Abstract
Depression is one of the most important and serious health problems in developing countries which affects millions of people. It is associated with the decrease of the quality of life as well as suicides and mortality. The disease may show recurrent episodes in some patients. Obviously, not all the patients with depression could be treated properly, because some individuals are drug-resistant and the options for the therapy are limited. Therefore, it is crucial to investigate new molecules and pathways that may have possible antidepressant activity. Sirtuin (SIRT), known as a class III histone deacetylase, which is regulated by nicotinamide adenine dinucleotide (NAD +), is one of these molecules. In the current study, we investigated the possible antidepressant-like effect of SIRT2 inhibitor AK-7. For this purpose, behavioral tests were performed in chronic AK-7-treated mice, and the expression levels of BDNF, NGF, NTF3, CREB, NTRK2, ERK1, ERK2, and GAP43 genes were evaluated by qRT-PCR analysis in brain tissues. Protein levels for BDNF, CREB1, and NTRK2 were determined by western blot. Our data showed that AK-7 significantly decreased immobility time and showed antidepressant-like effect. In addition, AK-7 treatment significantly increased mRNA levels of CREB and NTRK2 and protein levels of CREB1, BDNF, and NTRK2. Finally, our results suggest that SIRT2 and AK-7 may have a potential role in the cellular mechanisms of depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is one of the most important and serious psychiatric disorders in developing countries which affects approximately 350 million people worldwide. It is associated with the decrease of the quality of life as well as suicides and mortality [1, 2]. Even though the common treatment for depression is to use selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, at least 30% of the patients with major depressive disorder do not benefit from current antidepressant treatment [3]. In addition, there are also difficult-to-treat cases called treatment-resistant depression or refractory depression [4]. Electroconvulsive therapy (ECT) and vagus nerve stimulation (VNS) are the other options used in the treatment of depression which may have serious adverse effects, including retrograde amnesia [5]. Therefore, it is necessary to investigate new molecules and pathways that may have possible antidepressant activity.
Sirtuins (SIRTs), known as class III histone deacetylases, which are regulated by nicotinamide adenine dinucleotide (NAD +), modulate cellular functions via deacetylation of various proteins [6]. They are responsible for miscellaneous biological processes such as aging, metabolism, cancer, transcriptional silencing, chromosomal stability, cell differentiation, stress response, inflammation, apoptosis, and DNA repair [7,8,9]. Unlike from all other SIRTs, SIRT1 and SIRT2 are the most studied proteins which are mainly expressed in the brain tissues. While SIRT1 is expressed in the cerebellum, hippocampus, and hypothalamus, the expression of SIRT2 has been found in the spinal cord, brain stem, cortex, frontal lobe, hippocampus, striatum, and cerebellum [10]. The expression profiles of SIRT1 and SIRT2 in different regions of the brain indicate that they have an important role in the central nervous system. Regulation of SIRT1/SIRT2 activity with different molecules has been considered as a promising therapeutic approach for the treatment of neurodegenerative diseases [11, 12], such as Alzheimer’s disease [13], Parkinson’s disease [14], and Huntington’s disease [15]. In addition, evidence showed that SIRT1 and SIRT2 modulation could be useful in the pathophysiology of mood disorders. However, most of the studies with SIRTs mainly focused on SIRT1 modulation, and the effectiveness of SIRT2 modulators on depression is still unknown [16,17,18,19]. Thus, in the current study, we aimed to investigate the possible antidepressant-like effects of AK-7, a selective SIRT2 inhibitor, in mice.
Materials and Methods
Animals
Male Swiss albino mice weighing 20–25 g were obtained from the Animal Care Facility (SUDAM) at the University of Selcuk and were used for the experiments. Mice were housed 5 per cage in standard translucent plastic cages and kept in an environmentally controlled vivarium under a 12:12-h light–dark cycle. They were allowed food and water ad libitum. All experiments were carried out between 09:00 and 16:00. Mice were allowed for a 60-min adaptation period for the laboratory conditions before behavioral experiments to reduce possible stress. Animals were given a code to avoid bias, and also, each behavior was video-recorded for further analysis. The scoring was performed by a blind evaluator.
