light has opposite effect (Klemmer and Schneider 1979). Blue light irradiation also causes negative phototropic response in maize roots (Mullen et al. 2002). Auxin has been shown to promote the root development involving ZmPINa which regulates PAT. More amount of auxin in root suppresses primary root growth while lateral root emergence is enhanced, which result in drought resistance (Li et al. 2018). Although, the role of light and auxin have been reported in root development, but the nodal components between these two signaling pathways are not well established. In the recent review, we have presented a report which emphasizes the white light promoting U-turn formation in maize by regulating IAA level in root apex. How photoreceptors control the auxin regulated root growth and related gene expression needs to be studied extensively in this case. Rice has similar types of root as present in maize, where it has been reported that white light regulates the growth of seminal and crown root in different manner. Blue light has been shown to play predominant role in suppression of adventitious root emergence and its upward growth, red and FR light were also implicated in this phenomenon (Lin and Sauter 2018). OsPIL15, (orthologue of PIF family in rice) has been shown to promote seminal root, upon light irradiation (Zhou et al. 2014). It has also been shown in rice that, OsAUX1 gene, an efflux of auxin transport regulates gravitropic responses and primary and crown root angle, that also promotes root hair elongation (Giri et al. 2018). Very limited information is available concerning the photoreceptor’s involvement with auxin to regulate root growth in rice. The light dependent promoter regulation of most important PINs and other auxin related genes will help in understanding the detail of the root morphology. As, rice, wheat and maize are the staple food items, it would be beneficial to investigate the common components of light and auxin signaling modulating the root growth. This will help to generate improved varieties of cereals with strong and healthy root system with high yield. In case of dicot, Arabidopsis thaliana is the most studied model plant. Here, the PHYs, CRYs and PHOTs signaling have been reported to cross-talk with auxin to regulate root growth and HY5 is the most important central regulator of light and hormone interaction. PIFs have been shown to interact with auxin under different light conditions, specifically in presence of low R:FR light. The PIF-auxin interaction has presented clear idea about the hypocotyl growth while with respect to root development, their cross-talk is far from being understood. PIF4, PIF5 and PIF7 are the main PIFs which regulate auxin synthesis, signaling and transport in plant development (Hornitschek et al. 2012; Yang and Lin 2017). Few reports are available which present the cross-talk of PIFs and auxin in root development. In specific stress conditions such as in presence of aluminum and nitric oxide, their cross-talk has been documented (Bai et al. 2014; Liu et al. 2016). Several downstream genes of light signaling could be investigated which directly or indirectly affect root growth via modulating auxin related pathways. Studies in case of Nicotiana show that very limited information exist concerning light controlling the root growth. In a light intensity dependent experiment, it has been reported that root biomass increases with increasing white light intensity. It has also been shown that root responded more than shoot with increasing light intensity. The enhanced root growth under higher intensity of light has been correlated with enhanced sugar transport from shoot to root (Nagel et al. 2006). A report suggests higher amount auxin causes reduction in root growth and it affects more the root elongation than root density (Niu et al. 2013). Root growth with respect to photoreceptors and auxin signaling could be investigated in detail to elucidate more about light dependency in root patterning. The role of common players in the light and auxin signaling which affect root development such as HY5 could be analysed in tobacco. The root morphology in photoreceptor mutants and in some other transgenic lines such as photoreceptor overexpressing plants with mutation in auxin related genes of tobacco will help in understanding this field more clearly. The fruit yielding plants such as Vitis, the root is deeply spread but the density of root is very less. Handful of reports are present which explains the light dependent root growth in grapes. It has been reported that rooting frequency is enhanced by red light (Poudel et al. 2008). Root growth has also been shown to be affected by circadian clock and carbon supply suggests the photoperiod regulated root growth (Mahmud et al. 2018). Auxin induces rooting, it is accelerated with increasing concentration of auxin while at higher concentration, auxin shows an inhibitory effect (Galavi et al. 2013). More informations need to be established which can connect the role of light signaling and auxin homeostasis in root patterning. Hence, detailed study in this regard will help to improve the knowledge and to produce improved varieties of plant. Most of the studies have been done in respect of light regulation and auxin control over root growth separately, but the interaction of light-auxin involved in root growth has not been investigated in detail.
