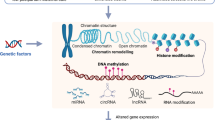
Abstract
Early life stress (ELS), characterized as abuse, neglect, and abandonment, can cause several adverse consequences in the lives of affected individuals. ELS experiences can affect an individual’s development in variable ways, persisting in the long term and promoting lasting impacts, considering that early exposure to stressors can be biologically incorporated, as prolonged stimulation of stress response systems affects the development of the brain structure and other body systems, increasing the risk of diseases associated with stress and cognitive impairment. This type of stress increases the risk of developing major depressive disorder (MDD) in a severe form that does not respond adequately to traditional antidepressant treatments. Several alterations are studied as mechanisms that relate ELS with MDD, such as epigenetic alterations, neurotransmitters, and neuronal signaling. This review discusses research that brings evidence about the ELS mechanisms involved in synaptic impairments and MDD. The processes involved in epigenetic changes and the HPA axis are highlighted, as well as changes in neurotransmitters and neuronal signaling mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The early years of life represent a period of considerable influence on mental health. While a childhood in an environment portrayed by care, learning, and support tends to be protective, on the contrary, experiencing adverse childhood experiences leads to an increased risk of experiencing mental health problems [1].
Stress in childhood can be triggered by different negative experiences, such as maltreatment, exposure to domestic violence [2], trauma from emotional, physical, emotional and sexual abuse, neglect [3], among others. Early exposure to stressful experiences such as these may be biologically incorporated, as prolonged stimulation of stress response systems affects the development of brain structure and other body systems, increasing the risk of stress-associated diseases and cognitive impairment [4].
A meta-analysis that included 37 studies and a total of 253,719 participants found that having multiple adverse childhood experiences, in addition to being a significant risk factor for the development of diseases, is strongly associated with posing a risk for the next generation, such as diseases. Mental disorders, violence, and substance use, suggest intergenerational effects [2]. Studies have already shown that experiencing stressful adversities in the early years is strongly linked to the development of psychiatric psychopathologies, such as major depressive disorder (MDD), and other health aggravating factors [5,6,7,8].
The multifactorial origin of depression is still little known. The impact it causes on individuals and, sometimes, the resistance to pharmacological treatments have led scientific research to investigate possible variables potentially associated with the development, severity, and outcomes of the condition, which is relevant to providing more assertive subsidies for therapeutic interventions. This article aims to gather relevant scientific evidence on epigenetic changes in neurotransmitters and neuronal signaling mechanisms related to ELS associated with MDD, discussing the ways in which stress in childhood can affect individual development, persisting in the long term and causing lasting impacts.
Early Life Stress and Major Depressive Disorder
Studies suggest that adverse childhood experiences may have repercussions as predictors of neuropsychiatric disorders [9]. There are multiple types of stressors in childhood, and scientific literature points to an association of physical and sexual abuse with anxiety disorders, post-traumatic stress disorder, and personality disorders, as well as an association between emotional abuse and MDD and suicidal ideation [10].
In this sense, traumatic and, consequently, stressful experiences in early life are considered risk factors for developing emotional and behavioral disorders that can be transgenerational [11]. A study with mice exposed to maternal separation demonstrated consequences such as depressive-like behaviors and epigenetic changes, such as DNA methylation in the lineage, and these characteristics have the potential to be transmitted through generations [12].
Depression is one of the most prevalent mental disorders, affecting around 4.4% of the world population and is responsible for disabling years of life, about 7.5% of the global index [13]. In addition to the high and increasing incidence over the years, anxiety and depression disorders increased the estimate in the population by about 25% in the first year of the COVID-19 pandemic [1].
MDD is characterized mainly by a persistent picture of sadness or loss of interest in previously pleasurable activities and causes significant impairment in the individual’s functioning. It has a multifactorial etiology, with a combination of biological, psychological, and social aspects [14], and, in many cases, people with MDD do not respond well to therapeutic measures [15].
The severity and increase in the percentage of people affected by MDD make longitudinal research relevant, such as a cohort study that followed for 10 years of 1351 people diagnosed with depression and found that different types of childhood maltreatment and stressful life events anticipated a depressive condition, providing evidence to support the impact of early and long-term psychosocial stressors in the development of depression [16].
Adverse childhood experiences such as abuse and neglect and other risk factors, such as discrimination, were associated with greater chances of developing a major depressive episode in individuals, according to a survey of 6081 black adults in the USA [17].
In the same way, a retrospective cohort study with 56,082 individuals identified a relationship between child maltreatment and mental disorders. Participants more exposed to types of abuse showed a higher risk of having mental disorders, and those who drank heavily or had few social connections showed a stronger association. Abuse and neglect were strongly associated with long-term mental disorders [18].
Greater severity, earlier age at first diagnosis, comorbid anxiety, post-traumatic stress disorder, poorer quality of life, and suicidal ideation were associated with childhood adversity and adult MDD [19]. A survey of 1084 patients with MDD identified that experiencing stress in childhood makes them more sensitive to the possibility of experiencing stressful events in adulthood, with the subject being more vulnerable to the development of psychiatric disorders and, when experiencing adversities in early life and adulthood, there is an association with higher rates of suicide ideation [6]. Another study found that high levels of traumatic experiences in the early years were identified in patients with MDD when they also had more significant suicide attempts and deliberate self-harm behavior [3].
MDD patients who had been maltreated in childhood had impaired recognition of facial emotions and altered functioning in frontostriatal circuits associated with reward in emotion recognition tasks and mood-regulating systems. Thus, experiencing maladaptive stressful and socio-emotional processes in childhood may indicate a risk for developing psychopathologies with even more damage at the nervous system level [5].
Predicting the response to antidepressant therapy in individuals with MDD was the objective of a randomized study, which identified that abuse occurring at ages 4 and 7 presented worse results in antidepressant treatment with sertraline. In addition, the study pointed out that the greater the exposure to trauma, the lower the probability of a remission response to antidepressant therapy, suggesting the importance of investigating the patient’s history of childhood trauma that may require therapeutic intervention complementary to antidepressants to address specifically the impact of experienced traumas [20].
Research shows that ELS, in combination with the polymorphism of brain-derived neurotrophic factor (BDNF), a factor involved in neuroplasticity, and 5-hydroxytryptamine transporter (5-HTT), a serotonin transporter, contribute to the risk of developing non-melancholic depression [21].
According to the literature, the recognition of the connection between the central nervous system (CNS) and the immune system has already been established [22], in which, for the release of inflammatory cytokines through stressors, it is necessary to stimulate cells of the central and peripheral nervous system, so that they are activated, allowing the release. The primary immune cells of the CNS are known as microglia, which act as inflammatory response cells in the CNS through the secretion of inflammatory cytokines and chemokines [23]. In addition, microglia can produce several substances, including reactive oxygen and nitrogen species [24] that, when they go into imbalance, characterize oxidative stress, an extremely harmful process to cells and tissues and intrinsically linked to the triggering of inflammatory processes [25].
Although cytokines play a fundamental role in the nervous system, where they are of paramount importance for the optimal functioning of the brain, their constant exposure can cause recognized brain changes in triggering MDD. Studies indicate the increase of these cytokines in the cerebrospinal fluid of depressed individuals [24].
There is already evidence regarding the relationship between inflammatory processes and MDD through the analysis of biological markers of depression, where it is possible to find the presence of inflammatory markers [26]. In addition to the cytokines themselves being inflammatory markers of MDD, interleukin-6 (IL-6) stimulate another marker commonly expressed in the brain during the MDD process, such as C-reactive protein (CRP) [27, 28].
Exposure to trauma in childhood-age individuals can cause different types of inflammation in response to the stress generated, resulting in other biological markers [29], mainly IL-6 and CRP, which were detected at high levels in children exposed to stress [27]. Although the etiology of depression is very heterogeneous and uncertain, recent studies indicate a correlation between these markers in childhood and MDD [30], and in addition to being linked with the development of MDD, there are indications that IL-6 can also affect the severity level of MDD [31, 32].
IL-6 is not only related to changes at the cellular level but also, according to research carried out by Kakeda et al. [33], to morphological changes in the brain of depressed individuals. Another study by Chamberlain et al. [34] relates treatment-resistant depression with the significant increase in CRP. The same research suggests the presence of a more considerable amount of CRP in cases of severe depression.
Furthermore, there is the possibility that different types of adverse situations in childhood influence the onset and intervals of MDD throughout life, which are correlated with the levels of biomarkers such as IL-6 and CRP, which are higher in patients who have suffered abuse than in patients who have gone through other traumatic experiences [35]. Therefore, the inflammatory pattern may be influenced and altered according to the trauma experienced, and the levels of IL-6 and CRP observed in these individuals and, consequently, in the developed MDD [35].