Forced Swim Test
The forced swim test was performed similar to that described by Porsolt et al. [20] and Inan et al. [21]. Briefly, each mouse was gently lowered into a glass cylinder (height 17 cm, diameter 14 cm) containing 11 cm of freshwater maintained at 23–25 °C and left there for 6 min. A mouse was judged to be immobile when it floated in the water, in an upright position, and made only small movements to keep its head above the water. Since little immobility was observed during the first 2 min, the duration of immobility was recorded during the last 4 min. A decrease in the duration of immobility was interpreted as indicating an antidepressant-like effect. In each test, fresh water was used.
Open Field Test
Spontaneous locomotor activity was investigated in the open field test as previously described by Inan et al. [22]. Briefly, the open field apparatus was a white square arena (40 cm × 40 cm × 15 cm) divided into 16 equal squares. Mice were gently placed individually into the open field facing one corner and allowed to explore the area for 5 min. The activity level was expressed as the total number of squares crossed.
Elevated Plus Maze
The elevated plus maze test was modified as previously described by Inan and Aksu [23]. Briefly, the maze was consisted of two open (10 cm × 50 cm) and two enclosed (10 cm × 50 cm × 50 cm) arms, and was elevated 50 cm above the floor. On the first day (acquisition trial), each mouse was gently placed on the end of an open arm facing the center of the plus maze and allowed to explore the apparatus for 3 min. After the acquisition trial, the mouse was taken from the maze and returned to its home cage until the next trial. Twenty four hours later, memory retention test was performed and the duration of entering to an enclosed arm was recorded (retention latency). Cutoff time for the retention session was set to 120 s for each mouse. A decrease in the retention latency was interpreted as indicating a memory enhancing effect.
Social Interaction Test
The social interaction test was modified as previously described by Venzala et al. [24]. Briefly, each mouse was gently placed into a rectangle open field (70 cm × 50 cm × 15 cm) facing one corner and allowed to explore the area for 5 min. The social interaction latency was expressed as the total duration of approaches to a social target (an unfamiliar female mouse) which was in a plexiglass mesh cage and was placed into an opposite corner of the open field. An increase in the social interaction latency was interpreted as indicating an antidepressant-like behavior.
Grip Strength Test
The mouse grip strength test was modified as previously described by Maurissen et al. [25]. Briefly, the apparatus was consisted of a wooden T-bar (100 cm long × 1 cm wide and 0.5 cm thick) elevated 25 cm above a foam bed. Each mouse was allowed to grasp the bar freely with its forepaws until the grip is broken. The test was performed 3 consecutive times, and the time between holding the bar and releasing it was recorded. An increase in the grip latency was interpreted as indicating an enhancement in the muscular strength.
RNA Extraction and qRT-PCR Analysis
Immediately after all behavioral testing, mice were euthanized with high dose of chloral hydrate (600 mg/kg) and their brain tissues were collected for further analysis. The expression levels of BDNF, NGF, NTF3 neurotrophic factors, CREB in CREB/BDNF signaling pathway, BDNF receptor NTRK2, ERK1, and ERK2 genes, and GAP43 gene which are important for axonal growth and synaptic plasticity were evaluated by qRT-PCR analysis. For this purpose, total RNA was isolated from the brain tissues and cDNA synthesis was performed according to the instructions of the manufacturer (iScriptTM cDNA synthesis kit, Bio-Rad, Cat. No. 1708891). Then, qRT-PCR analysis was performed with primers designed for each target gene region. Primers for target genes were designed using the IDT Primer Quest (https://eu.idtdna.com/Primerquest/Home/Index) program. The primer sequences of target genes and beta-actin (ACTB) gene are presented in Table 1. qRT-PCR analysis was performed by using qPCR mastermix (BrightGreen 2 × qPCR MasterMix—ROX, ABM, MasterMix-R) containing BrightGreen dye. For this purpose, 5 µl of BrightGreen 2 × qPCR MasterMix, 5 pMol forward primer, 5 pMol reverse primer, and 2 µl cDNA were used and the total volume was made up to 10 µl with nuclease-free water. Then, PCR protocol consisting of enzyme activation (10 min at 95 °C), denaturation (15 s at 95 °C), and bonding/extension (60 s at 60 °C) steps were applied in the real-time PCR system (Bio-Rad, CFX Connect) with 40 cycles. At the end of the reaction, threshold cycle values (Ct) were recorded and normalization was performed with the ACTB reference gene.
