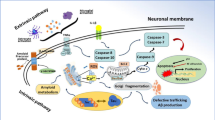
Abstract
Bone marrow-derived neural stem cells (BM-NSCs) have shed light on novel therapeutic approaches for PD with the potential to halt or even reverse disease progression. Various strategies have been developed to promote therapeutic efficacy via optimizing implanted cells and the microenvironment of transplantation in the central nervous system (CNS). This current study further proved that the combination of fasudil, a Rho-kinase inhibitor, and BM-NSCs exhibited a synergetic effect on restoring neuron loss in the MPTP-PD mice model. It simultaneously unveiled cellular mechanisms underlying synergistic neuron-protection effects of fasudil and BM-NSCs, which included promoting the proliferation, and migration of endogenous NSCs, and contributing to microglia shift into the M2 phenotype. Corresponding molecular mechanisms were observed, including the inhibition of inflammatory responses, the elevation of neurotrophic factors, and the induction of WNT/β-catenin and PI3K/Akt/mTOR signaling pathways. Our study provides evidence for the co-intervention of BM-NSCs and fasudil as a promising therapeutic method with enhanced efficacy in treating neurodegenerative diseases.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a prevalent, debilitating neurological disorder [6]. It has long been characterized by the classical motor dysfunctions of parkinsonism associated with the degeneration of dopaminergic neurons (DA) in the substantia nigra compacta (SNpc) [16]. Though current pharmacotherapies and surgical treatments for PD appear effective in controlling symptoms, these strategies have shown some limitations, such as undesirable side effects and long-term inefficiency [5, 12, 22]. Based on the theory of using self-renewal cells to replace the progressive loss of DA neurons during PD, neural stem cell-based therapies emerged as a promising disease-modifying therapy, thus restoring normal function in striatopallidal circuitry [1, 40].
Among different neural stem cell types for application in PD patients, bone marrow derived-neural stem cells (BM-NSCs) are accessible with minimally invasive procedures, easily cultured, and expanded in vitro for several passages. Moreover, BM-NSCs are used for autologous transplantation due to hypo-immunogenicity with few ethical concerns [38]. However, clinical applications of NSCs are mainly hindered by the poor survival rates of grafted DA caused by the host response to the graft [48]. The microenvironment in CNS provides extracellular signals to modulate NSCs behaviors, including survival, migration, and differentiation, thus underlying the discrepancy between theoretical efficacy and practical outcome [10, 43]. Multiple studies have been investigating approaches to promote the protective behaviors of transplanted NSCs via alleviating oxidative stress and inflammatory response [15, 42].
In PD rodent models, Rho-kinase has been proven effective in modulating the CNS aberrant microenvironment, thereby protecting DA neurons from death. [19, 32]. Fasudil, a Rho kinase inhibitor, was reported to enhance the survival of dopaminergic neurons and attenuate axonal loss due to its effects on anti-inflammation anti-oxidation and promote the expression of neurotrophic factors [29].
Our previous work has validated the synergistic effects of fasudil and BM-NSCs on restoring dopaminergic neurons and improving behavior function in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mouse model [26]. In the current study, we further investigated the cellular and molecular mechanisms underlying the additional efficacy of fasudil in BM-NSCs-based therapy in the MPTP mouse model.
Materials and Methods
Animals
Male C57BL/6 mice (aged 10- to 12-week-old and 26–30 g weight) were provided by Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). All animal experiments were carried out according to the guidelines of the Ethics Committee of Fudan University, Shanghai, China. The mice were randomly raised with food and water and maintained in a 12/12-h light/dark cycle in a temperature control room (25 ± 2 °C).
Cultivation of BM-NSCs
Bone marrow-derived neural stem cells (BM-NSCs) were given from Tianjin Medical College, China. Briefly, bone marrow-derived neural stem cells (BM-NSCs) were harvested from the femurs of 8-week-old green fluorescent protein (GFP) transgenic mice (C57BL/ 6-Tg; ACTB-EGFP), as in previously described protocols [26]. NSCs were expanded in vitro. At the 5th–10th passages, neurospheres were dissociated into single cells and validated for Nestin expression. Single dissociated Nestin+ BM-NSCs were suspended in sterile PBS for transplantation.
MPTP-PD Model and Experimental Design
The PD mouse model was developed by intraperitoneally administrating MPTP (Sigma-Aldrich Chemical Co., USA) (15 mg/kg) four times at 2-h intervals in one day. The mice were divided into 4 groups (15 mice/group): (1) PBS (saline) group; (2) fasudil (fasudil) group; (3) BM-NSCs (NSCs) group; (4) fasudil + BM-NSCs (fasudil/NSCs) group.
Administration of Fasudil and BM-NSCs
For NSCs inoculation, mice were lightly anesthetized with diethyl ether 48 h after the last MPTP administration (Sinopharm, Shanghai, China). To facilitate cell entry, we applied two doses of 3 μl hyaluronidase/PBS (total 100 U; Sigma-Aldrich Chemical Co., USA) in each nostril to increase the barrier permeability of nasopharyngeal mucosa. Subsequently, mice received a total of 1.5x105 BM-NSCs in 12 μl PBS in each nostril or the same volume of PBS.