The current review will help to understand most of the existing mechanisms and evolution of known molecular players underlying light and auxin signaling to regulate the rhizoid/root development in lower as well as higher plants. Light regulated auxin signaling genes in root growth have been shown to be conserved as they have been found in different group of plants from lower to higher. Light has been shown to control the root development by modulating the auxin synthesis, signaling and transport. It will also help in finding out the conserved mechanisms and genes that depict the land plant evolution. A mechanistic cross-talk of light and auxin signaling that regulate the different aspects of root growth has been shown in figure 8. Though, many of the components involved in the light-auxin interaction in root pattering have been identified, still more studies could to be done to decipher in-depth knowledge on this mechanism.
Abbreviations
- ARF7:
-
auxin response factor 7
- AXR2:
-
auxin resistant 2
- BFA:
-
brefeldin A
- bZIP:
-
basic leucine zipper
- bHLH:
-
basic helix loop helix
- COP1:
-
constitutive photomorphogenic 1
- CRYs:
-
cryptochromes
- CRYox:
-
cryptochrome overexpression
- DFL1:
-
dwarf in light 1
- FR:
-
far-red
- GLK:
-
golden 2-like
- HYH:
-
homolog of HY5
- HY5:
-
elongated hypocotyl 5
- IAA:
-
indole-3 acetic acid
- IAA14:
-
indole-3-acetic acid inducible 14
- IPA:
-
indole-3 pyruvic acid
- NPH3:
-
non-phototropic hypocotyl 3
- OsRAA1:
-
Oryza sativa root architecture associated 1
- PAT:
-
polar auxin transport
- PGPs:
-
phosophoglycoproteins
- PHOTs:
-
phototropins
- PHYs:
-
phytochromes
- PID:
-
protein kinase PINOID
- PIF3:
-
phytochrome interacting factor 3
- PKS1:
-
phytochrome kinase substrate 1
- PP2A:
-
protein phosphatase 2A
- P. patens :
-
Physcomitrella patens
- PpGH3L1:
-
Physcomitrella patens GH3-like protein 1
- PpIAA1:
-
Physcomitrella patens indole-3-acetic acid 1
- PpRSL1:
-
Physcomitrella patens root hair defective six-like 1
- R:
-
red
- RPT2:
-
root phototropism 2
- SLR:
-
solitary root
- SPA1:
-
suppressor of PHYA 1
- TCN1:
-
taichung native 1
- TNG67:
-
tainung 67
- UVR8:
-
UV-B resistance 8
References
Arongaus AB, Chen S, Pireyre M, Glöckner N, Galvão VC, Andreas A, Winkler JB, Fankhauser C, Harter K and Ulm R 2018 Arabidopsis RUP2 represses UVR8-Mediated Flowering in noninductive photoperiods. Genes Dev. 32 1332–1343
Bai S, Yao T, Li M, Guo X, Zhang Y, Zhu S and He Y 2014 PIF3 is involved in the primary root growth inhibition of Arabidopsis induced by nitric oxide in the light. Mol. Plant 7 616–625
Boccalandro HE, De Simone S N, Bergmann-Honsberger A, Schepens I, Fankhauser C and Casal JJ 2008 PKS1 regulates root phototropism and gravitropism. Plant Physiol. 146 108–115
Bohn-Courseau I 2010 Auxin: A major regulator of organogenesis. CR Biol. 333 290–206
Briggs W and Olney M 2001 Photoreceptors in plant photomorphogenesis to date. Five phytochromes, two cryptochromes, one phototropin, and one superchrome. Plant Physiol. 125 85–88
Canamero R, Bakrim N, Bouly J, Garay A, Dudkin E, Habricot Y and Ahmad M 2006 Cryptochrome photoreceptors cry1 and cry2 antagonistically regulate primary root elongation in Arabidopsis thaliana. Planta 224 995–1003
Chattopadhyay S, Ang L, Puente P, Deng X and Wei N 1998 Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell 10 673–683
Cluis C, Mouchel C and Hardtke C 2004 The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J. 38 332–347
Correll M and Kiss J 2005 The roles of phytochromes in elongation and gravitropism of roots. Plant Cell Physiol. 46 317–323