Western Blot Analysis
Protein isolation from brain tissues was performed by using RIPA solution (RIPA buffer (10 ×), Cell Signaling, Cat. No. 9806). Protein concentration was determined by Bradford method using BSA standards. For this purpose, 30 µg of protein was loaded on 8–15% SDS-PAGE. After electrophoresis, proteins were transferred to PVDF membrane (Porablot PVDF, MN, Cat. No. 741260). After blocking of the membrane with 5% non-fat milk, the membrane was incubated with anti-BDNF (Elabscience, Cat. No. E-AB-18244, 1:1000), anti-CREB1 (Elabscience, Cat. No. E-AB-63474, 1:1000), anti-NTRK2 (Elabscience, Cat. No. E-AB-70155, 1:1000), and anti-ACTB (Bioss Antibodies, Cat. No. BS-0061R, 1:1000) primary antibodies at 4 °C for overnight. After incubation period, the membrane was incubated with secondary antibody (Jackson Immuno Research, Cat. No. 211–035-109) for 2 h. Afterwards, chemiluminescence solution (Biovision, Cat. No. K820-500) was added to the membrane, and imaging was performed on the Azure Biosystems™ c280. The density of each band was quantified with ImageJ software, and ACTB was used as an internal control.
Drugs
AK-7 was purchased from Tocris Bioscience (UK) and dissolved in DMSO (15 mg/mL), and the final volume was made up with saline solution. AK-7 was injected intraperitoneally at the doses of 5, 10, or 20 mg/kg for 21 days. The doses were chosen based on our preliminary studies and the literature [14, 15, 26, 27]. In order to reduce animal numbers for ethical concerns, we only tested forced swim test for the 5 and 10 mg/kg doses in 20 mice. Since we did not find any promising effects with these doses, we continued our study with the most effective dose (20 mg/kg). Control animals received vehicle solution. Behavioral studies were performed 30 min after the last AK-7 or vehicle injection.
Statistical Analysis
Results were presented as means ± SEM. Statistical analyses were performed with GraphPad Prism software (Version 6.0, San Diego, CA). The comparison between two separate groups was analyzed by using unpaired t test for behavioral experiments. Quantitation analysis of genes was performed with 2(−∆∆CT) method. qRT-PCR and Western blot data were evaluated by using unpaired t test. p < 0.05 was considered statistically significant.
Results
Forced Swim Test
Chronic administration of AK-7 for 21 days significantly decreased immobility time and showed antidepressant-like effect (Fig. 1, t = 16.9, df = 18, p < 0.0001). Mean latencies for control and AK-7 groups were found as 214.3 ± 3.8 s and 117.7 ± 4.3 s, respectively.
Open-Field Test
AK-7 had no effect on spontaneous locomotor activity in the open-field test (Fig. 2, t = 0.084, df = 18, p = 0.934). This means AK-7 does not have neurotoxic properties at the dose of administration. Mean line crossings for control and AK-7 groups were found as 224.4 ± 2.7 and 225.2 ± 9.1, respectively.
Elevated Plus Maze
Even though chronic administration of AK-7 slightly decreased retention latency which reflects long-term memory, we did not find significant differences between groups (Fig. 3, t = 0.708, df = 18, p = 0.488). Mean retention latencies for control and AK-7 groups were found as 36.9 ± 4.8 s and 31.9 ± 5.2 s, respectively.
