Abstract
Membrane remodelling or the bending and rupture of the lipid bilayer occurs during diverse cellular processes such as cell division, synaptic transmission, vesicular transport, organelle biogenesis and sporulation. These activities are brought about by the localized change in membrane curvature, which in turn causes lipid-packing stress, of a planar lipid bilayer by proteins. For instance, vesicular transport processes are typically characterized by the cooperative recruitment of proteins that induce budding of a planar membrane and catalyse fission of the necks of membrane buds to release vesicles. The analysis of such membrane remodelling reactions has traditionally been restricted to electron microscopy–based approaches or force spectroscopic analysis of membrane tethers pulled from liposome-based model membrane systems. Our recent work has demonstrated the facile creation of tubular model membrane systems of supported membrane tubes (SMrTs), which mimic late-stage intermediates of typical vesicular transport reactions. This review addresses the nature of such an assay system and a fluorescence-intensity-based analysis of changes in tube dimensions that is indicative of the membrane remodelling capacity of proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The hydrophobic effect confers lipids with the ability to self-assemble into a stable bilayer. The lipid bilayer was chosen over the course of evolution as the material to contain life for its remarkable ability to resist rupture (Antonny et al. 2015). Nevertheless, a well-orchestrated and a controlled process of membrane bending and rupture manifests in diverse cellular processes such as cell division, synaptic transmission, vesicular transport, organelle biogenesis and sporulation. In the case of vesicular transport, such a controlled process of bending and rupture or ‘membrane fission’ typically involves the application of localized curvature stresses to first deform the planar membrane into a bud and later to constrict the neck of the bud to cause scission and release of a vesicle (Antonny 2006). Since these shape changes require the lipid bilayer to deviate from its preferred planar configuration, membrane budding and fission are energetically unfavourable processes, and cells have evolved multiple proteins and mechanisms to catalyse such processes. The identification, understanding and recreation of such mechanisms represent an extensively researched topic in contemporary cell biology. The complex environment of the cell, however, presents a hurdle in discovering and appreciating the design principles by which a singular protein or combinations of proteins manage membrane remodelling. Thus, methods that allow reconstitution of membrane remodelling using purified proteins on model membrane systems of defined compositional and topological complexity as templates have emerged as an attractive approach to address these outstanding questions.
2 Traditional templates to analyse membrane remodelling
The use of liposomes, the preferred model system for most membrane-based reconstitution studies, has provided a wealth of information on the ability of proteins to cooperatively bind and remodel lipid bilayers (Takei et al. 1998; Kinuta et al. 2002; Boucrot et al. 2012; Inaba et al. 2016). Nevertheless, visualizing liposome remodelling requires tedious and often artefact-prone use of electron microscopic methods. The use of alternative model membrane systems has considerably helped overcome many of the challenges inherent in liposome-based assays. Supported lipid bilayers with excess reservoir (SUPER) is an assay system that presents investigators with the option of analysing membrane budding and fission using simultaneous microscopic and biochemical approaches (Pucadyil and Schmid 2008, 2010; Neumann et al. 2013).
In contrast to liposomes and SUPER templates, membrane tethers pulled from a giant unilamellar vesicle or SUPER templates using micromanipulators or optical traps have led to a significant advancement in specifically understanding the pathway to membrane rupture and fission (Frolov et al. 2003; Bashkirov et al. 2008; Roux et al. 2010; Morlot et al. 2012; Shnyrova et al. 2013; Bassereau et al. 2014). Membrane tethers provide a convenient option to assess forces exerted on the membrane using force spectroscopy. Using the knowledge of tension in the vesicle and the bending rigidity of the membrane, such methods allow precise estimation of forces necessary to deform the membrane tube (Koster et al. 2003). The use of such model membrane systems has significantly contributed to our understanding of the physics of membrane curvature generation (Baumgart et al. 2011), mechanism by which proteins sense membrane curvature (Sorre et al. 2012) and the pathway to membrane fission (Morlot et al. 2012; Shnyrova et al. 2013; Renard et al. 2015; Simunovic et al. 2017).
3 Supported membrane tubes
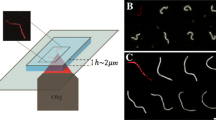
The traditional methods to form membrane tethers demand a high degree of technical expertise and are experimentally challenging, since they allow monitoring a single membrane tether at a time. In contrast, a simpler method to produce membrane tethers is by subjecting hydrated lipids spotted on a passivated glass coverslip to the flow of buffer (figure 1). The flow-induced extrusion of the lipid reservoir results in the formation of long membrane tubes, which eventually settle down and get pinned on to defects on the glass surface. Our recent efforts have led to the systematic characterization of such templates, referred to as supported membrane tubes (SMrT) (Dar et al. 2015; Holkar et al. 2015; Pucadyil and Holkar 2016; Dar et al. 2017). SMrT templates can be formed from any lipid that can self-assemble into a bilayer, and the initial stages in the formation of these templates appear similar to how vesicles respond to hydrodynamic flow (Rossier et al. 2003). In many ways, SMrT templates are architecturally similar to the previously described motor protein-pulled membrane tethers (Roux et al. 2002; Koster et al. 2003; Leduc et al. 2004) but can be set up with far less biochemical complexity, technical skill and expertise.