Dai X, Mashiguchi K, Chen Q, Kasahara H, Kamiya Y, Ojha S, Du Bios J, Ballou D and Zhao Y 2013 The biochemical mechanism of auxin biosynthesis by an Arabidopsis YUCCA flavin containing monooxygenase. J. Biol. Chem. 288 1448–1457
Devlin PF 2000 Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. Plant Cell Online 12 2499–2510
El-Din El-Assal S, Alonso-Blanco C, Peeters AJM, Wagemaker C, Weller JL and Koornneef M 2003 The role of cryptochrome 2 in flowering in arabidopsis. Plant Physiol. 133 1504–1516
Fahad S, Hussain S, Bano A, Saud S, Hassan S, Shan D, Khan FA, Khan F, Chen Y, Wu C, Tabassum MA, Chun MX, Afzal M, Jan A, Jan MT and Huang J 2015 Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: consequences for changing environment. Environ. Sci. Pollution Res. 22 4907–4921
Fasano R, Gonzalez N, Tosco A, Dal Piaz F, Docimo T, Serrano R, Grillo S, Leone A and Inzé D 2014 Role of Arabidopsis UV RESISTANCE LOCUS 8 in plant growth reduction under osmotic stress and low levels of UV-B. Mol. plant 7 773–791
Franklin KA and Quail PH 2010 Phytochrome functions in Arabidopsis development. J. Exp. Bot. 61 11–24
Fu X and Harberd NP 2003 Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421 740
Fukaki H, Yoko O and Masao T 2007 Auxin-mediated lateral root formation in higher plants. Int. Rev. Cytol. 256 111–137
Galavi M, Karimian MA and Mousavi SR 2013 Effects of different auxin (IBA) concentrations and planting-beds on rooting grape cuttings (Vitis vinifera). Annu. Rev. Res. Biol. 3 517–523
van Gelderen K, Kang C and Pierik R 2018 Light signaling, root development and plasticity. Plant Physiol. 176 1049–1060
Giri J, Bhosale R, Huang G, Pandey BK, Parker H, Zappala S, Yang J, Dievart A, Bureau C, Ljung K and Price A 2018 Rice auxin influx carrier OsAUX1 facilitates root hair elongation in response to low external phosphate. Nat. Commun. 9 1408
Giuliani S, Sanguineti MC, Tuberosa R, Bellotti M, Salvi S and Landi P, 2005 Root-ABA1, a major constitutive QTL, affects maize root architecture and leaf ABA concentration at different water regimes. J. Exp. Bot. 56 3061–3070
Goffinet B, Buck WR and Shaw AJ 2009 Morphology, anatomy and classification of the Bryophyta. Bryophyte Biol. https://doi.org/10.1017/CBO9780511754807.003
Halliday KJ, Martínez-García JF and Josse EM 2009 Integration of light and auxin signaling. Cold Spring Harb. Perspect. Biol. 1 1–11
Heschel MS, Selby J, Butler C, Whitelam GC, Sharrock RA, Donohue K 2007 A New Role for Phytochromes in Temperature-Dependent Germination. New Phytologist. 174 735–741
Holm M, Ma L, Qu L and Deng X 2002 Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev. 16 1247–1259
Hornitschek P, Kohnen MV, Lorrain S, Rougemont J, Ljung K, LópezVidriero I, FrancoZorrilla JM, Solano R, Trevisan M, Pradervand S and Xenarios I 2012 Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J. 71 699–711
Imaizumi T, Kadota A, Hasebe M and Wada M 2002 Cryptochrome light signals control development to suppress auxin sensitivity in the moss Physcomitrella patens. Plant Cell 14 373–386
Inada S, Ohgishi M, Mayama T, Okada K and Sakai T 2004 RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. Plant Cell 16 887–896
Jang G and Dolan L 2011 Auxin promotes the transition from chloronema to caulonema in moss protonema by positively regulating PpRSL1and PpRSL2 in Physcomitrella patens. New Phytol. 192 319–327
Kelly M and Leopold A 1992 Light regulation of the growth response in corn root gravitropism. Plant Physiol. 98 835–839