Social Interaction Test
As expected, AK-7 significantly increased social interaction latency which also reflects an antidepressant-like activity (Fig. 4, t = 12.6, df = 18, p < 0.0001). Mean latencies for control and AK-7 groups were found as 95.2 ± 5.2 s and 179.3 ± 4.2 s, respectively.
Grip Strength Test
Physical activity and muscular strength are important parameters that associate with depression. Therefore, the more enhancement in the muscular strength, the less depression-related symptoms. Chronic administration of AK-7 for 21 days significantly increased grip latency (Fig. 5, t = 12.0, df = 18, p < 0.0001). Mean latencies for control and AK-7 groups were found as 11.5 ± 0.2 s and 21.5 ± 0.8 s, respectively.
Neuroplasticity-Associated Genes and Proteins
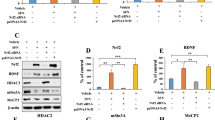
The effect of chronic AK-7 treatment on the expression levels of neuroplasticity-related genes at mRNA level was evaluated by qRT-PCR. Our data showed that AK-7 treatment significantly increased mRNA levels of CREB and NTRK2 as 2.35-fold and 2.98-fold, respectively, when compared to control group. No significant differences were detected in the expression levels of BDNF, NGF, NTF3, ERK1, ERK2, and GAP43 genes (Fig. 6A: BDNF: unpaired t test, t = 0.2641, df = 10, p = 0.7971; NGF: unpaired t test, t = 0.3787, df = 10, p = 0.7129; NTF3: unpaired t test, t = 0.5611, df = 10, p = 0.5871; CREB: unpaired t test, t = 6.448, df = 10, p < 0.0001; NTRK2: unpaired t test, t = 5.164, df = 10, p = 0.0004; ERK1: unpaired t test, t = 0.09632, df = 10, p = 0.9252; ERK2: unpaired t test, t = 1.036, df = 10, p = 0.3245; GAP43: unpaired t test, t = 1.632, df = 10, p = 0.1338).
Effect of chronic AK-7 treatment on neuroplasticity-associated genes and proteins. (A) The expression levels of BDNF, NGF, NTF3, CREB, NTRK2, ERK1, ERK2, and GAP43 genes were evaluated by qRT-PCR analysis. (B) BDNF, CREB-1, and NTRK2 protein levels were evaluated by western blot analysis. N = 6 for each group; *p < 0.05, unpaired t test
After qRT-PCR analysis, we thought that chronic administration of AK-7 may modulate the CREB1, BDNF, and NTRK2 pathway, and we evaluated levels of BDNF, CREB1, and NTRK2 proteins by western blot analysis. Our data showed that AK-7 significantly increased the levels of BDNF, CREB1, and NTRK2 proteins compared to control group (Fig. 6B: BDNF: unpaired t test, t = 2.384, df = 10, p = 0.0384; CREB: unpaired t test, t = 3.930, df = 10, p = 0.0028; NTRK2: unpaired t test, t = 2.354, df = 10, p = 0.0404).
Discussion
Depression is one of the most common and serious neuropsychiatric disorders in developing countries which affects millions of people. It is associated with the decrease of the quality of life as well as high suicide and mortality rates. Nevertheless, its pathophysiology and limited effectiveness of the treatment methods are still unclear [2], since the disease may show recurrent episodes in some patients. The accepted routine therapy for depression is the use of serotonin and/or norepinephrine re-uptake inhibitors which are based on the monoamine hypothesis and aim to increase monoamine concentrations in the synapses [28]. However, long-term use of these drugs is required for a better healing, and yet only one-third of the patients completely resolve the symptoms [29]. Therefore, it is crucial to investigate new molecules and signaling pathways associated with depression which may contribute to the elucidation of pathogenesis as well as development of new therapeutic targets. In the present study, we aimed to investigate the possible relationship of SIRT2, an alpha NAD + -dependent deacetylase, with depression. For this purpose, we examined antidepressant-like effects of AK-7, a SIRT2 inhibitor, in mice.