On day 4 of MPTP injection, mice received fasudil (Tianjin Chase Sun Pharmaceutical Co., Ltd, China) 400 μg/mice every day for 6 days. Saline groups received PBS injections of the same volume. Two days after the final fasudil injection, mice were anesthetized and rapidly perfused with PBS, and the brains were removed for Western Blot analysis. Total proteins were extracted from the midbrain with 1 mM PMSF in 1 ml ice-cold RIPA buffer (Beyotime) and added to an EDTA-free protease inhibitor cocktail. Protein concentrations were determined using the bicinchoninic acid protein assay (Beyotime). 4% paraformaldehyde followed PBS perfusion for some animals, and the brains were carefully harvested for immunofluorescence (IF) staining. Ninety sequential 10-μm-thick coronal sections (-2.54 to -3.88 mm posterior to bregma) were cut on freezing microtome using a Leica cryostat for immunohistochemistry.
Immunofluorescence Staining
The serial sections were blocked with 5% BSA/0.2%TritonX-100 (Multiscience, China) at room temperature (RT) for 30 min and then incubated overnight at 4 °C with primary antibodies. The primary antibodies include Dcx (Abcam, USA), Ki-67 (Abcam, USA), anti-GFAP (Sigma, USA), and anti-Nestin (Abcam, USA). Subsequently, the sections were washed with PBS three times and incubated with the corresponding fluorescein-labeled secondary antibodies (Invitrogen, USA) for 1 h at RT. Positively stained cells were quantified under a fluorescence microscope with Image-Pro Plus 6.0 software. Immunopositive cells in 4 coronal slices at the same position were counted, respectively, and the average value was taken as the number of positively expressing cells of each mouse.
Western Blot Analysis
The mouse brain was extracted using protein extraction buffer (T-PER, Thermo Fisher, USA) containing protease and phosphatase inhibitors (Thermo Fisher, USA). Equal amounts of protein extracts (20 µg) were separated by SDS-PAGE and transferred onto a nitrocellulose filter (NC) membrane (GE Healthcare Life Sciences, USA). After being blocked with 5% non-fat milk, membranes were incubated at 4 °C overnight with primary antibodies (all at 1:1000) as follows: anti-β-actin (Abcam, USA), anti-p-mTOR(Abcam, USA), anti-GDNF(Abcam, USA), anti-BNDF(Abcam, USA), anti-CNTF(Abcam, USA), anti-Bcl-2(Abcam, USA), anti-Bax (Abcam, USA), anti-p110α-PI3K (C73F8) (Cell Signaling Technology, MA), anti-p-Akt (Ser473) (Cell Signaling Technology, MA), anti-β-catenin (Cell Signaling Technology, MA), anti- Fzd1 (R&D system, USA), anti- NLRP3 (R&D system, USA), anti-WNT1 (ABGENT, USA), anti- iNOS/NOS(BD, USA) or anti-Arginase1 (BD, USA). After being washed in TBST, the immunoblots underwent incubation with horseradish peroxidase-conjugated secondary antibodies (Jackson Lab, USA) for 1 h. Bands were visualized and analyzed using a ChemiDoc XRS (Bio-Rad, USA) and Image Lab(Bio-Rad, USA).
Statistical Analysis
Data were statistically analyzed and presented as means ± SEM. One-way ANOVA assessed multiple comparisons among groups. P < 0.05 was accepted to be statistically significant. All statistical analyses and graphs were performed or generated with GraphPad Prism v5.0 (GraphPad Prism Software, Inc., USA).
Results
The Combination of Fasudil and BM-NCSs Contributes to NSCs Proliferation and Migration
Our previous work showed the synergistic effects of fasudil and BM-NSCs on improving behavior function and protecting DA in the SNpc of mouse brain injected MPTP [26]. To investigate the effect of fasudil on the proliferation and migration of BM-NSCs in MPTP-induced mice, we conducted immunofluorescent staining of DCX (a neurogenesis marker) and Ki-67 (a proliferation marker) to compare proliferating NSCs in SVZ (subventricular zone) and the hippocampus. The combination of fasudil and NSCs significantly elevated both numbers of Dcx+ and Ki-67+ cells in the SVZ region, compared to the numbers in the saline group (p < 0.001 for both) and the group treated with NSCs or fasudil, respectively (p < 0.05 for both, Fig. 1a). Meanwhile, none of GFP+ NSCs were observed in SVZ (Fig. 1a), indicating that the combination of fasudil and NCSs promotes the proliferation of endogenous cells.