Kim K, Shin J, Lee S-H, Kweon H-S, Maloof JN and Choi G 2011 Phytochromes inhibit hypocotyl negative gravitropism by regulating the development of endodermal amyloplasts through phytochrome-interacting factors. Proc. Nat. Acad. Sci. 108 1729–1734
Kimura Y, Kimura I and Kanegae T 2018 Phototropins of the moss Physcomitrella patens function as blue-light receptors for phototropism in Arabidopsis. Plant Signal. Behav. 13 e1525995
Kinoshita T, Michio D, Noriyuki S, Takatoshi K, Masamitsu W and Ken IS 2001 Phot1 and Phot2 mediate blue light regulation of stomatal opening. Nature 414 656–660
Kiss JZ 2003 Phytochromes A and B mediate red-light-induced positive phototropism in roots. Plant Physiol. 131 1411–1417
Klemmer R and Schneider HA 1979 On a blue light effect and phytochrome in the stimulation of georesponsiveness of maize roots. Zeitschrift für Pflanzenphysiologi. 95 189–197
Kobayashi K, Baba S, Obayashi T, Sato M, Toyooka K, Keranen M, Aro E, Fukaki H, Ohta H, Sugimoto K and Masuda T 2012 Regulation of root greening by light and auxin/cytokinin signaling in Arabidopsis. Plant Cell 10 1105
Kurepin L, Walton L, Hayward A, Emery R, Pharis R and Reid D 2012 Interactions between plant hormones and light quality signaling in regulating the shoot growth of Arabidopsis thaliana seedlings. Botany 90 237–246
Lahti M, Aphalo PJ, Finér L, Ryyppö A, Lehto T and Mannerkoski H 2005 Effects of soil temperature on shoot and root growth and nutrient uptake of 5-year-old Norway spruce seedlings. Tree Physiol. 25 115–122
Lariguet P, Schepens I, Hodgson D, Pedmale U, Trevisan M, Kami C, Carbonnel M, Alonso J, Ecker J, Liscum E and Fankhauser C 2006 PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. PNAS 103 10134–10139
Li C, Xu Z, Dong R, Chang S, Wang L, Rehman M and Tao J 2017 An RNA-seq analysis of grape plantlets grown in vitro reveals different responses to blue, green, red LED light, and white fluorescent light. Front. Plant Sci. 8 78
Li J, Gang L, Haiyang W and Xing WD 2011 Phytochrome signaling mechanisms. Arabidopsis Book 9 e0148
Li J, Li Y, Dan J, Nezames CD, Terzaghi W and Xing WD 2013 UV-B-Induced Photomorphogenesis in Arabidopsis. Protein Cell 4 485–492
Li Z, Zhang X, Zhao Y, Li Y, Zhang G, Peng Z and Zhang J 2018 Enhancing auxin accumulation in maize root tips improves root growth and dwarfs plant height. Plant Biotechnol. J. 16 86–99
Lin C 2000 Update on development photoreceptors and regulation of flowering time 1. Plant Physiol. 123 39–50
Lin C and Sauter M 2018 Control of adventitious root architecture in rice by darkness, light, and gravity. Plant Physiol. 176 1352–1364
Liu H, Bin L, Zhao C, Pepper M and Lin C 2012 The action mechanisms of plant cryptochromes. Trends Plant Sci. 16 684–691
Liu G, Gao S, Tian H, Wu W, Robert HS and Ding Z 2016 Local transcriptional control of YUCCA regulates auxin promoted root-growth inhibition in response to aluminium stress in Arabidopsis. PLoS Genet. 12 1006360
Lee HJ, Ha JH, Kim SG, Choi HK, Kim ZH, Han YJ, Kim JI, Oh Y, Fragoso V, Shin K and Hyeon T 2016 Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots. Sci. Signal. 9 106–106
Lynch JP and Brown KM 2012 New roots for agriculture: exploiting the root phenome. Phil. Trans. R. Soc. B 367 1598–1604
Mahmud KP, Holzapfel BP, Guisard Y, Smith JP, Nielsen S and Rogiers SY 2018 Circadian regulation of grapevine root and shoot growth and their modulation by photoperiod and temperature. J. Plant Physiol. 222 86–93
Martínez-García JF, Gallemí M, Molina-Contreras MJ, Llorente B, Bevilaqua MRR and Quail PH 2014 The shade avoidance syndrome in Arabidopsis: The antagonistic role of phytochrome A and B differentiates vegetation proximity and canopy shade. PLoS One. https://doi.org/10.1371/journal.pone.0109275