Some studies point to a correlation between IL-6 and the time to develop MDD. However, more precise and conclusive studies are needed to deal with the possibilities presented, making it necessary to carry out more studies on the subject [35].
Studies suggest that the activation of emotional nuclei involved in stress can affect other body systems, especially the cardiovascular system, leading to various diseases. In addition to aggravating myocardial ischemia–reperfusion injury, severe stress is associated with a greater risk of developing cardiovascular diseases, such as hypertension, heart disease, and stroke. Prolonged exposure to stress hormones can damage blood vessels, leading to atherosclerosis or restricting blood flow to the heart and brain. Stress can also contribute to unhealthy behaviors such as overeating, smoking, and alcohol abuse, further increasing the risk of cardiovascular problems [36, 37].
Taking into account the relationship between stress, inflammation, and MDD, it is important to understand that stressors in early life can induce the development of MDD where the stressor trauma leads to the induction of production of inflammatory cytokines, CRP, and reactive oxygen and nitrogen species that result in the triggering of inflammatory processes widely studied by individuals with MDD.
Early Life Stress, Epigenetics, and Major Depressive Disorder
Stress-induced epigenetic alterations in depressed individuals are noted, where alterations in DNA methylation, histones, and non-coding RNAs are found [38]. In epigenetic research, it has been studied how genetic interactions with the environment can interfere with the phenotype of individuals from childhood stress and the consequences for adult life [39]. DNA methylation is a change in gene expression without a difference in the sequence of the DNA nucleotide chain. The process involves adding a methyl group to CpG islands in gene promoters [40]. Excessive DNA methylation can inhibit transcription and reduce gene expression [41], implying reduced function of the respective gene. This modification is influenced by stress and can trigger or propitiate MDD [42]. In addition to raising levels of markers and inflammatory processes, childhood trauma is associated with epigenetic changes, such as increased DNA methylation and increased risk of MDD [42]. Other epigenetic variations associated with childhood trauma include histone modification, upregulation of multiple exon one transcripts, and DNA hypermethylation of exon 1F [43].
The hypothalamic–pituitary–adrenal (HPA) axis, the primary stress response system, is closely associated with MDD, a relationship supported by multiple studies [44,45,46]. Epigenetic changes triggered by stress can precipitate changes in the HPA axis, affecting the response of these individuals to external stressors. In patients who suffered childhood sexual abuse and maltreatment, an increase in hypermethylation of the glucocorticoid receptor gene, nuclear receptor subfamily 3, group C, member 1 gene (NR3C1) responsible for controlling endocrine responses (HPA axis) to stress was observed. Alteration of this promoter has also been identified in suicidal individuals and those with borderline personality disorder [43, 47]. These epigenetic changes can define neuropsychiatric disorders’ severity, duration, and treatment [48].
The NR3C1 gene is located on chromosome 5q31-q32. It contains eight coding exons (exons 2 to 9) [49] and seven first non-coding alternative exons (1D, 1 J, 1E, 1B, 1F, 1C, and 1H) found within the GpG island [50] and three splice variants with different functions (GRalpha, GRbeta, and GR-P). Studies in rats utilize the rodent exon 17 promoter, corresponding to human exon 1F [49]. In MDD, NR3C1 is decreased, and its 1F exon is methylated [51]. In addition, total messenger RNA of human glucocorticoid receptor (hGR) expression is decreased in the hippocampus in suicidal individuals [50]. In vivo research with rats found that decreased maternal care may increase methylation at the CpG site of NR3C1, at exon 17, and decrease binding to nerve growth factor I-A (NGFI-A) [51].
In the hippocampus postmortem of suicidal individuals who had experienced childhood abuse, exons 1B, 1C, and 1H are decreased compared to non-abused suicidal individuals and controls. Besides, the hGR messenger RNA negatively correlates with exons 1B and 1C methylation [50].
A postmortem brain study of children between 1 and 4 years of age found that severe childhood physical abuse was associated with increased DNA methylation in the NGFI-A binding region of the 1F promoter of NR3C1 in hippocampal pyramidal cells. However, in the same series of studies, no DNA methylation was observed in the brains of children who suffered childhood stress, possibly less traumatic than severe physical abuse. Thus, a possible explanation is that the period between the stress suffered in childhood and the child’s death was not enough time to trigger sustained epigenetic changes [52].
Other genes are also studied as they are altered when childhood adversity happens. F506 binding protein 5 (FKBP5), a mediator of GR binding by glucocorticoids, is increased in individuals who presented suicidal idealization of childhood adversities through demethylation in intron 7 that induces positive feedback to the HPA axis, increasing its potential [39]. The FKBP5 gene encodes the FKPB51 protein, an immunophilin being investigated as a target in glucocorticoid resistance. The protein appears to play an essential role in glucocorticoid resistance mechanisms [53]. It is one of the components of a cytoplasmic multiprotein complex, which retains the glucocorticoid receptor (GR) in the cytoplasm. The protein complex, including FKBP51, also provides a high GR affinity for glucocorticoids [54]. However, when the glucocorticoid hormone binds to the receptor on the protein complex, the FKBP51 is replaced by the FKBP52 immunophilin (FKBP4 gene), which allows the translocation of the receptor to the nucleus, where the hormone will carry out its functions of transcription [55, 56]. Both immunophilins compete for the binding site of the complex, but FKBP52 has a higher affinity when the glucocorticoid is bound to the GR. The activated GR acts in the nucleus as a transcription factor for FKBP5, among other genes, increasing the release of FKBP51 in cells, which makes it possible to increase its competitiveness with FKBP52. Thus, an increased release of glucocorticoids during stressful periods may increase FKBP51 levels and thus prevent the GR from being transported to the nucleus, hindering the action of glucocorticoids in other functions, such as negative feedback on the HPA axis [57] (Fig. 1).
GR activation in the cytoplasm of individuals with and without MDD. In the cytoplasm, FKBP51 is associated with the GR and retains the receptor in the cytoplasm, preventing its translocation to the nucleus and, therefore, the transcriptional function of the glucocorticoid. On the other hand, FKBP52 competes with FKBP51 for the binding site on the protein complex, and when the hormone binds to the GR, FKBP52 replaces FKBP51, allowing the receptor to be transported to the nucleus and carries out its transcription functions. In MDD with hyperactivation of the HPA axis and large amounts of circulating glucocorticoids, there is an increase in FKBP51 levels and, therefore, less coupling with FKBP52. Thus, there is a reduction in the sensitivity to its ligands, affecting the action of glucocorticoids and the negative feedback on the HPA axis. FKBP51, 51 kDa FK506 binding protein; FKBP52, 52 kDa FK506 binding protein; GR, glucocorticoid receptor; HPA, hypothalamus–pituitary–adrenal; MDD, major depressive disorder
Some researchers studied the correlation of attachment in adults with the OXTR promoter region that encodes the human oxytocin receptor for hormone signaling and the NR3C1. There was evidence of the correlation between gene methylation and affective dysregulation. Attachment avoidance was evident in patients with hypermethylation of the OXTR promoter [40]. The same happened with the NR3C1 gene, which was hypermethylated in individuals with high attachment avoidance. Given the significant action of this gene for the functionality of negative feedback on the HPA axis, when hypermethylated, it can deregulate the functioning of emotions and stress. Thus, the article reflects that these individuals with decreased emotions and increased stress are reluctant to avoid social contact and attachment as they do not have the physiological capacity to withstand stressful events [40].
Inflammatory genes in the amygdala brain region were increased in childhood-abused individuals. IFNy was analyzed in the amygdala of humans in a genetics imaging study in individuals who went through traumatic situations in childhood. The genetic expressions of interferons variant rs1861494, rs2069718, and rs2430561 were analyzed. The latter was associated with damage-prevention behaviors. The research identified that only the genetic variant rs1861494 correlated with ELS. In addition, a strong relationship was found between the rs1861494 genotype and ELS, culminating in amygdala reactivity to negative emotional stimuli, which is associated with reduced IFNy, compared to the primary allele without alteration by ELS. Therefore, gene alteration can interfere with its transcription and, thus, cause depletion in serotonergic and noradrenergic transmission from changes in IFNy [58].
Other research studied the methylation of genes related to inflammation and panic disorder (PD), which also correlates with MDD. The study did not show a significant difference in the hypermethylation of inflammatory cytokine genes between patients with PD and the control group. However, it concludes that, according to the more severe PD level, more methylation is present in the inflammatory genes IL-4, indicating that inflammatory genes can be altered in mood disorders [41].
Methylation was also increased in genes linked to neuronal growth and development, such as the BDNF gene, responsible for encoding the protein responsible for neural growth, differentiation, and plasticity. According to Kowiański et al. [59], BDNF significantly interferes with neural and glial development, neuroprotection, and synapses. Synapses are responsible for transmitting information quickly from one neuron to another, and studies propose the idea that trans-synaptic cell adhesion molecules organize synapses (CAMs) encoded in frequently described genes in neuropsychiatric disorders [60].