Combination of fasudil and BM-NSCs exhibits synergistic contributions to the proliferation and migration of endogenous NCSs in both SVZ and hippocampus. Brain sections of SVZ or hippocampus in hippocampus from each mouse (n = 4) were stained with anti-Dcx and anti-Ki-67 antibodies, followed by the corresponding fluorescein-labeled secondary antibodies. Dcx and Ki-67 expression in a.SVZ and b.hippocampus was determined by IFH. Numbers of Dcx + cells and Ki-67 + cells were observed in group treated with combination of fasudil and NSC. The magnified figure showed Dcx + /Ki-67 + cells (arrows). GFP + cells were not merged with Dcx or Ki-67(blank arrows). Numbers of Dcx + cells and Ki-67 + cells per high-power field (/HP) were quantified respectively in SVZ and hippocampus. Quantitative results are mean ± SD (n = 4 in each group) and comparisons were analyzed by one-way ANOVA. Asterisk: comparisons with the NSCs + PD group. ns: not significant *p < 0.05 **p < 0.01, ***p < 0.001
In the SG zone of hippocampus, more Dcx + and Ki-67 + cells were observed in the fasudil/NSCs group than in the fasudil or NSCs group (p < 0.001 for both, Fig. 1b). None of the GFP+ cells was Dcx or Ki-67 positive, indicating proliferation of endogenous NSCs but not BM-NSCs. These results showed that in the combination of the fasudil and NCSs group, endogenous NSCs have proliferated in both SVZ and the hippocampus.
The Combination of Fasudil and BM-NSCs Induces Astrocytes to Express Neuronal Marker
A previous study in the PD mouse model observed some astrocytes that were giant in morphology and expressed Nestin, a protein expressed in neuronal precursor cells, which was considered to be differentiating into NSCs, thereby contributing to restoring DA neurons [36]. Here we double-stained GFAP (an astrocyte marker) with Nestin (a neural stem/progenitor cell marker). There were a low number of GFAP+/Nestin+ cells in the saline group and slightly increased by fasudil or BM-NSCs. The combination of fasudil and BM-NSCs has further elevated (Fig. 2). It is indicated that fasudil and NSCs alone promoted GFAP + astrocytes expressing Nestin. In contrast, the combination of fasudil and NSCs exerted a synergistic effect.
Combination of fasudil and BM-NSCs promotes neuronal marker expression in astrocytes. Brain sections were stained with anti-GFAP and anti-Nestin antibodies, followed by the corresponding fluorescein-labeled secondary antibodies. Two investigators independently and blindly examined all slices. Double immunostaining of GFAP and Nestin in the brain. The magnified figure showed GFAP + /Nestin + cells (arrows) and GFAP + cells(blank arrows). Elevated number of GFAP + /Nestin + cells were observed in group treated with combination of fasudil and NSC. Quantitative results are mean ± SEM (n = 8 in d each group), and one-way ANOVA analyzed multiple comparisons. Asterisk: comparisons between saline and combination of fasudil/NSCs groups. ns: not significant. *p < 0.05
The Combination of Fasudil and BM-NSCs Alleviates Inflammation in MPTP Model
Previous studies confirmed that the combination of fasudil and NSCs inhibited microglia activation around SNpc and striatum [26]. Here, we studied the influences of fasudil and NSCs treatment on microglia subtypes. The expression of iNOS (a marker for the microglia M1 subtype) and arginase1 (a marker for the microglia M2 subtype) were used to evaluate the microglia polarization. Either fasudil or BM-NSCs treatment alone downregulated iNOS expression and increased the expression of arginase1 to a similar level (Fig. 3a, b). The combined treatment further declined the expression of iNOS and elevated the expression of arginase1(Fig. 3a, b).
Combination of fasudil and BM-NSCs attenuates inflammatory responses in MPTP-PD mouse brain. a iNOS, b arginase-1, and c NLPR3 expression in the brain in different groups deternmined by Western Blot. iNOS expression was downregulated and arginase-1 expression was increased when treated with either fasudil or NSCs. The combined treatment further declined the expression of iNOS and elevated the expression of arginase-1. d The level of IL-1β, TNF-a, IL-10, and IL-6 per milligram of brain tissue (pg/mg) in different groups were measured by ELISA. Pro-inflammatory cytokines such as IL-1β and TNF-a were inhibited and IL-10, a representative anti-inflammatory cytokine, was elevated with combined treatment of fasudil/NSCs. Difference was insignificant in IL-6 levels among each group. Quantitative results are mean ± SD (n = 4 in each group) and comparisons were analyzed by one-way ANOVA. Asterisk: comparisons with the NSCs + PD group. ns: not significant *p < 0.05 **p < 0.01, ***p < 0.001
The polarization of microglia M1 to M2 should be accompanied by a decreased inflammatory response and increased anti-inflammatory response. Consistent with this phenomenon. The expression of NLRP3 (NLR family pyrin domain containing 3) was inhibited in all three treated groups. However, the most significant reduced expression was observed in the fasudil/NSCs group (p < 0.001–0.05, Fig. 3c). Simultaneously, pro-inflammatory cytokines such as IL-1β (P < 0.01, Fig. 3d) and TNF-a (P < 0.001–0.05, Fig. 3d) were inhibited with fasudil/NSCs combination compared to the control or individual treatment by using ELISA. Increased expression of IL-10, a representative anti-inflammatory cytokine, was observed in the fasudil/NSCs combined treatment group compared with control (p < 0.001) and fasudil treatment alone (p < 0.05, Fig. 3d). However, there is little difference in IL-6 in each group (Fig. 3d). Thus, combined fasudil and NSCs attenuate inflammatory responses and promote microglial polarization toward the M2 phenotype.