Meng L, Song W, Liu S, Dong J, Zhang Y, Wang C, Xu Y and Wang S 2015 Light quality regulates lateral root development in tobacco seedlings by shifting auxin distributions. J. Plant Growth Regul. 34 574–583
Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F, Schwab R, Weigel D, Meyerowitz EM, Luschnig C, Offringa R and Friml J 2007 Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130 1044–1056
Mller D and Ottoline L 2011 Auxin, cytokinin and the control of shoot branching. Annal. Bot. 107 1203–1212
Moni AA, Lee Y, Briggs WR and Han IS 2015 The blue light receptor phototropin 1 suppresses lateral root growth by controlling cell elongation. Plant Biol. 17 34–40
Muday GK 2001 Auxins and tropisms. J. Plant Growth Regul. 20 226–243
Mullen JL, Wolverton C, Ishikawa H, Hangarter RP and Evans ML 2002 Spatial separation of light perception and growth response in maize root phototropism. Plant Cell Environ. 25 1191–1196
Nagel KA, Schurr U and Walter A 2006 Dynamics of root growth stimulation in Nicotiana tabacum in increasing light intensity. Plant Cell Environ. 29 1936–1945
Nakazawa M, Yabe N, Ichikawa T, Yamamoto Y, Yoshizumi T, Hasunuma K and Matsui M 2001 DFL1, an auxin-responsive GH3 gene homologue, negatively regulates shoot cell elongation and lateral root formation, and positively regulates the light response of hypocotyl length. Plant J. 25 213–221
Niu S, Li Z, Yuan H, Fang P, Chen X and Li W 2013 Proper gibberellin localization in vascular tissue is required to regulate adventitious root development in tobacco. J. Exp. Bot. 64 3411–3424
Oyama T, Shimura Y and Okada K 1997 The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 11 2983–2995
Ronseaux S, Clément C and Barka E 2013 Interaction of Ulocladium atrum, a potential biological control agent, with Botrytis cinerea and grapevine Plantlets. Agronomy 3 632–647
Pitts RJ, Alex C and Mark E 1998 Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J. 16 553–560
Poudel PR, Kataoka I and Mochioka R 2008 Effect of red-and blue-light-emitting diodes on growth and morphogenesis of grapes. Plant Cell Tissue Organ Culture 92 147–153
Sakakibara K, Nishiyama T, Sumikawa N, Kofuji R, Murata Tand Hasebe M 2003 Involvement of auxin and a homeodomain-leucine zipper I gene in rhizoid development of the moss Physcomitrella patens. Development 130 4835–4846
Salisbury FJ, Hall A, Grierson CS, and Halliday KJ 2007 Phytochrome coordinates Arabidopsis shoot and root development. Plant J. 50 429–438
Satiat-Jeunemaitre B and Hawes C 1992 Reversible dissociation of the plant golgi apparatus by brefeldin A. Biol. Cell 74 325–328
Shimizu H, Tanabata T, Xie X, Inagaki N, Takano M, Shinomura T and Yamamoto KT 2009 Phytochrome-mediated growth inhibition of seminal roots in rice seedlings. Physiol. Plant 137 289–297
Sibout R, Sukumar P, Hettiarachchi C, Holm M, Muday G and Hardtke C 2006 Opposite root growth phenotypes of hy5 versus hy5 hyh mutants correlate with increased constitutive auxin signaling. PLoS Genet. 2 202
Simonetta S, Avidano L and Berta G 2007 Morphogenetic effects induced by pathogenic and non-pathogenic Rhizoctonia solani Kühn strains on tomato roots. Caryologia 60 141–145
Suzuki H, Yokawa K, Nakano S, Yoshida Y, Fabrissin I, Okamoto T, Baluška F and Koshiba T 2016 Root cap-dependent gravitropic U-turn of maize root requires light-induced auxin biosynthesis via the YUC pathway in the root apex. J. Exp. Bot. 67 4581–4591