Given its importance and connection with MDD, some studies have linked DNA methylation with decreased BDNF expression. Research on adult rats that underwent maternal maltreatment in the puerperium and childhood reduced the BDNF expression gene in the prefrontal cortex. The mouse exon III region (human IV) was evaluated, and it was identified that after methylation in childhood, the BDNF gene decreased in adulthood, as well as restored with the treatment of a compound that inhibits DNA methylation, which culminated in the mistreatment of their descendants with their children [61]. One example may be individuals with MDD, whereas studies have shown that BDNF correlates with depressive disorders [48].
Although BDNF has several exons, there is a more significant relationship between exon I and exon IV in stressful and depressive processes, where exon I has high levels of methylation of its promoter while a reduction in exon IV promoter methylation occurs. The link between BDNF and stress lies in the systems on which the two act in stress-associated and reward-associated systems. Feelings similar to depression can affect the expression of BDNF transcripts, decreasing the amount of BDNF in different brain regions [61].
Changes in histone structure can also be observed in individuals who have experienced childhood stress, along with alterations in other gene expressions such as CpG sites of arginine vasopressin, the precursor of adrenocorticotropic hormone, methylation of metabotropic and ionotropic subunits of glutamate receptors, methylation of the serotonin transporter gene. It is also linked with factors such as sex, type of stressor, tissue-specific genetic cell, time, and type of childhood distress [39]. Thus, it is evidenced and proven that epigenetic modulation may have an essential relationship with traumatic situations that occur in childhood, which may affect gene expressions involved in inflammation and depression (Fig. 2).
Early life stress and epigenetic modulation. According to the trauma involved in childhood, changes are identified in adults. The hippocampus has methylation and a decrease in the hCR receptor. In the amygdala, there is an increase in inflammation from alterations in the Interferon y (IFNy) rs1861494 gene. The increase in methylation also affects the OXTR promoter genes in exon 1F of the nuclear receptor subfamily 3, group C, member 1 gene (NR3C1) gene and exon I of the brain-derived neurotrophic factor (BDNF) gene, and a decrease in its exon IV
In future studies, it is essential to focus on more advanced methods to improve the understanding of this complex condition and its relationship with genetic and epigenetic changes, using, for example, genome-wide association studies (GWAS). The absence of genes such as FKBP5, BDNF, and OXTR in large-scale studies such as GWAS highlights the need to re-evaluate the validity of these markers in future studies. Incorporating these findings into new research improves the accuracy of results and helps lay a solid foundation for more effective interventions and treatments for depression [62].
Early Life Stress, Neurotransmitter, and Major Depressive Disorder
Adults who experience childhood maltreatment have more significant cognitive deficits in acute depression, less treatment-associated cognitive improvements, and poorer cognitive performance in remission. A multicenter survey of individuals diagnosed with MDD who received 16 weeks of standardized antidepressant treatment identified that childhood maltreatment is associated with cognitive deficits that can become more severe and persistent in adults with MDD [63].
Neurotransmitters released and functioning balanced provide the proper functioning of neuronal circuits. Neurotransmitter levels decline with age increasing the risk of MDD [64]. Among the therapeutic strategies, the practice of physical exercises increases circulating neurotransmitters in the CNS and, consequently, a reduction in depressive symptoms and an improvement in quality of life with advancing age [65].
The paraventricular nucleus of the hypothalamus is responsible, among other functions, for regulating the neuroendocrine stress response, a process mediated by the corticotropin-releasing factor (CRF) present in high concentrations at the site. CRF has neurotransmitter potential in the cortico-limbic pathways related to fear and anxiety, and its concentrations are elevated in individuals with untreated MDD [66, 67]. ELS is related to altered CRF function, causing persistent neurodevelopmental changes in adults [68]. Negative feedback inhibition to the HPA axis causes elevated CRF levels in MDD. The axis is stimulated in response to stress and releases cortisol, activating specific receptors responsible for negative feedback signals. Depression interferes with this mechanism and thus causes an increase in CRF and cortisol levels, generating an imbalance in the stress response system [69].
The disruption of hormones, apart from causing MDD, can give rise to various other personality and behavioral disorders. According to studies, ELS implies dysfunction in learning and memory and the manifestation of aggressive behaviors [12]. Similarly, cortisol hypersecretion is associated with mental disorders such as psychosis in MDD and memory alteration. This is comparable to the fact that individuals with schizophrenia have higher cortisol levels, which are also linked to increased delusions [69]. Consequently, dysregulation of the HPA axis exacerbates the severity of MDD, with individuals potentially displaying not only depressive symptoms but also aggressive behaviors, cognitive dysfunction, and psychoses.
Consequences of this HPA axis dysfunction resulting from ELS may also be related to patients with chronic pain. While chronic stress generates axis dysfunction, it can result in chronically elevated CRH levels and heightened pain responses, evidenced in patients with fibromyalgia, chronic back pain, and rheumatoid arthritis [70]. Based on the fact that patients with fibromyalgia present depressive symptoms, in addition to being correlated with inflammatory factors and neurotransmitters (dopamine, serotonin, and opioids) [71], patients who have suffered ELS may be willing to present fibromyalgia and, consequently, MDD.
ELS is related to genetic variants that result in poor antidepressant response [72]. A survey identified that ELS is associated with a polymorphism in the solute carrier family 6 member 2 (SLC6A2) gene, which encodes the sodium-dependent noradrenaline transporter of the noradrenaline transporter (NET), which negatively influences the response of individuals to antidepressant treatment, decreasing their response to traditional antidepressants. The SLC6A2 gene is a human gene that encodes the NET. The NET protein regulates the amount of noradrenaline available at the neural synapse, removing the noradrenaline released from the synapse and returning it to the nerve cells. However, the mechanism by which the NET gene's polymorphism negatively affects individuals’ response to antidepressant therapies is poorly understood. Therefore, more research is needed [73]. Studies also demonstrate a correlation between the serotonin transporter gene (SLC6A4) methylation and chronic pain in individuals who have suffered ELS [70].
Another study analyzed the expression of the ELS-related SLC6A2 gene from epigenetic alterations, which may contribute to the development of stress-related psychiatric disorders, such as post-traumatic stress disorder, schizophrenia, anxiety, and MDD. It has been identified that exposure to childhood trauma is associated with increased promoter methylation of the SLC6A2 gene, which in turn results in a decrease in gene expression and the amount of NET protein. This can negatively affect noradrenaline regulation and contribute to developing stress-related psychiatric disorders such as depression [74].
Another genetic polymorphism in adults who suffered childhood stress involves the single nucleotide rs2171363 of the tryptophan hydroxylase 2 (TPH2) gene, also related to MDD unresponsive to classical antidepressants. TPH2 is a rate-limiting enzyme of serotonin, and its levels are inadequate in depression and are associated with a poor response to antidepressants. Xu et al. [75] identified that the presence of ELS potentiates changes in the TPH2 enzyme from epigenetic changes that influence its gene transcription.
The serotonergic system influences the microbiome-gut-brain axis on male germ-free animals exposed to ELS. These animals have high levels of 5-hydroxytryptamine (5-HT) and 5-hydroxy indole acetic acid (5-HIAA) (its main metabolite) in the hippocampus compared to control animals. They have higher tryptophan concentrations, a serotonin precursor, in the plasma. The results indicate that an alteration of the intestinal flora from the beginning of life negatively affects the neurotransmission in the CNS, increasing the risk of individuals developing disorders related to the system [46].
Research suggested that the imbalance between the activity of 5-hydroxytryptamine 1A (5-HT1A) and 5-HT2A receptors, involving the desensitization of the 5-HT1A receptor, is implicated in developing psychiatric disorders. Early weaning in female pups pigs culminated in behavioral inhibition, besides lowered 5-HT1A messenger RNA (mRNA) expression in the hippocampus, amygdala, and hypothalamic paraventricular nucleus and higher 5-HT2A mRNA expression in the hippocampus and amygdala in adulthood. These results show that ELS can cause lasting harmful effects in different species [76].
Women who have experienced stress early in life show alterations in serotonergic circuits related to cognitive function but without necessarily having difficulties associated with this process. During menopause, estradiol levels decrease, changing the serotonergic function, mainly in the dorsolateral prefrontal cortex (dlPFC). This change affects the maintenance of working memory processes. Therefore, ELS generates lasting effects on serotonergic circuits related to executive functions, an alteration unmasked by the loss of estradiol during menopause [77, 78].
The dopaminergic system is also modified by stress in childhood, which can cause several behavioral and biochemical changes. Studies have shown that ELS reduces dopamine levels in the PFC of individuals with psychotic disorders. One hypothesis is that the reduction of dopamine levels may be related to an adaptation in brain circuits caused by the psychotic disorder [79].