The Combination of Fasudil and BM-NSCs Contributes to NSCs Neural Migration in V-SVZ by Enhancing WNT/Fzd1/β-Catenin Pathway
The Wnt signaling pathway plays a vital role in embryonic development, cell proliferation, migration, differentiation, survival, adhesion, and renewal of stem cells to maintain adult tissue homeostasis [4]. Western Blot evaluated the expression of major Wnt signaling molecules, Wnt, Fzd1, and β-catenin. The results showed that expression of Wnt, Fzd1, and β-catenin was increased with treatment of fasudil or NSCs, respectively (p < 0.05, Fig. 4a–c). The combination of NSCs and fasudil further elevated the expression of Wnt, Fzd1, and β-catenin (Fig. 4a–c).
Combination of fasudil and BM-NSCs upregulates WNT/Fzd1/β-catenin pathway. Western Blot were conducted to measure the expression level of a WNT, b Fzd1, and c β-catenin. The combination of NSCs and fasudil further elevated the expression of Wnt, Fzd1, and β-catenin. Quantitative results are mean ± SD (n = 4 in each group) and comparisons were analyzed by one-way ANOVA. Asterisk: comparisons with the NSCs + PD group. *p < 0.05, **p < 0.01, ***p < 0.001
These data suggested that combining NSCs and fasudil enhances the Wnt signaling pathway, which should be related to endogenous cell proliferation and migration.
The Combination of Fasudil and BM-NSCs Influences PI3K/Akt/mTOR Pathway and Apoptosis
Mounting evidence suggests that loss of dopaminergic neurons in PD patients is related to inhibition of the PI3K/Akt pathway and apoptosis [28]. To explore the effects of different interventions on apoptosis and the PI3K/Akt pathway, we detected the expressions of Bcl-2, Bax, and upstream molecular signals, such as PI3K, pAkt, and p-mTOR were detected by Western Blot. The results showed that fasudil/NSCs combined treatment promoted the expression of PI3K, pAkt, and p-mTOR compared to the other groups (Fig. 5a–c). Furthermore, Bcl-2 expression was elevated in the fasudil/NSCs group, while expression of Bax was inhibited. Increased Bcl-2/Bax ratio suggested combination of fasudil and NSCs decreased cell susceptibility to apoptosis. (Fig. 5d). The combined treatment of fasudil and NSCs protects against MPTP neurotoxin, possibly by inhibiting apoptosis via PI3K/Akt/mTOR pathway.
Combination of fasudil and BM-NSCs inhibits autophagy via enhancing PI3K/Akt/mTOR pathway. a PI3K expression, b pAkt expression, c p-mTOR expression, d Bcl-2 and Bax expression by Western Blot in the brain of different groups. (n = 4 in each group) Combined treatment promoted the expression of PI3K, pAkt, and p-mTOR compared to the other groups. Increased Bcl-2/Bax ratio were observed with combination of fasudil and NSCs. Quantitative results are mean ± SD (n = 4 in each group) and comparisons were analyzed by one-way ANOVA. Asterisk: comparisons with the NSCs + PD group. *p < 0.05 **p < 0.01, ***p < 0.001
The Combination of Fasudil and BM-NSCs Enhances the Expression of Neurotrophic Factors
In recent years, transplanted stem cells’ regenerative effects have been correlated to the panel of bioactive soluble factors and vesicles with neuro-regulatory properties released to the extracellular environment, including multiple neurotrophic factors [20], [39]; [44]. This study showed that fasudil and NSCs alone could significantly increase BDNF, GDNF, and CNTF in the brain compared to saline treatment (Fig. 6a–c). The combination of fasudil and NSCs exhibited a synergistic effect, further increasing the expression of these neurotrophic factors (Fig. 6a–c).
Combination of fasudil and BM-NSCs enhances neurotrophic factors expression. a Pro-BNDF and mature BNDF, b GNDF, c CNDF expression in the brain by Western Blot in different groups (n = 4). Fasudil and NSCs alone could significantly increase BDNF, GDNF, and CNTF in the brain compared to saline treatment. The combination of fasudil and NSCs further increased the expression of these neurotrophic factors. Quantitative results are mean ± SD (n = 4 in each group), and comparisons were analyzed by one-way ANOVA. Asterisk: comparisons with the NSCs + PD group. *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
Cell-based therapy has shed light on novel therapeutic approaches for PD with the potential to halt or even reverse disease progression. Nevertheless, a few transplanted cells survive in vivo, partially explaining why PD patients only receive limited benefits from transplantation therapeutics [34]. Poor differentiation and maturation of DA neurons and decreased cell survival after transplantation remain challenging. Therefore, various strategies have been developed to promote therapeutic efficacy from different perspectives.