4 Quantitative analysis of protein-induced remodelling of tubular membrane templates
Based on the premise that the brightness of a diffraction-limited membrane tube labelled with a fluorescent lipid is directly proportional to the net membrane area within the field of illumination (Kunding et al. 2008) (figure 2), wide-field fluorescence microscopy can be used to conveniently and quantitatively measure the extent of change in the radial dimension of the tube in response to membrane remodelling. A distinct advantage with SMrT templates is the availability of an in situ calibration standard in the form of a planar supported bilayer at the source of every SMrT template preparation using one which can equate fluorescence intensities to membrane area and therefore to tube radius (Pucadyil and Holkar 2016). For this purpose, SMrT templates are labelled with trace amounts of a specific fluorescent lipid probe p-Texas Red-DHPE, which displays partitioning properties that are insensitive to membrane curvature and fluorescence properties that are insensitive to protein binding (Jung et al. 2009; Hsieh et al. 2012).
5 Applications
SMrT templates are housed inside a flow cell, thus making it convenient to flow in proteins and accurately monitor reaction kinetics. Since the membrane tube is supported on a coverslip and not free-standing, out-of-focus movements are reduced and the assay system is amenable to quantitative light microscopic analysis. Contrary to traditional techniques that generate a single membrane tube at a given time, SMrT templates represent an array of membrane tubes and overcome the experimental challenge in recording statistically significant numbers of membrane remodelling events. Due to the ill-defined nature of the lipid reservoir at the source, extrusion by buffer flow results in the formation of tubes with highly variable dimensions. In conjunction with the planar supported bilayer present at source, SMrT templates represent an assay system that displays membrane surfaces with variable degree of curvatures, thus allowing the systematic characterization of how the membrane curvature influences the binding and scaffolding of proteins (Dar et al. 2015; Holkar et al. 2015; Pucadyil and Holkar 2016). Furthermore, any localized change in tube dimension causes a change in tube fluorescence and allows for the convenient monitoring of membrane remodelling (Dar et al. 2015). The arrayed architecture of SMrT templates allows for a high-throughput analysis of membrane fission, which manifests in the tube undergoing scission at multiple points along its length. Unlike free-standing membrane tubes where the first scission event results in tension-induced retraction of the tube, the presence of multiple pinning sites restricts such effects and allows scoring for multiple scission events in a single microscope field (Dar et al. 2015).
6 Future perspectives
The vastly simplified lipid compositions used for the design of the assay systems described earlier fail to mimic the compositional heterogeneity inherent in biological membranes. Quite understandably, an important question then is whether such assays misrepresent real biological phenomena. Remarkably, the bulk of literature on reconstitution of protein-induced membrane remodelling, membrane fusion and fission suggests otherwise. In fact, while simplified model membrane systems may fail to recapitulate certain processes, rarely do they misrepresent protein function. This is because model membranes and biological membranes are formed by the same principles of self-assembly and that the two display very similar material properties. So if a cellular process under investigation recognizes or responds to a change in the bulk physical properties such as charge, shape and tension of the membrane, then the assay system described earlier is a good starting point, and it offers the definite possibility of a systematic identification and evaluation of the diversity of proteins that exhibit the capacity to remodel membranes. Naturally, having identified these, the real challenge and excitement is to decipher how such activities manifest in and regulate functions of complex living systems.