In adolescence, there is a high risk of psychopathologies from the interference of early stress in the maturation of the dopaminergic system [80]. In an animal protocol of social isolation during adolescence, an increase in vulnerability to alcoholism was observed, with a significant reduction in dopamine levels. Dopamine reduction was reversed after chronic administration of one kappa opioid receptor (KOR) antagonist, the nor-binaltorphimine (norBNI); therefore, the authors suggest that the use of KOR antagonists may help to prevent the downregulation of dopamine receptors and thus prevent or treat drug addiction [81]. Other research carried out in animal models found that stress in early life compromises dopamine receptor function in the prelimbic cortex and basal ganglia of females during adolescence, predisposing them to psychopathologies [80]. Thus, individuals who experience stress early in life may exhibit a higher inclination towards rewards associated with alcohol and other drugs while decreasing motivation in seeking healthy rewards [12].
The catecholamines norepinephrine (NE) and dopamine are synthesized from different genes. Therefore polymorphisms in these genes can alter the physiology of the CNS. A survey of 308 Chinese individuals diagnosed with MDD identified 13 single nucleotide polymorphisms (SNPs) in coding regions of six genes (monoamine oxidase A (MAOA), SLC6A2, tyrosine hydroxylase (TH), catechol-O-methyltransferase (COMT), dopamine receptor D2 (DRD2), DRD3), and polymorphisms in SLC6A2 are related to stress in the brain and early life identified by the Life Events Scale (LES) and Childhood Trauma Questionnaire-Short Form (CTQ-SF) [73].
The enzyme MAOA has the function of metabolizing neurotransmitters, including NE, 5-HT, and dopamine, central neurotransmitters for multiple functional brain circuits associated with stress regulation. Children who have experienced stress early in life have genetic deficiencies on the X chromosome (Xp11.123–11.4), which encodes MAOA, and are at increased risk of developing behavioral disorders. Low levels of MAOA are linked to childhood maltreatment, increasing the chances of developing mental health problems [68].
MDD individuals without medication showed a strong relationship between ELS and glutamine levels [82]. Individuals with higher scores on a childhood trauma inventory had a concomitant increase in glutamine levels in the occipital region, and those who suffered childhood emotional abuse had a more significant alteration of glutamate neurotransmission in the occipital region, which consequently increased the glutamate/glutamine cycle and glutamine level [83].
Gamma-aminobutyric acid (GABA) is an inhibitory neurotransmitter associated with the pathophysiology of MDD. Research in animal models indicates that ELS tends to reduce GABA expression in adolescence and adulthood and decrease the expression of GABAAR subunits in adulthood [84, 85].
Mice submitted to ELS presented impulsivity and excessive alcohol consumption in adulthood. ELS increased levels of GABAAR a2 subunit expression in central stress circuits. The CRF1 receptor antagonist antalarmine and a new GABAA R a2 subunit ligand, 3-PBC, were infused into the central amygdala and medial PFC (mPFC) of the animals, culminating in the pharmacological control of the CRF and GABAAR, reversing the effects of ELS in mice [86].
It is important to emphasize that the scientific literature presents a gap between neurotransmitters and ELS as a pathophysiological mechanism involved with MDD. Thus, research relating neurotransmitters and ELS is essential for an in-depth study of the long-term effects of stress, which may allow the creation of new therapeutic strategies for these individuals.
Early Life Stress, Neuronal Signaling, and Major Depressive Disorder
Individuals who have suffered ELS are at greater risk of developing psychiatric disorders and/or cognitive impairments in young or adult life. The mechanisms by which ELS triggers disorders or disabilities are not fully understood. Still, one hypothesis is related to changes in neuronal signaling and brain synaptic plasticity, especially in the hippocampal region. Synaptic function changes in individuals who undergo ELS, and a decrease in synaptic proteins may accompany this change. Synaptic protein synthesis is associated with the function of the mammalian target of the rapamycin (mTOR) signaling pathway, among other regulatory mechanisms [87].
One research was carried out with a mice model of ELS by a model of maternal deprivation and chronic restraint stress. The animals showed anxious behaviors and cognitive deficiencies, in addition to showing inhibition of the mTOR pathway by the S6 kinase signaling pathway (s6 path). Physiologically, mTOR activation causes s6 phosphorylation, which increases the expression of postsynaptic density protein 95 (PSD95) and synaptophysin and contributes to neuronal signaling and synaptic plasticity. In the research, there was also a decrease in the expression of PSD95 mRNA and protein and synaptophysin in the hippocampus. The results indicate that ELS impairs neuronal signaling and synaptic plasticity by altering mTOR signaling via the s6 pathway [87].
Animals that underwent a maternal deprivation protocol and therefore suffered from ELS show reduced body weight early in life, altered glutamate transmission, and a negative impact on the development of synaptic plasticity in the CA1 hippocampus in both female and male rats in comparison with animals in the control group that did not undergo the maternal deprivation protocol [88].
ELS influences the brain response during affective processing in the cortical region. Physiologically, visual stimuli with emotional contents drive cortical processing. One research carried out with individuals diagnosed with MDD who suffered ELS verified alterations in the affective response in the cortical region, considering that the stimuli presented less affective modulation when compared to the control group. This change is related to the increased risk and severity of MDD in individuals who have undergone ELS [89].
Globally based connectivity (GBC) is a method that describes brain interconnection. Some researchers evaluated GBC in individuals from neuroimaging exams to identify possible changes caused by ELS in neuronal signaling. It was determined that ELS causes sequelae in neuronal signaling from increased thalamic activity in healthy individuals and individuals diagnosed with MDD [90].
DRD3 signaling modulates neuronal activity in multiple areas of the brain, even relating to the behavior of social interaction. ELS culminates in changes in neuronal signaling and may contribute to the onset and severity of MDD. ELS downregulates DRD3 signaling in the lateral septum (LS), triggering social abnormality in individuals. The LS assists in processing emotional information and modulating behavioral responses to stress and is related to the reward system. The maternal deprivation protocol mimics ELS in animal models and triggers MDD in adulthood. A research carried out this protocol and identified the change in DRD3 signaling, a change reversed by pharmacological treatment with PD128907, a DRD3 agonist substance. Therefore, ELS negatively alters DRD3 signaling in LS and is a potential therapeutic target for affected individuals [91].
A study carried out in mice found that the strain of mice with high HPA axis reactivity to stress and exposed to ELS had HPA axis hyperactivity and cognitive impairments related to hippocampal function, in addition to altered corticotropin-releasing hormone (CRH) levels and BDNF compared to the strain of mice with low HPA axis reactivity [44].
Physiologically, the HPA axis stimulates the responses of the limbic regions to emotional stimuli, especially in the hippocampus and amygdala areas. Individuals who have undergone ELS show decreased reactivity of the amygdala and hippocampus during stressful situations experienced in adulthood, characterized by reduced cortisol concentrations and limbic deactivation. Neural effects can be positively modulated by work-related social support, but behavioral effects are not altered with this intervention [92].
Changes in the immune response contribute to the pathophysiology of MDD and are related to the effects caused by ELS. Individuals who have suffered ELS may have high levels of inflammatory markers chronically, which may be further exacerbated in cases of exposure to an acute stress situation. A study with adult men found higher levels of IL-6 and nuclear factor kappa B (NF-κB) in the serum of individuals who suffered ELS [93].
Metabolites produced by the kynurenine pathway (KP) have neuromodulatory functions involving neuroprotective, neurotoxic, and inflammatory substances. The presence of indoleamine 2,3-dioxygenase (IDO) stimulates KP, but when the immune system is overactivated, IDO levels are increased, potentiating the action of KP. Research verified the effect of variable maternal foraging demand as an ELS protocol on KP-related substances in the cerebrospinal fluid in female monkeys. Kynurenic acid (KynA) levels and the KynA/kynurenine (KYN) ratio were low in the stressed group, characterizing a reduction in KP neuroprotective substances. The results indicate that ELS is related to decreased neuroprotective substances and an increase in KP-related neurotoxic substances [94].
As mentioned above, several mechanisms related to neuronal signaling are associated with ELS and MDD (Fig. 3). It is possible to identify that the changes in MDD in individuals who have undergone ELS are exacerbated compared to individuals diagnosed with MDD without a history of ELS. In the scientific literature, there is still a lack of research that relates ELS with other mechanisms present in the pathophysiology of MDD, such as changes in the purinergic system and oxidative imbalance.