First, several studies have focused on optimizing the delivery route of cells [9, 13]. Conventional cell therapies for CNS diseases include intravenous, intraperitoneal, intraventricular, and intracerebral stereotactic injections. Among them, intravenous infusion is the most acceptable route of administration. However, most NSCs may be trapped in microvessels of pulmonary circulation after intravenous infusion because of their larger volume. In addition, studies have shown that intravenous NSCs were transferred from the lung to the liver, spleen, kidney, and bone marrow within 48 h (Gao et al., 2001). Stem cells for therapy may avoid the first clearance and bypass BBB directly into the brain, which helps to increase the number of implanted cells in the brain and migrate from the nasal cavity to the repair site. Compared with other routes, this method is simple, non-invasive, and proved ideal for cell-based therapy in other neuronal diseases likeAlzheimer'ss disease [37]. Our results confirmed that intranasal administration could deliver exogenous BM-NSCs to the hippocampus and SVZ zone.
Second, both the characteristics of stem cells and their living environments affect the therapeutic efficiency of NSCs [9]. NSCs implanted into the brain should survive, proliferate, and migrate to the defect area and differentiate into the appropriate functional phenotype to participate in tissue repair and regeneration. The molecular and cellular mechanisms underneath are complex and remain poorly understood. Accumulating evidence has demonstrated that ROCK regulates cell proliferation, adhesion, and migration [45]. Therefore, the ROCK inhibitor appears to enhance the yield and survival of stem cells from various sources and inhibit the apoptosis of stem cell-derived neuronal progenitors following animal transplantation. The migration of NSCs is indispensable during the repair and regeneration process. In earlier studies, dental pulp cells (DPCs) migration was increased by inhibition of ROCK [3]. In this study, fasudil supplementation increased the number of Ki-67 + cells or Dcx + cells, indicating that NSCs combined with fasudil promoted the proliferation and migration of endogenous NSCs in both SVZ and hippocampus areas, which was consistent with another study that cell migration was increased by inhibition of ROCK [3].
Besides, the microenvironment controlling transplanted stem cell fate is vital for better application of stem cell-based therapies in vivo. Many challenges limit the successful use of stem cell translation in clinical practice, such as low cell retention and engraftment and poor long-term maintenance of stem cell function [52]. Therefore, a supportive microenvironment of seed soil is necessary to regulate the function of NSCs by activating or potentiating intrinsic host repairment after transplantation. This microenvironment contains two beneficial or harmful elements to tissue repair and neuronal function. Increasing studies reveal that the therapeutic effect of transplanted NSCs is mainly related to the impact of the NSCs secretome on endogenous stem cells and the host microenvironment and, to a lesser extent, the direct differentiation of MSCs into neural cells. Recent investigations have turned to how cellular components of the local stromal microenvironment (the “soil to the stemcells” seed), such as the dynamic re-balance of local inflammatory reactions and neurotrophic factors, contribute to successful tissue regeneration.
In addition to understanding the biological characteristics of transplanted cells (or seeds), it may also be necessary to modify the recipient tissue matrix (soil) and provide a suitable environment for the successful integration or repair of transplanted cells, including inhibiting neuroinflammation and promoting neurotrophic factor [8]. In the pathogenesis of PD, inflammatory M1 microglia play an essential role in the loss of DA neurons [2]. Endogenous microglial polarization to M2 phenotype improved neuronal function [18, 54]. Bone marrow mesenchymal stem cells induce M2 microglia polarization in a rat middle cerebral artery occlusion (MCAO) model [49]. In this study, fasudil or NSCs intervention alone can inhibit neuroinflammation by inducing M2 microglia, and combined interventions have a synergistic effect on the inhibition of inflammation and polarization of M2 microglia. Astrocytes stimulate microglial proliferation and M2 polarization in vitro through crosstalk between astrocytes and microglia [17]. M2 microglia regulated NSC survival, migration, and differentiation differently [33]. The integrity of NSCs, microglia, and astrocytes in the brain microenvironment under specific conditions affects the fate of DA neurons and the effect of NSCs treatment. Our results provide limited information on NSCs therapy in treating PD models, while the relationship between NSCs, microglia, and astrocytes remains further explored. In recent years, whole-genome transcriptomic and epigenomic analysis revealed a neurodegenerative signature termed M0-homeostatic microglia, MGnD (neurodegenerative microglia),and DAM (disease-associated microglia) in neeurodegeration disease. Therefore, we will do further work to explore polarization of M0 and MGnD and DAM microglia phenotypes and functions in our experiment.[30]
Thirdly, we tried to further understand the molecular mechanisms involved in the proliferation and migration of NSCs after the combined intervention of fasudil and NSCs. The Wnt signaling pathway plays a vital role in regulating the proliferation, differentiation, and regeneration of embryonic and adult neurons via the transcriptional coactivator b-catenin during development and adult tissue homeostasis [7]. Activating the Wnt signaling pathway promotes dopaminergic neuron proliferation. Downregulation of the WNT/β-catenin pathway was found in PD, and upregulation contributes to DA neuron restoration and behavioral improvement in PD animal models [31]. The treatment of fasudil or NSCs alone upregulated the Wnt signaling pathway in this study.