In various studies with neurodegenerative disease models, it has been reported that AK-7, a sulfobenzoic acid derivative, may inhibit neurodegenerative processes and exert neuroprotective effects. The inhibition of SIRT2 pathway with AK-7 improved cognitive performance and modulate molecular mechanisms associated with Alzheimer’s disease [13]. In addition, it has been demonstrated that AK-7 shows neuroprotective effects by attenuating alpha synuclein toxicity and reducing dopaminergic neuron loss in a Parkinson’s disease model [14]. Similarly, evidence displayed that AK-7 improved motor functions, prolonged survival, and reduced brain atrophy in a mouse model of Huntington’s disease [15]. However, there is limited information about the possible cellular and/or molecular mechanisms of SIRT2 inhibitors in depression. Recently, it has been shown that 33i, another SIRT2 inhibitor, causes an antidepressant-like effect by modulating glutamate and serotonin systems in mice [18]. Moreover, it has also been reported that co-treatment of 33i with MC1568 increases synaptic plasticity in the prefrontal cortex [30]. In addition, sirtinol, a SIRT1 and SIRT2 inhibitor, has been found to reduce anhedonic behavior in rats [16].
In the present study, we examined the possible antidepressant-like effects of AK-7 and its underlying molecular mechanisms in mice. As it is well known, 4 to 6 weeks are required for clinical improvement for the antidepressant therapy [31]. Therefore, we administered AK-7 for 21 days. According to our results, AK-7 treatment significantly reduced immobility time in the forced swim test and increased the duration of social interaction and grip latency. However, it did not change spontaneous locomotor activity in the open-field test and retention latency in the elevated plus maze. Moreover, we did not find any significant differences between groups in acute AK-7 experiments (unpublished preliminary data).
There are various pathophysiological mechanisms thought to be effective in depression, and different combinations of these mechanisms can be found in patients [32]. Decrement in the neurotrophic factors and the level of neuroplasticity are one of the significant mechanisms of depression [33]. As it is well identified, the adaptation of neurons and neural elements to internal and external signals is defined as neuroplasticity [34]. Furthermore, post-mortem investigations claimed that depression may inhibit neuroplasticity in the hippocampus and prefrontal cortex and reduce the concentrations of various neurotrophic factors such as brain-derived neurotrophic factor (BDNF). On the contrary, antidepressant treatment has been found to increase the concentrations of neurotrophic factors and neuroplasticity in the hippocampus and prefrontal cortex [35]. Various growth factors such as BDNF are known to be effective in neuroplasticity [36]. Besides, a decrease in the expression of nerve growth factors may also cause a decrease in the volume of hippocampus and prefrontal cortex [37]. The expression of BDNF, one of the most important neurotrophic factors associated with depression, is regulated by cAMP-response element binding protein (CREB). Post-mortem studies with major depression patients who committed suicide have shown that CREB levels decrease in the hippocampus [38].
Other members of the neurotrophic factor family, such as nerve growth factor (NGF) and neurotrophin-3 (NT-3), act through tyrosine kinase receptors. One of the best characterized neurotrophin-activated signaling pathways is the mitogen-activated protein (MAP) kinase cascade involving extracellular signal-regulated protein kinase (ERK) [39]. Evidence showed that patients with depression who committed suicide have low ERK activity in their hippocampus and prefrontal cortex [40]. The MAPK/ERK pathway also modulates the level of growth-associated protein 43 (GAP-43), a presynaptic protein expressed in the hippocampus and association cortex [41, 42]. GAP-43 plays a role in the regulation of axonal growth, synaptic plasticity, and learning and memory functions, and it is thought that this protein may be associated with long-term depression [43].