Stress-altered systems in early life. The figure compares the affected brain regions and which mechanism of action is modified. On the left is a normal brain with no changes, and on the right is a brain that has suffered ELS. In the prefrontal cortex, synaptic changes increase the activation of 5-HT1A and 5-HT2A receptors and dopamine (A2) levels. In the hippocampus, phosphorylation does not occur, and the s6 pathway is decreased, thus decreasing expression of PSD95 mRNA and synaptophysin (B2), which results in synaptic impairment. In the pituitary, the hippocampus pituitary adrenal system is affected by the increase in the axis and thus dysregulation of cortisol, the connection with the amygdala, and the neuroplasticity from brain-derived neurotrophic factor (BDNF) (C2). The color of the entire brain region is also modified because neuroplasticity and the whole brain's limbic system are altered and impaired
Considerations and Conclusions
The multifactorial nature of the etiology of MDD was supported and strengthened by the research presented in this article, which emphasizes the relevant interaction between human biology and environmental factors, with preponderance for the initial phase of life. Several studies point to the correlation between early stress and psychiatric disorders involving marker changes [68, 95], such as telomere length [96], dysregulation in central alpha2 noradrenergic receptors [97], and inflammatory changes among other biological mechanisms [29] (Fig. 4).
Molecular mechanisms and synaptic impairments related to ELS and MDD. Traumas suffered in childhood can lead to changes in the immune system, neurotransmitters, and, thus, signaling. In the immune system (A), it can increase cytokines like interleukin-6 (IL-6), increase C-reactive protein (CRP) inflammation factor, oxidative stress, and nuclear factor kappa B (NF-KB), and still can lead to brain-derived neurotrophic factor (BDNF) dysfunction as inflammation increases. The neurotransmitters system affected by ELS (B), resulting in its decrease, are serotonin, dopamine, GABA, GABAAR, and the MAO enzyme. However, there is an increase in 5-HT1A, 5-HT2A, glutamine and glutamate receptors, GABAA receptor, and corticotropin-releasing factor (CRF). As there is a change in neurotransmitters, signaling and synaptic plasticity are also altered (C) through a decrease in the mammalian target of the rapamycin (mTOR) pathway and dopamine receptors and an increase in the hypothalamus–pituitary–adrenal (HPA) axis, causing hormonal dysregulation of cortisol
Besides interfering with biological mechanisms, ELS can hinder the development of healthy coping skills [98]. Without adequate strategies to deal with stress, some people may resort to alcohol and other drugs [99]. Escape from drug use is one of the ways to seek pleasure and emotional relief, considering that the brain mechanisms of reward, pleasure, and motivation are impaired in many individuals who have suffered ELS [100]. The whole context can also be associated with self-medication and unfavorable social situations such as lack of social support, poverty, and domestic violence [101].
A study of individuals who underwent early loss experience (ELE), such as death or separation from parents, identified an association between this ELS and altered dynamics of the HPA axis, which could be reflected in reduced cortisol responses upon awakening. The study also highlighted that the effect was more pronounced in individuals who experienced multiple types of ELE. Furthermore, no significant differences were observed between men and women, indicating that this altered pattern of cortisol response was consistent between the sexes [102].
However, epidemiological studies show more significant evidence of MDD after ELS in women than men [103]. Research has concluded that men have higher levels of 5-HT1A receptor synthesis in cortical and subcortical brain regions, while women correlate positively with the receptor only in the hippocampus. Regarding the sexual genome, XY mice with decreased testosterone levels had lower expression of GABA, 5-HT, and dopamine-related genes in the prefrontal lobe compared to XX mice. This suggests that lower testosterone levels increase the predisposition to depression and anxiety [103]. Even though estrogen and testosterone levels influence MDD and schizophrenia, the effects of these hormones are transformative for individuals during puberty [104], not characterized as events in early childhood.
Furthermore, after maternal neglect, inflammatory factors such as TNFα and IL-6 in the prefrontal cortex and hippocampus increased in males. However, BDNF expression in the hippocampus and striatum of male mice was increased after maternal neglect, while the same expression was decreased in the striatum of females [103]. Few current studies tend to show the difference between the sexes and ELS and MDD, while it is conclusive that ELS affects brain plasticity and psychiatric disorders in both sexes. The difference between the sexes is the brain site involved and the modified transcriptomic signatures, but the stress response is similar [105, 106].
Early stress associated with changes in serotonergic and BDNF function has a potential risk for the lifelong manifestation of depression [21]. In addition to these mechanisms, studies indicate that ELS can culminate in increased epigenetic changes, leading to gene expressions involved in increased inflammation and favoring a greater risk of developing MDD [107, 108].
Recent studies have shown that epigenetic changes may also occur in sperm and eggs [109,110,111]. These inherited epigenetic changes may influence the stress response and susceptibility to MDD in future generations, including children of mothers who suffered ELS and did not experience any stress in childhood [112, 113]. An important example to consider is that the MAOA enzyme, encoded by the X chromosome, which metabolizes monoaminergic neurotransmitters, undergoes changes from the ELS [68]. Therefore, it is fundamental that new studies study this relationship and help to elucidate the mechanisms related to intergenerational epigenetics and the diagnosis of MDD so that it is possible to think of new therapeutic strategies that consider the history of the individuals' parents. New studies are also essential to investigate, for example, whether the effect of stress on the offspring of stressed mothers can increase the risk of triggering more severe symptoms of depression and refractoriness to different treatment strategies.
The neurotransmitter systems need a greater field of investigation regarding the correlation between MDD and ELS [83, 108]. The verified studies demonstrate relevant data. However, a considerable lack of research goes into the pathophysiological mechanisms linked to depression and adverse situations in childhood. It is worth noting that classic drug therapy for MDD acts on neurotransmitters and, therefore, significantly impacts the treatment of patients [114]. Classic antidepressants work mainly by increasing levels of monoaminergic neurotransmitters in some brain regions [115]. However, when an imbalance in neurotransmitter systems occurs due to ELS, the response to antidepressants can be affected [108, 116]. This diminished response to antidepressants is known as treatment resistance or inadequate response [117].
Another situation hypothesized to contribute to the poor response to traditional antidepressants is resistance to glucocorticoids released by the HPA axis [118]. Glucocorticoid resistance is stimulated by chronic stress, with potentiated effects when it occurs early in life [119]. It may interfere with the ability of glucocorticoids to modulate the inflammatory response and synaptic impairments related to MDD [120]. In cases of glucocorticoid resistance, this dysfunction may indirectly affect the effectiveness of antidepressants because glucocorticoids are also involved in regulating neurotransmitter levels, synaptic plasticity, and neurogenesis [121]. Therefore, when there is resistance to glucocorticoids, these processes can be impaired, making it difficult to respond to antidepressants [122].
With this, it is essential to emphasize that further research needs to be carried out to elucidate the mechanisms by which ELS potentiates the changes present in the pathophysiology of MDD both at the biological and behavioral levels so that, in this way, it is possible to think of new effective therapeutic strategies for this group of patients.
Data Availability
The cited studies are publicly available.
References
World Health Organization (2022) World Mental Health Report: transforming mental health for all. Geneva: World Health Organization. https://www.who.int/publications/i/item/9789240049338. Accessed 02 June 2023
Hughes K, Bellis MA, Hardcastle KA et al (2017) The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. The Lancet Public Health 2:e356–e366. https://doi.org/10.1016/S2468-2667(17)30118-4
Celik FGH, Hocaoglu H (2022) Implications of childhood trauma on suicidal behavior and deliberate self-harm in patients with major depressive disorder. Psychiatr Danub 34:57–63. https://doi.org/10.24869/psyd.2022.57
Nelson CA, Bhutta ZA, Burke Harris N et al (2020) Adversity in childhood is linked to mental and physical health throughout life. BMJ m3048. https://doi.org/10.1136/bmj.m3048
Nagy SA, Kürtös Z, Németh N et al (2021) Childhood maltreatment results in altered deactivation of reward processing circuits in depressed patients: a functional magnetic resonance imaging study of a facial emotion recognition task. Neurobiology of Stress 15:100399. https://doi.org/10.1016/j.ynstr.2021.100399
Lin J, Su Y, Lv X et al (2022) Childhood adversity, adulthood adversity and suicidal ideation in Chinese patients with major depressive disorder: in line with stress sensitization. Eur Arch Psychiatry Clin Neurosci 272:887–896. https://doi.org/10.1007/s00406-021-01375-4
Miao H, Zhong S, Liu X et al (2022) Childhood trauma history is linked to abnormal brain metabolism of non-medicated adult patients with major depressive disorder. J Affect Disord 302:101–109. https://doi.org/10.1016/j.jad.2021.12.103
Ahn YD, Jang S, Shin J, Kim J-W (2022) Psychological aspects of child maltreatment. J Korean Neurosurg Soc 65:408–414. https://doi.org/10.3340/jkns.2021.0300
Martins JSTO, Dinis MAP, Caridade SMM et al (2021) Adverse childhood experiences and delinquent behaviour: Predictors and mediating variables. Arch Clin Psychiatry (São Paulo) 48:75–82. https://doi.org/10.15761/0101-60830000000284
Waters RC, Gould E (2022) Early life adversity and neuropsychiatric disease: differential outcomes and translational relevance of rodent models. Front Syst Neurosci 16:860847. https://doi.org/10.3389/fnsys.2022.860847
Yehuda R, Lehrner A (2018) Intergenerational transmission of trauma effects: putative role of epigenetic mechanisms. World Psychiatry 17:243–257. https://doi.org/10.1002/wps.20568
Nishi M (2020) Effects of Early-Life Stress on the Brain and Behaviors: Implications of Early Maternal Separation in Rodents. IJMS 21:7212. https://doi.org/10.3390/ijms21197212
World Health Organization (2017). Depression and other common mental disorders: global health estimates. Geneva: World Health Organization. https://apps.who.int/iris/handle/10665/254610. Accessed 02 June 2023
American Psychiatric Association – APA (2014). Manual diagnóstico e estatístico dos transtornos mentais: DSM – V. Porto Alegre: Artmed.