Furthermore, the combined intervention enhanced this effect in vivo. These data support that Fasudil/NSCs combined intervention plays a synergistic role in the regeneration of endogenous NSCs, consistent with the behavioral improvement in MPTP-PD mice. A series of studies showed that fasudil differentiated bone marrow mesenchymal stem cells into neuron-like cells via the Wnt/β-catenin pathway, which was eliminated when the Wnt/β-catenin pathway was eliminated was inhibited[11]. In addition, the inhibition of ROCK with RhoA(DN) plasmid and Y-27632 caused the upregulation of Wnt pathway activity[45], identifying that the existence of a crosstalk between the ROCK pathway and the Wnt-signaling pathway [25]. Besides, intracerebroventricular (ICV) delivery of NSCs promoted endogenous NSC proliferation and migration and ultimately enhanced neuronal survival and neurological functional recovery by the upregulation of the Wnt signaling pathway [46], revealing that Wnt/β-catenin signaling is required to rescue midbrain dopaminergic progenitors and promote neuro repair in mouse PD model [21]. It was reported that tetrahydroxystilbene glucoside TSG could induce the DA neuronal differentiation and maturation of mouse NSCs, mediated by triggering the Wnt/β-catenin signaling pathway [51]. However, how the combined intervention of fasudil and NSCs induces Wnt/β-catenin signaling cascades still needs to be further studied.
In addition to the induction of the Wnt/β-catenin pathway, the intervention of fasudil or NSCs alone was insufficient to cause a significant increase in PI3K and pAkt expressions. In contrast, the combined intervention can upregulate the PI3K/Akt pathway and elevate the mTOR and Bcl-2 / Bax ratio, thus effectively protecting the DA neurons. The PI3K/AKT/mTOR complex is a signaling pathway that has recently emerged as a critical regulator of stem cell properties and functions that essentially regulates the survival, proliferation, differentiation, and maturation of NSCs [50]. The activation of the PI3K/AKT/mTOR pathway has a protective effect on the destruction of DA neurons caused by the MPTP model [53], and also promotes the survival and growth of DA neurons by inhibiting apoptosis, thus preventing PD [23]. Increasing evidence has demonstrated that fasudil can induce the PI3L/Akt pathway activation.
The question is how to explain that combined intervention of fasudil with NSCs further activates the PI3K/AKT/mTOR pathway more effectively. Classical activation of the PI3K/Akt/mTOR network is triggered by extracellular stimuli (such as growth factors) at the plasma membrane. BDNF binds to the tropomyosin receptor kinase B (TRKB) receptor and triggers a variety of downstream signaling cascades, including the PI3K/Akt pathway [14]. GDNF can activate the tyrosine kinase receptor, stimulating the PI3K pathway, which is the primary signaling pathway for GDNF [24, 41]. CNTF-derived peptidergic compound P021 also activates PI3K/Akt by increasing BDNF in 4-month-old 3xTg-AD mice [47]. In vitro cultured dorsal root ganglion (DRG) with gp120-induced neurotoxicity, CNTF improved neurite outgrowth and neuronal migration by promoting PI3K/Akt signaling pathways [27]. Based on these studies, we speculate that combined intervention of fasudil and NSCs can significantly increase the formation of BDNF, GDNF, and CNTF, which should be one reason for the activation PI3K/AKT/mTOR signaling pathway. However, because the structure and function of the PI3K/AKT/mTOR signaling pathway and its relationship with upstream and downstream molecules are complex, further research is needed for a complete understanding.
The previous study demonstrated the potentiality of astrocytes to differentiate into DA neurons, thereby contributing to alleviating Parkinsonism symptoms [35]. These astrocytes are giant in morphology and express Nestin, a protein expressed in neuronal stem cells, which are considered to be in the differentiation process towards NSCs. This study observed that fasudil and NSCs promoted Nestin expression in astrocytes. Differentiating astrocytes into neurons may play a potential role in the protective effects of fasudil/NSCs interventions. Whether these GFAP+ cells further differentiate into DA neurons needs to be validated.