Takase T, Nakazawa M, Ishikawa A, Kawashima M, Ichikawa T, Takahashi N, Shimada H, Manabe K and Matsui M 2004 ydk1-D, an auxin-responsive GH3 mutant that is involved in hypocotyl and root elongation. Plant J. 37 471–483
Tilbrook K, Arongaus AB, Binkert M, Heijde M, Yin R and Ulm R 2013 The UVR8 UV-B photoreceptor: perception, signaling and response. Arabidopsis Book 11 e0164
Usami T, Mochizuki N, Kondo M, Nishimura M and Nagatani, A 2004 Cryptochromes and phytochromes synergistically regulate Arabidopsis root greening under blue light. Plant Cell Physiol. 45 1798–1808
Wan Y, Jasik J, Wang L, Hao H, Volkmann D, Menzel D, Mancuso S, Baluška F and Lin J 2012 The signal transducer NPH3 integrates the phototropin1 photosensor with PIN2-based polar auxin transport in Arabidopsis root phototropism. Plant Cell 24 551–565
Wang S, Ho C and Chen H 2011 Rice develop wavy seminal roots in response to light stimulus. Plant Cell Rep. 30 1747–1758
Waters MT, Moylan EC and Langdale JA 2008 GLK transcription factors regulate chloroplast development in a cell-autonomous manner. Plant J. 56 432–444
Waters MT, Wang P, Korkaric M, Capper RG, Saunders NJ and Langdale JA 2009 GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell 21 1109–1128
Wu G and Spalding EP 2007 Separate functions for nuclear and cytoplasmic cryptochrome 1 during photomorphogenesis of Arabidopsis seedlings. Proc. Nat. Acad. Sci. 104 18813–18818
Yamamoto M and Yamamoto KT 1999 Effects of natural and synthetic auxins on the gravitropic growth habit of roots in two auxin-resistant mutants of Arabidopsis, axr1 and axr4: evidence for defects in the auxin influx mechanism of axr4. J. Plant Res. 112 391–396
Yang C and Lin L 2017 Hormonal regulation in shade avoidance. Front. Plant Sci. 8 1–8
Yu X, Hongtao L, John K and Lin C 2010 The cryptochrome blue light receptors. Arabidopsis Book 8 0135
Zeng J, Wang Q, Lin J, Deng K, Zhao X, Tang D and Liu X 2010 Arabidopsis cryptochrome-1 restrains lateral roots growth by inhibiting auxin transport. J. Plant Physiol. 167 670–673
Zhang K, Xu H, Yuan T, Zhang L and Lu Y 2013 Blue-light induced PIN3 polarization for root negative phototropic response in Arabidopsis. Plant J. 76 308–321
Zhang K, Xu H, Gong W, Jin Y, Shi Y, Yuan T, Li J and Lu Y 2014 Proper PIN1 distribution is needed for root negative phototropism in Arabidopsis. PLOS One 9 85720
Zhao X, Wang Y-L, Qiao X-R, Wang J, Wang L-D, Xu C-S and Zhang X 2013 Phototropins function in high-intensity blue light-induced hypocotyl phototropism in Arabidopsis by altering cytosolic calcium. Plant Physiol. 162 1539–1551
Zhou J, Liu Q, Zhang F, Wang Y, Zhang S, Cheng H, Yan L, Li L, Chen F and Xie X 2014 Overexpression of OsPIL15, a phytochrome-interacting factor-like protein gene, represses etiolated seedling growth in rice. J. Integr. Plant Biol. 56 373–387
Acknowledgements
We would like to thank Dr. Madhusmita Panigrahy, Durga Prasad Biswal, Debadutta Patra and Aaram A. Kumar for critical reading of this manuscript. We acknowledge the financial support received from the Department of Biotechnology (DBT), India for the funding to K.C.P. (D.O. No. BT/PBA/MF2014). S.K. expresses gratitude to Department of Atomic Energy (DAE), Govt. of India for her fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: BJ Rao
Rights and permissions
About this article
Cite this article
Kumari, S., Panigrahi, K.C.S. Light and auxin signaling cross-talk programme root development in plants. J Biosci 44, 26 (2019). https://doi.org/10.1007/s12038-018-9838-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-018-9838-2