Papp M, Cubała WJ, Swiecicki L et al (2022) Perspectives for therapy of treatment-resistant depression. British J Pharmacology 179:4181–4200. https://doi.org/10.1111/bph.15596
Su YY, D’Arcy C, Li M et al (2022) Specific and cumulative lifetime stressors in the aetiology of major depression: A longitudinal community-based population study. Epidemiol Psychiatr Sci 31:e3. https://doi.org/10.1017/S2045796021000779
Cavanaugh C, Nelson T (2022) A national study of the influence of adverse childhood experiences on depression among Black adults in the United States. J Affect Disord 311:523–529. https://doi.org/10.1016/j.jad.2022.05.112
Macpherson JM, Gray SR, Ip P et al (2021) Child maltreatment and incident mental disorders in middle and older ages: a retrospective UK Biobank cohort study. The Lancet Regional Health - Europe 11:100224. https://doi.org/10.1016/j.lanepe.2021.100224
Zisook S, Planeta B, Hicks PB et al (2022) Childhood adversity and adulthood major depressive disorder. Gen Hosp Psychiatry 76:36–44. https://doi.org/10.1016/j.genhosppsych.2022.03.008
Williams LM, Debattista C, Duchemin A-M et al (2016) Childhood trauma predicts antidepressant response in adults with major depression: data from the randomized international study to predict optimized treatment for depression. Transl Psychiatry 6:e799–e799. https://doi.org/10.1038/tp.2016.61
Nestor PG, O’Donovan K, Lapp HE et al (2019) Risk and protective effects of serotonin and BDNF genes on stress-related adult psychiatric symptoms. Neurobiology of Stress 11:100186. https://doi.org/10.1016/j.ynstr.2019.100186
Morimoto K, Nakajima K (2019) Role of the immune system in the development of the central nervous system. Front Neurosci 13:916. https://doi.org/10.3389/fnins.2019.00916
Muzio L, Viotti A, Martino G (2021) Microglia in neuroinflammation and neurodegeneration: from understanding to therapy. Front Neurosci 15:742065. https://doi.org/10.3389/fnins.2021.742065
Hassamal S (2023) Chronic stress, neuroinflammation, and depression: an overview of pathophysiological mechanisms and emerging anti-inflammatories. Front Psychiatry 14:1130989. https://doi.org/10.3389/fpsyt.2023.1130989
Sharifi-Rad M, Anil Kumar NV, Zucca P et al (2020) Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol 11:694. https://doi.org/10.3389/fphys.2020.00694
Nobis A, Zalewski D, Waszkiewicz N (2020) Peripheral markers of depression. J Clin Med 9:3793. https://doi.org/10.3390/jcm9123793
Slavich GM, Irwin MR (2014) From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull 140:774–815. https://doi.org/10.1037/a0035302
Ting EY-C, Yang AC, Tsai S-J (2020) Role of interleukin-6 in depressive disorder. IJMS 21:2194. https://doi.org/10.3390/ijms21062194
Kuhlman KR, Cole SW, Craske MG et al (2023) Enhanced immune activation following acute social stress among adolescents with early-life adversity. Biol Psychiatry Glob Open Sci 3:213–221. https://doi.org/10.1016/j.bpsgos.2022.03.001
Flouri E, Francesconi M, Midouhas E, Lewis G (2020) Prenatal and childhood adverse life events, inflammation and depressive symptoms across adolescence. J Affect Disord 260:577–582. https://doi.org/10.1016/j.jad.2019.09.024
Fan N, Luo Y, Ou Y, He H (2017) Altered serum levels of TNF-α, IL-6, and IL-18 in depressive disorder patients. Hum Psychopharmacol Clin Exp 32:e2588. https://doi.org/10.1002/hup.2588
Hodes GE, Ménard C, Russo SJ (2016) Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiol Stress 4:15–22. https://doi.org/10.1016/j.ynstr.2016.03.003
Kakeda S, Watanabe K, Katsuki A et al (2018) Relationship between interleukin (IL)-6 and brain morphology in drug-naïve, first-episode major depressive disorder using surface-based morphometry. Sci Rep 8:10054. https://doi.org/10.1038/s41598-018-28300-5
Chamberlain SR, Cavanagh J, de Boer P et al (2019) Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry 214:11–19. https://doi.org/10.1192/bjp.2018.66
Dittrich K, Boedeker K, Kluczniok D et al (2021) Elevated inflammatory markers in women with remitted major depressive disorder and the role of early life maltreatment. Brain Behav Immun 97:219–225. https://doi.org/10.1016/j.bbi.2021.07.024
Zhou Y, Liu Z, Liu Z et al (2021) Ventromedial hypothalamus activation aggravates hypertension myocardial remodeling through the sympathetic nervous system. Front Cardiovasc Med 8:737135. https://doi.org/10.3389/fcvm.2021.737135
Liu Z, Liu Z, Zhou H et al (2023) Increased sympathetic outflow induced by emotional stress aggravates myocardial ischemia–reperfusion injury via activation of TLR7/MyD88/IRF5 signaling pathway. Inflamm Res 72:901–913. https://doi.org/10.1007/s00011-023-01708-0
Barbon A, Magri C (2020) RNA editing and modifications in mood disorders. Genes 11:872. https://doi.org/10.3390/genes11080872
Burns SB, Szyszkowicz JK, Luheshi GN et al (2018) Plasticity of the epigenome during early-life stress. Semin Cell Dev Biol 77:115–132. https://doi.org/10.1016/j.semcdb.2017.09.033
Ein-Dor T, Verbeke WJMI, Mokry M, Vrtička P (2018) Epigenetic modification of the oxytocin and glucocorticoid receptor genes is linked to attachment avoidance in young adults. Attach Hum Dev 20:439–454. https://doi.org/10.1080/14616734.2018.1446451
Zou Z, Huang Y, Wang J et al (2020) DNA methylation of IL-4 gene and the association with childhood trauma in panic disorder. Psychiatry Res 293:113385. https://doi.org/10.1016/j.psychres.2020.113385
Peng H, Zhu Y, Strachan E et al (2018) Childhood trauma, DNA methylation of stress-related genes, and depression: findings from two monozygotic twin studies. Psychosom Med 80:599–608. https://doi.org/10.1097/PSY.0000000000000604
Li M, Fu X, Xie W et al (2020) Effect of early life stress on the epigenetic profiles in depression. Front Cell Dev Biol 8:867. https://doi.org/10.3389/fcell.2020.00867
McIlwrick S, Pohl T, Chen A, Touma C (2017) Late-onset cognitive impairments after early-life stress are shaped by inherited differences in stress reactivity. Front Cell Neurosci 11. https://doi.org/10.3389/fncel.2017.00009
Cherian K, Schatzberg AF, Keller J (2019) HPA axis in psychotic major depression and schizophrenia spectrum disorders: Cortisol, clinical symptomatology, and cognition. Schizophr Res 213:72–79. https://doi.org/10.1016/j.schres.2019.07.003
Caspani G, Kennedy S, Foster JA, Swann J (2019) Gut microbial metabolites in depression: understanding the biochemical mechanisms. Microb Cell 6:454–481. https://doi.org/10.15698/mic2019.10.693
Chmielewska N, Szyndler J, Maciejak P, Płaźnik A (2019) Epigenetic mechanisms of stress and depression. Psychiatr Pol 53:1413–1428. https://doi.org/10.12740/PP/94375
Czarny P, Białek K, Ziółkowska S et al (2021) The importance of epigenetics in diagnostics and treatment of major depressive disorder. J Pers Med 11:167. https://doi.org/10.3390/jpm11030167
Martín-Blanco A, Ferrer M, Soler J et al (2014) Association between methylation of the glucocorticoid receptor gene, childhood maltreatment, and clinical severity in borderline personality disorder. J Psychiatr Res 57:34–40. https://doi.org/10.1016/j.jpsychires.2014.06.011
Labonte B, Yerko V, Gross J et al (2012) Differential glucocorticoid receptor exon 1B, 1C, and 1H expression and methylation in suicide completers with a history of childhood abuse. Biol Psychiat 72:41–48. https://doi.org/10.1016/j.biopsych.2012.01.034
Yuan M, Yang B, Rothschild G et al (2023) Epigenetic regulation in major depression and other stress-related disorders: molecular mechanisms, clinical relevance and therapeutic potential. Sig Transduct Target Ther 8:309. https://doi.org/10.1038/s41392-023-01519-z