In conclusion, the current study further proved that the combination of fasudil and NSCs has a synergetic effect on protecting neuron loss, accompanied by NSCs survival, proliferation and migration, M2 microglia polarization, and anti-inflammation as well as production of neurotrophic factors, possibly via the upregulation of Wnt/β-catenin and PI3K/Akt/mTOR signaling pathways in the MPTP-PD mice model. This report provides a novel strategy for promoting the development of stem cell therapies that could be applied safely and effectively in clinical settings.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Barker RA (2019) Designing stem-cell-based dopamine cell replacement trials for Parkinson’s disease. Nat Med 25(7):1045–1053
Bartels T, De Schepper S, Hong S (2020) Microglia modulate neurodegeneration in Alzheimer’s and Parkinson’s diseases. Science 370(6512):66–69
Cheng L et al (2017) Interaction between mDia1 and ROCK in Rho-induced migration and adhesion of human dental pulp cells. Int Endod J 50(1):15–23
Clevers H, Nusse R (2012) Wnt/β-catenin signaling and disease. Cell 149(6):1192–1205
Elkouzi A et al (2019) Emerging therapies in Parkinson disease - repurposed drugs and new approaches. Nat Rev Neurol 15(4):204–223
Erkkinen MG, Kim MO, Geschwind MD (2018) Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol 10(4):a033118. https://doi.org/10.1101/cshperspect.a033118
Fan R et al (2020) Wnt/Beta-catenin/Esrrb signalling controls the tissue-scale reorganization and maintenance of the pluripotent lineage during murine embryonic diapause. Nat Commun 11(1):5499
Forbes SJ, Rosenthal N (2014) Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med 20(8):857–869
Fričová D, Korchak JA, Zubair AC (2020) Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson’s disease. NPJ Regen Med 5(1):20
Gantner CW et al (2020) Viral delivery of GDNF promotes functional integration of human stem cell grafts in Parkinson’s disease. Cell Stem Cell 26(4):511-526 e5
Hu Y et al (2019) Fasudil may induce the differentiation of bone marrow mesenchymal stem cells into neuron-like cells via the Wnt/β-catenin pathway. Mol Med Rep 19(4):3095–3104
Jankovic J, Tan EK (2020) Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry 91(8):795–808
Jiaming M, Niu C (2015) Comparing neuroprotective effects of CDNF-expressing bone marrow derived mesenchymal stem cells via differing routes of administration utilizing an in vivo model of Parkinson’s disease. Neurol Sci 36(2):281–287
Julius D, Basbaum AI (2001) Molecular mechanisms of nociception. Nature 413(6852):203–210
Kahroba H et al (2021) The role of Nrf2 in neural stem/progenitors cells: From maintaining stemness and self-renewal to promoting differentiation capability and facilitating therapeutic application in neurodegenerative disease. Ageing Res Rev 65:101211
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386(9996):896–912
Kim S, Son Y (2021) Astrocytes stimulate microglial proliferation and M2 polarization in vitro through crosstalk between astrocytes and microglia. Int J Mol Sci 22(16):8800. https://doi.org/10.3390/ijms22168800
Kobashi S et al (2020) Transplantation of M2-deviated microglia promotes recovery of motor function after spinal cord injury in mice. Mol Ther 28(1):254–265
Koch JC et al (2018) ROCK inhibition in models of neurodegeneration and its potential for clinical translation. Pharmacol Ther 189:1–21
Kolar MK, Kingham PJ (2014) Regenerative effects of adipose-tissue-derived stem cells for treatment of peripheral nerve injuries. Biochem Soc Trans 42(3):697–701
L’Episcopo F et al (2014) Wnt/β-catenin signaling is required to rescue midbrain dopaminergic progenitors and promote neurorepair in ageing mouse model of Parkinson’s disease. Stem Cells 32(8):2147–2163
Lang AE, Espay AJ (2018) Disease modification in Parkinson’s disease: current approaches, challenges, and future considerations. Mov Disord 33(5):660–677
Leikas JV et al (2017) Brief isoflurane anesthesia regulates striatal AKT-GSK3β signaling and ameliorates motor deficits in a rat model of early-stage Parkinson’s disease. J Neurochem 142(3):456–463
Li B et al (2020) Abdominal massage reduces visceral hypersensitivity via regulating GDNF and PI3K/AKT signal pathway in a rat model of irritable bowel syndrome. Evid Based Complement Alternat Med 2020:3912931
Li L et al (2011) Wnt-signaling mediates the anti-adipogenic action of lysophosphatidic acid through cross talking with the Rho/Rho associated kinase (ROCK) pathway. Biochem Cell Biol 89(6):515–521
Li YH et al (2017) Fasudil enhances therapeutic efficacy of neural stem cells in the mouse model of MPTP-induced Parkinson’s disease. Mol Neurobiol 54(7):5400–5413
Liu H, Liu G, Bi Y (2014) CNTF regulates neurite outgrowth and neuronal migration through JAK2/STAT3 and PI3K/Akt signaling pathways of DRG explants with gp120-induced neurotoxicity in vitro. Neurosci Lett 569:110–115
Long HZ et al (2021) PI3K/AKT signal pathway: a target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Front Pharmacol 12:648636