References
Antonny B 2006 Membrane deformation by protein coats. Curr. Opin. Cell Biol. 18 386–394
Antonny B, Vanni S, Shindou H and Ferreira T 2015 From zero to six double bonds: phospholipid unsaturation and organelle function. Trends Cell Biol. 25 427–436
Bashkirov PV, Akimov SA, Evseev AI, Schmid SL, Zimmerberg J and Frolov VA 2008 GTPase cycle of dynamin is coupled to membrane squeeze and release, leading to spontaneous fission. Cell 135 1276–1286
Bassereau P, Sorre B and Lévy A 2014 Bending lipid membranes: experiments after W. Helfrich’s model. Adv. Colloid Interface Sci. 208 47–57
Baumgart T, Capraro BR, Zhu C and Das SL 2011 Thermodynamics and mechanics of membrane curvature generation and sensing by proteins and lipids. Annu. Rev. Phys. Chem. 62 483–506
Boucrot E, Pick A, Çamdere G, Liska N, Evergren E, McMahon HT and Kozlov MM 2012 Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent bar domains. Cell 149 124–136
Dar S, Kamerkar SC and Pucadyil TJ 2015 A high-throughput platform for real-time analysis of membrane fission reactions reveals dynamin function. Nat. Cell Biol. 17 1588–1596
Dar S, Kamerkar SC and Pucadyil TJ 2017 Use of the supported membrane tube assay system for real-time analysis of membrane fission reactions. Nat. Protocols 12 390–400
Frolov VA, Lizunov VA, Dunina-Barkovskaya AY, Samsonov AV and Zimmerberg J 2003 Shape bistability of a membrane neck: a toggle switch to control vesicle content release. Proc. Natl. Acad. Sci. USA 100 8698–8703
Holkar SS, Kamerkar SC and Pucadyil TJ 2015 Spatial control of epsin-induced clathrin assembly by membrane curvature. J. Biol. Chem. 290 14267–14276
Hsieh W-T, Hsu C-J, Capraro BR, Wu T, Chen C-M, Yang S and Baumgart T 2012 Curvature sorting of peripheral proteins on solid-supported wavy membranes. Langmuir 28 12838–12843
Inaba T et al. 2016 Phospholipase Cβ1 induces membrane tubulation and is involved in caveolae formation. Proc. Natl. Acad. Sci. USA 113 7834–7839
Jung H, Robison AD and Cremer PS 2009 Detecting protein−ligand binding on supported bilayers by local ph modulation. J. Am. Chem. Soc. 131 1006–1014
Kinuta M, Yamada H, Abe T, Watanabe M, Li S-A, Kamitani A, Yasuda T, Matsukawa T, Kumon H and Takei K 2002 Phosphatidylinositol 4,5-bisphosphate stimulates vesicle formation from liposomes by brain cytosol. Proc. Natl. Acad. Sci. USA 99 2842–2847
Koster G, VanDuijn M, Hofs B and Dogterom M 2003 Membrane tube formation from giant vesicles by dynamic association of motor proteins. Proc. Natl. Acad. Sci. USA 100 15583–15588
Kunding AH, Mortensen MW, Christensen SM and Stamou D 2008 A fluorescence-based technique to construct size distributions from single-object measurements: application to the extrusion of lipid vesicles. Biophys. J. 95 1176–1188
Leduc C, Campàs O, Zeldovich KB, Roux A, Jolimaitre P, Bourel-Bonnet L, Goud B, Joanny J-F, Bassereau P and Prost J 2004 Cooperative extraction of membrane nanotubes by molecular motors. Proc. Natl. Acad. Sci. USA 101 17096–17101
Morlot S, Galli V, Klein M, Chiaruttini N, Manzi J, Humbert F, Dinis L, Lenz M, Cappello G and Roux A 2012 Membrane shape at the edge of the dynamin helix sets location and duration of the fission reaction. Cell 151 619–629
Neumann S, Pucadyil TJ and Schmid SL 2013 Analyzing membrane remodeling and fission using supported bilayers with excess membrane reservoir. Nat. Protocols 8 213–222
Pucadyil TJ and Holkar SS 2016 Comparative analysis of adaptor-mediated clathrin assembly reveals general principles for adaptor clustering. Mol. Biol. Cell 27 3156–3163
Pucadyil TJ and Schmid SL 2008 Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell 135 1263–1275
Pucadyil TJ and Schmid SL 2010 Supported bilayers with excess membrane reservoir: a template for reconstituting membrane budding and fission. Biophys. J. 99 517–525
Renard H-F et al. 2015 Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature 517 493–496
Rossier O, Cuvelier D, Borghi N, Puech PH and Langmuir ID 2003 Giant vesicles under flows: Extrusion and retraction of tubes. Langmuir 19 575–584
Roux A, Cappello G, Cartaud J, Prost J, Goud B and Bassereau P 2002 A minimal system allowing tubulation with molecular motors pulling on giant liposomes. Proc. Natl. Acad. Sci. USA 99 5394–5399
Roux A, Koster G, Lenz M, Sorre B, Manneville J-B, Nassoy P and Bassereau P 2010 Membrane curvature controls dynamin polymerization. Proc. Natl. Acad. Sci. USA 107 4141–4146
Shnyrova AV, Bashkirov PV, Akimov S, Pucadyil TJ, Zimmerberg J, Schmid SL and Frolov VA 2013 Geometric catalysis of membrane fission driven by flexible dynamin rings. Science 339 1433–1436
Simunovic M et al. 2017 Friction mediates scission of tubular membranes scaffolded by BAR proteins. Cell 170 172–184.e11
Sorre B, Callan-Jones A, Manzi J, Goud B, Prost J, Bassereau P and Roux A 2012 Nature of curvature coupling of amphiphysin with membranes depends on its bound density. Proc. Natl. Acad. Sci. USA 109 173–178
Takei K, Haucke V, Slepnev V, Farsad K, Salazar M, Chen H and De Camilli P 1998 Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell 94 131–141
Acknowledgements
TJP acknowledges financial support from the Wellcome Trust–DBT India Alliance and the Howard Hughes Medical Institute. TJP was a Senior Fellow of the Wellcome Trust–DBT India Alliance and is currently an international scholar of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
corresponding editor: xxxx
Rights and permissions
About this article
Cite this article
Pucadyil, T.J. A novel fluorescence microscopic approach to quantitatively analyse protein-induced membrane remodelling. J Biosci 43, 431–435 (2018). https://doi.org/10.1007/s12038-018-9767-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-018-9767-0