Takahashi Y, Kubo R, Sano R et al (2018) DNA methylation of the NR3C1 promoter region in brains of pediatric victims of physical abuse. Neurocase 24:269–275. https://doi.org/10.1080/13554794.2019.1582678
Jiang S, Postovit L, Cattaneo A et al (2019) Epigenetic modifications in stress response genes associated with childhood trauma. Front Psychiatry 10:808. https://doi.org/10.3389/fpsyt.2019.00808
Timmermans S, Souffriau J, Libert C (2019) A general introduction to glucocorticoid biology. Front Immunol 10:1545. https://doi.org/10.3389/fimmu.2019.01545
Zannas AS, Wiechmann T, Gassen NC, Binder EB (2016) Gene–stress–epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacol 41:261–274. https://doi.org/10.1038/npp.2015.235
Zgajnar N, De Leo S, Lotufo C et al (2019) Biological actions of the Hsp90-binding immunophilins FKBP51 and FKBP52. Biomolecules 9:52. https://doi.org/10.3390/biom9020052
Merkulov VM, Merkulova TI, Bondar NP (2017) Mechanisms of brain glucocorticoid resistance in stress-induced psychopathologies. Biochemistry Moscow 82:351–365. https://doi.org/10.1134/S0006297917030142
Redlich R, Stacey D, Opel N et al (2015) Evidence of an IFN-γ by early life stress interaction in the regulation of amygdala reactivity to emotional stimuli. Psychoneuroendocrinology 62:166–173. https://doi.org/10.1016/j.psyneuen.2015.08.008
Kowiański P, Lietzau G, Czuba E et al (2018) BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol 38:579–593. https://doi.org/10.1007/s10571-017-0510-4
Südhof TC (2018) Towards an understanding of synapse formation. Neuron 100:276–293. https://doi.org/10.1016/j.neuron.2018.09.040
Pathak H, Borchert A, Garaali S et al (2022) BDNF exon IV promoter methylation and antidepressant action: a complex interplay. Clin Epigenet 14:187. https://doi.org/10.1186/s13148-022-01415-3
Flint J (2023) The genetic basis of major depressive disorder. Mol Psychiatry. https://doi.org/10.1038/s41380-023-01957-9
Chakrabarty T, Harkness KL, McInerney SJ et al (2020) Childhood maltreatment and cognitive functioning in patients with major depressive disorder: a CAN-BIND-1 report. Psychol Med 50:2536–2547. https://doi.org/10.1017/S003329171900268X
Dallé E, Mabandla MV (2018) Early life stress, depression and Parkinson’s disease: a new approach. Mol Brain 11:18. https://doi.org/10.1186/s13041-018-0356-9
Kim Y-S, O’Sullivan DM, Shin S-K (2019) Can 24 weeks strength training reduce feelings of depression and increase neurotransmitter in elderly females? Exp Gerontol 115:62–68. https://doi.org/10.1016/j.exger.2018.11.009
Jalene S, Pharr J, Shan G, Poston B (2019) Estimated cardiorespiratory fitness is associated with reported depression in college students. Front Physiol 10:1191. https://doi.org/10.3389/fphys.2019.01191
Kageyama K, Iwasaki Y, Daimon M (2021) Hypothalamic regulation of corticotropin-releasing factor under stress and stress resilience. IJMS 22:12242. https://doi.org/10.3390/ijms222212242
Syed SA, Nemeroff CB (2017) Early life stress, mood, and anxiety disorders. Chronic Stress 1:247054701769446. https://doi.org/10.1177/2470547017694461
Mikulska J, Juszczyk G, Gawrońska-Grzywacz M, Herbet M (2021) HPA axis in the pathomechanism of depression and schizophrenia: new therapeutic strategies based on its participation. Brain Sci 11:1298. https://doi.org/10.3390/brainsci11101298
Burke NN, Finn DP, McGuire BE, Roche M (2017) Psychological stress in early life as a predisposing factor for the development of chronic pain: clinical and preclinical evidence and neurobiological mechanisms. J Neurosci Res 95:1257–1270. https://doi.org/10.1002/jnr.23802
Ansari AH, Pal A, Ramamurthy A et al (2021) Fibromyalgia pain and depression: an update on the role of repetitive transcranial magnetic stimulation. ACS Chem Neurosci 12:256–270. https://doi.org/10.1021/acschemneuro.0c00785
Maul S, Giegling I, Fabbri C et al (2020) Genetics of resilience: implications from genome-wide association studies and candidate genes of the stress response system in posttraumatic stress disorder and depression. Am J Med Genet B Neuropsychiatr Genet 183:77–94. https://doi.org/10.1002/ajmg.b.32763
Xu Z, Zhang Z, Shi Y et al (2011) Influence and interaction of genetic polymorphisms in catecholamine neurotransmitter systems and early life stress on antidepressant drug response. J Affect Disord 133:165–173. https://doi.org/10.1016/j.jad.2011.04.011
Mourtzi N, Sertedaki A, Charmandari E (2021) Glucocorticoid signaling and epigenetic alterations in stress-related disorders. Int J Mol Sci 22:5964. https://doi.org/10.3390/ijms22115964
Xu Z, Reynolds GP, Yuan Y et al (2016) TPH-2 polymorphisms interact with early life stress to influence response to treatment with antidepressant drugs. IJNPPY 19:pyw070. https://doi.org/10.1093/ijnp/pyw070
Sumner BEH, D’Eath RB, Farnworth MJ et al (2008) Early weaning results in less active behaviour, accompanied by lower 5-HT1A and higher 5-HT2A receptor mRNA expression in specific brain regions of female pigs. Psychoneuroendocrinology 33:1077–1092. https://doi.org/10.1016/j.psyneuen.2008.05.004
Shanmugan S, Loughead J, Cao W et al (2017) Impact of tryptophan depletion on executive system function during menopause is moderated by childhood adversity. Neuropsychopharmacol 42:2398–2406. https://doi.org/10.1038/npp.2017.64
Shanmugan S, Satterthwaite TD, Sammel MD et al (2017) Impact of early life adversity and tryptophan depletion on functional connectivity in menopausal women: a double-blind, placebo-controlled crossover study. Psychoneuroendocrinology 84:197–205. https://doi.org/10.1016/j.psyneuen.2017.07.239
Kasanova Z, Hernaus D, Vaessen T et al (2016) Early-life stress affects stress-related prefrontal dopamine activity in healthy adults, but not in individuals with psychotic disorder. PLoS One 11:e0150746. https://doi.org/10.1371/journal.pone.0150746
Majcher-Maślanka I, Solarz A, Wędzony K, Chocyk A (2017) The effects of early-life stress on dopamine system function in adolescent female rats. Int j dev neurosci 57:24–33. https://doi.org/10.1016/j.ijdevneu.2017.01.001
Karkhanis AN, Rose JH, Weiner JL, Jones SR (2016) Early-life social isolation stress increases kappa opioid receptor responsiveness and downregulates the dopamine system. Neuropsychopharmacol 41:2263–2274. https://doi.org/10.1038/npp.2016.21
Truong V, Cheng PZ, Lee H-C et al (2021) Occipital gamma-aminobutyric acid and glutamate-glutamine alterations in major depressive disorder: An mrs study and meta-analysis. Psychiatry Res Neuroimaging 308:111238. https://doi.org/10.1016/j.pscychresns.2020.111238
Averill LA, Abdallah CG, Fenton LR et al (2020) Early life stress and glutamate neurotransmission in major depressive disorder. Eur Neuropsychopharmacol 35:71–80. https://doi.org/10.1016/j.euroneuro.2020.03.015
Lukkes JL, Meda S, Thompson BS et al (2017) Early life stress and later peer distress on depressive behavior in adolescent female rats: Effects of a novel intervention on GABA and D2 receptors. Behav Brain Res 330:37–45. https://doi.org/10.1016/j.bbr.2017.04.053
Everington EA, Gibbard AG, Swinny JD, Seifi M (2018) Molecular characterization of GABA-A receptor subunit diversity within major peripheral organs and their plasticity in response to early life psychosocial stress. Front Mol Neurosci 11:18. https://doi.org/10.3389/fnmol.2018.00018
Gondré-Lewis MC, Warnock KT, Wang H et al (2016) Early life stress is a risk factor for excessive alcohol drinking and impulsivity in adults and is mediated via a CRF/GABA A mechanism. Stress 19:235–247. https://doi.org/10.3109/10253890.2016.1160280