Lopez-Lopez A et al (2020) Rho kinase inhibitor fasudil reduces l-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. Br J Pharmacol 177(24):5622–5641
Madore C et al (2020) Microglia, lifestyle stress, and neurodegeneration. Immunity 52(2):222–240
Marchetti B et al (2022) “Reframing” dopamine signaling at the intersection of glial networks in the aged Parkinsonian brain as innate Nrf2/Wnt driver: Therapeutical implications. Aging Cell 21(4):e13575
Moskal N et al (2020) ROCK inhibitors upregulate the neuroprotective Parkin-mediated mitophagy pathway. Nat Commun 11(1):88
Osman AM et al (2019) The secretome of microglia regulate neural stem cell function. Neuroscience 405:92–102
Parmar M, Grealish S, Henchcliffe C (2020) The future of stem cell therapies for Parkinson disease. Nat Rev Neurosci 21(2):103–115
Qian H et al (2020) Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature 582(7813):550–556
di Val R, Cervo P et al (2017) Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson’s disease model. Nat Biotechnol 35(5):444–452
Santamaria G et al (2021) Intranasal delivery of mesenchymal stem cell secretome repairs the brain of Alzheimer’s mice. Cell Death Differ 28(1):203–218
Schiess M et al (2021) Allogeneic bone marrow-derived mesenchymal stem cell safety in idiopathic Parkinson’s disease. Mov Disord 36(8):1825–1834
Silvestro S, Bramanti P, Trubiani O, Mazzon E (2020) Stem cells therapy for spinal cord injury: An overview of clinical trials. Int J Mol Sci 21(2):659. https://doi.org/10.3390/ijms21020659
Sonntag KC et al (2018) Pluripotent stem cell-based therapy for Parkinson’s disease: current status and future prospects. Prog Neurobiol 168:1–20
Srinivasan S et al (2005) Enteric neuroblasts require the phosphatidylinositol 3-kinase/Akt/Forkhead pathway for GDNF-stimulated survival. Mol Cell Neurosci 29(1):107–119
Tian Y et al (2020) Proinflammatory S100A9 regulates differentiation and aggregation of neural stem cells. ACS Chem Neurosci 11(21):3549–3556
Tsai RY (2018) Creating a graft-friendly environment for stem cells in diseased brains. J Clin Invest 128(1):116–119
Wagenaar N et al (2018) Promoting neuroregeneration after perinatal arterial ischemic stroke: neurotrophic factors and mesenchymal stem cells. Pediatr Res 83(1–2):372–384
Wang T et al (2017) Rho-kinase inhibitor Y-27632 facilitates the proliferation, migration and pluripotency of human periodontal ligament stem cells. J Cell Mol Med 21(11):3100–3112
Wang Z et al (2021) Intracerebroventricular administration of hNSCs improves neurological recovery after cardiac arrest in rats. Stem Cell Rev Rep 17(3):923–937
Wei W et al (2021) Neurotrophic treatment initiated during early postnatal development prevents the Alzheimer-like behavior and synaptic dysfunction. J Alzheimers Dis 82(2):631–646
Wenker SD, Pitossi FJ (2020) Cell therapy for Parkinson’s disease is coming of age: current challenges and future prospects with a focus on immunomodulation. Gene Ther 27(1–2):6–14
Yang F et al (2020) Bone marrow mesenchymal stem cells induce M2 microglia polarization through PDGF-AA/MANF signaling. World J Stem Cells 12(7):633–658
Yu JS, Cui W (2016) Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 143(17):3050–3060
Zhang L, Yang H (2021) Promotive effects of tetrahydroxystilbene glucoside on the differentiation of neural stem cells from the mesencephalon into dopaminergic neurons. Neurosci Lett 742:135520
Zhao X et al (2021) Constructing a cell microenvironment with biomaterial scaffolds for stem cell therapy. Stem Cell Res Ther 12(1):583
Zhong Z et al (2021) Fecal microbiota transplantation exerts a protective role in MPTP-induced Parkinson’s disease via the TLR4/PI3K/AKT/NF-κB pathway stimulated by α-synuclein. Neurochem Res 46(11):3050–3058
Zhu D et al (2016) M2 Macrophage transplantation ameliorates cognitive dysfunction in amyloid-β-treated rats through regulation of microglial polarization. J Alzheimers Dis 52(2):483–495
Funding
Author Wen-Bo Yu has received research support from the National Natural Science Foundation of China (Grant Numbers: 81971194).
Author information
Authors and Affiliations
Contributions
J-Y X and W-B Y were involved in the conception and design of the study. Y-H L, J-Y X, and W-B Y collaborated to conduct the experiments in this study. Y-C Y was responsible for the analysis and interpretation of the acquired data. Y-C Y wrote the draft of the manuscript. J-W, W-B Y and B-G X revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics Approval
All animal experiments were carried out according to the guidelines of the Ethics Committee of Fudan University, Shanghai, China.
Consent to Participate and Consent for Publication
This study contains no human subjects. Neither consent to participate nor consent to publish applies to this research.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, YC., Li, Yh., Xiao, BG. et al. Cellular and Molecular Mechanisms Underly the Combined Treatment of Fasudil and Bone Marrow Derived-Neuronal Stem Cells in a Parkinson’s Disease Mouse Model. Mol Neurobiol 60, 1826–1835 (2023). https://doi.org/10.1007/s12035-022-03173-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03173-y