Wang A, Zou X, Wu J et al (2020) Early-life stress alters synaptic plasticity and mTOR signaling: correlation with anxiety-like and cognition-related behavior. Front Genet 11:590068. https://doi.org/10.3389/fgene.2020.590068
Derks NAV, Krugers HJ, Hoogenraad CC et al (2016) Effects of early life stress on synaptic plasticity in the developing hippocampus of male and female rats. PLoS One 11:e0164551. https://doi.org/10.1371/journal.pone.0164551
Peters AT, Burkhouse KL, Kinney KL, Luan Phan K (2019) The roles of early-life adversity and rumination in neural response to emotional faces amongst anxious and depressed adults. Psychol Med 49:2267–2278. https://doi.org/10.1017/S0033291718003203
Philip NS, Tyrka AR, Albright SE et al (2016) Early life stress predicts thalamic hyperconnectivity: a transdiagnostic study of global connectivity. J Psychiatr Res 79:93–100. https://doi.org/10.1016/j.jpsychires.2016.05.003
Shin S, Pribiag H, Lilascharoen V et al (2018) Drd3 signaling in the lateral septum mediates early life stress-induced social dysfunction. Neuron 97:195-208.e6. https://doi.org/10.1016/j.neuron.2017.11.040
Leicht-Deobald U, Bruch H, Bönke L et al (2018) Work-related social support modulates effects of early life stress on limbic reactivity during stress. Brain Imaging Behav 12:1405–1418. https://doi.org/10.1007/s11682-017-9810-z
Pace TWW, Mletzko TC, Alagbe O et al (2006) Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. AJP 163:1630–1633. https://doi.org/10.1176/ajp.2006.163.9.1630
Coplan JD, George R, Syed SA et al (2021) Early life stress and the fate of kynurenine pathway metabolites. Front Hum Neurosci 15:636144. https://doi.org/10.3389/fnhum.2021.636144
Smith KE, Pollak SD (2020) Early life stress and development: potential mechanisms for adverse outcomes. J Neurodevelop Disord 12:34. https://doi.org/10.1186/s11689-020-09337-y
Ridout KK, Levandowski M, Ridout SJ et al (2018) Early life adversity and telomere length: a meta-analysis. Mol Psychiatry 23:858–871. https://doi.org/10.1038/mp.2017.26
Kuehl LK, Deuter CE, Nowacki J et al (2021) Attentional bias in individuals with depression and adverse childhood experiences: influence of the noradrenergic system? Psychopharmacology 238:3519–3531. https://doi.org/10.1007/s00213-021-05969-7
Pallarés ME, Monteleone MC, Pastor V et al (2021) Early-life stress reprograms stress-coping abilities in male and female juvenile rats. Mol Neurobiol 58:5837–5856. https://doi.org/10.1007/s12035-021-02527-2
Júnior EBC, Fernandes MNDF, Gimenez LBH et al (2020) Influence of early life stress on alcohol and crack dependents. OJN 10:490–512. https://doi.org/10.4236/ojn.2020.105034
Volkow ND, Michaelides M, Baler R (2019) The neuroscience of drug reward and addiction. Physiol Rev 99:2115–2140. https://doi.org/10.1152/physrev.00014.2018
Anda RF, Felitti VJ, Bremner JD et al (2006) The enduring effects of abuse and related adverse experiences in childhood: a convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci 256:174–186. https://doi.org/10.1007/s00406-005-0624-4
Meinlschmidt G, Heim C (2005) Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinology 30:568–576. https://doi.org/10.1016/j.psyneuen.2005.01.006
An X, Guo W, Wu H et al (2022) Sex differences in depression caused by early life stress and related mechanisms. Front Neurosci 16:797755. https://doi.org/10.3389/fnins.2022.797755
Eck SR, Bangasser DA (2020) The effects of early life stress on motivated behaviors: A role for gonadal hormones. Neurosci Biobehav Rev 119:86–100. https://doi.org/10.1016/j.neubiorev.2020.09.014
Parel ST, Peña CJ (2022) Genome-wide signatures of early life stress: influence of sex. Biol Psychiatry 91:36–42. https://doi.org/10.1016/j.biopsych.2020.12.010
Shobeiri P, Kalantari A, Teixeira AL, Rezaei N (2022) Shedding light on biological sex differences and microbiota–gut–brain axis: a comprehensive review of its roles in neuropsychiatric disorders. Biol Sex Differ 13:12. https://doi.org/10.1186/s13293-022-00422-6
Ochi S, Dwivedi Y (2023) Dissecting early life stress-induced adolescent depression through epigenomic approach. Mol Psychiatry 28:141–153. https://doi.org/10.1038/s41380-022-01907-x
Cheng Z, Su J, Zhang K et al (2022) Epigenetic mechanism of early life stress-induced depression: focus on the neurotransmitter systems. Front Cell Dev Biol 10:929732. https://doi.org/10.3389/fcell.2022.929732
Fitz-James MH, Cavalli G (2022) Molecular mechanisms of transgenerational epigenetic inheritance. Nat Rev Genet 23:325–341. https://doi.org/10.1038/s41576-021-00438-5
Lismer A, Kimmins S (2023) Emerging evidence that the mammalian sperm epigenome serves as a template for embryo development. Nat Commun 14:2142. https://doi.org/10.1038/s41467-023-37820-2
Donkin I, Barrès R (2018) Sperm epigenetics and influence of environmental factors. Molecular Metabolism 14:1–11. https://doi.org/10.1016/j.molmet.2018.02.006
Dias BG, Ressler KJ (2014) Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci 17:89–96. https://doi.org/10.1038/nn.3594
Rodgers AB, Morgan CP, Bronson SL et al (2013) Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 33:9003–9012. https://doi.org/10.1523/JNEUROSCI.0914-13.2013
Li Z, Ruan M, Chen J, Fang Y (2021) Major depressive disorder: advances in neuroscience research and translational applications. Neurosci Bull 37:863–880. https://doi.org/10.1007/s12264-021-00638-3
Hillhouse TM, Porter JH (2015) A brief history of the development of antidepressant drugs: From monoamines to glutamate. Exp Clin Psychopharmacol 23:1–21. https://doi.org/10.1037/a0038550
Jiang Y, Zou D, Li Y et al (2022) Monoamine neurotransmitters control basic emotions and affect major depressive disorders. Pharmaceuticals 15:1203. https://doi.org/10.3390/ph15101203
Haroon E, Daguanno AW, Woolwine BJ et al (2018) Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology 95:43–49. https://doi.org/10.1016/j.psyneuen.2018.05.026
Menke A (2019) Is the HPA Axis as Target for Depression Outdated, or Is There a New Hope? Front Psychiatry 10:101. https://doi.org/10.3389/fpsyt.2019.00101
Finsterwald C, Alberini CM (2014) Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiol Learn Mem 112:17–29. https://doi.org/10.1016/j.nlm.2013.09.017
Zunszain PA, Anacker C, Cattaneo A et al (2011) Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry 35:722–729. https://doi.org/10.1016/j.pnpbp.2010.04.011
Madalena KM, Lerch JK (2017) The effect of glucocorticoid and glucocorticoid receptor interactions on brain, spinal cord, and glial cell plasticity. Neural Plast 2017:1–8. https://doi.org/10.1155/2017/8640970
Anacker C, Zunszain PA, Carvalho LA, Pariante CM (2011) The glucocorticoid receptor: Pivot of depression and of antidepressant treatment? Psychoneuroendocrinology 36:415–425. https://doi.org/10.1016/j.psyneuen.2010.03.007
Funding
Zuleide Maria Ignácio is supported by research grants from the Santa Catarina State Research and Innovation Support Foundation—FAPESC and Federal University of Southern Frontier—UFFS.
Author information
Authors and Affiliations
Contributions
Conceptualization, Zuleide Maria Ignácio; writing, Amanda Gollo Bertollo, Agatha Carina Leite Galvan, Claudia Dallagnol, and Arthur Dellazeri Cortez; editing, Amanda Gollo Bertollo; reviewing and supervision, Zuleide Maria Ignácio. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publcation
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bertollo, A.G., Galvan, A.C.L., Dallagnol, C. et al. Early Life Stress and Major Depressive Disorder—An Update on Molecular Mechanisms and Synaptic Impairments. Mol Neurobiol 61, 6469–6483 (2024). https://doi.org/10.1007/s12035-024-03983-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-024-03983-2