In the present study, we also evaluated the effects of AK-7 at the molecular level through the BDNF, NGF, NTF3, CREB, NTRK2, ERK1, ERK2, and GAP43 genes associated with both neuroplasticity and neurotrophic factors. According to our qRT-PCR results, chronic AK-7 treatment led to a significant increase in gene expression levels of CREB and NTRK2 which encodes the BDNF receptor. Based on these results, we investigated the effects of AK-7 treatment on BDNF, CREB, and NTRK2 protein levels by western blot. As expected, chronic AK-7 treatment significantly increased BDNF, CREB, and NTRK2 protein levels. As for the qRT-PCR and western blot results for acute experiments, we did not find any differences between groups (unpublished preliminary data).
One of the long-term effects of antidepressant therapy is the induction of various transcription factors such as CREB [44]. Increased CREB levels [45] and NTK2 expression [46, 47] are associated with antidepressant-like activity. Likewise, in the present study, our results indicated that chronic AK-7 treatment increased the BDNF, CREB, and NTRK2 levels which might contribute to an antidepressant-like effect.
Taken together, our results suggest that AK-7 and SIRT2 pathway in the brain may be involved in depression and that AK-7 could be a potentially novel antidepressant agent. The possible mechanisms for the antidepressant activity seem to be associated with the upregulation of CREB1, BDNF, and NTRK2. However, further studies are needed to understand the mechanistic effects of AK-7 in the central nervous system.
Data Availability
Data and materials will be made available on reasonable request.
Code Availability
Not applicable.
References
Sobocki P, Jönsson B, Angst J, Rehnberg C (2006) Cost of depression in Europe. J Ment Health Policy Econ 9(2):87–98
Misztak P, Pańczyszyn-Trzewik P, Sowa-Kućma M (2018) Histone deacetylases (HDACs) as therapeutic target for depressive disorders. Pharmacol Rep 70(2):398–408
Ionescu DF, Rosenbaum JF, Alpert EA (2015) Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues Clin Neurosci 17:111–126
Fava M (2003) Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 53:649–659
Goto S, Terao T, Hoaki N, Wang Y, Tsuchiyama K, Araki Y, Kohno K (2012) Is serotonergic function associated with the antidepressant effects of modified-electroconvulsive therapy? J Affect Disord 136(3):1062–1066
Whittle JR, Powell MJ, Popov VM, Shirley LA, Wang C, Pestell RG (2007) Sirtuins, nuclear hormone receptor acetylation and transcriptional regulation. Trends Endocrinol Metab 18(9):356–364
Kaeberlein M, McVey M, Guarente L (1999) The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 13(19):2570–2580
Michan S, Sinclair D (2007) Sirtuins in mammals: insights into their biological function. Biochem J 404(1):1–13
Lu G, Li J, Zhang H, Zhao X, Yan LJ, Yang X (2018) Role and possible mechanisms of Sirt1 in depression. Oxid Med Cell Longev 2018:8596903
Chandramowlishwaran P, Vijay A, Abraham D, Li G, Mwangi SM, Srinivasan S (2020) Role of sirtuins in modulating neurodegeneration of the enteric nervous system and central nervous system. Front Neurosci 14:614331
Yeong KY, Berdigaliyev N, Chang Y (2020) Sirtuins and their implications in neurodegenerative diseases from a drug discovery perspective. ACS Chem Neurosci 11(24):4073–4091
Zhang Y, Anoopkumar-Dukie S, Arora D, Davey AK (2020) Review of the anti-inflammatory effect of SIRT1 and SIRT2 modulators on neurodegenerative diseases. Eur J Pharmacol 867:172847
Biella G, Fusco F, Nardo E, Bernocchi O, Colombo A, Lichtenthaler SF, Forloni G, Albani D (2016) Sirtuin 2 inhibition improves cognitive performance and acts on amyloid-β protein precursor processing in two Alzheimer’s disease mouse models. J Alzheimers Dis 53(3):1193–1207
Chen X, Wales P, Quinti L, Zuo F, Moniot S, Herisson F, Rauf NA, Wang H, Silverman RB, Ayata C, Maxwell MM, Steegborn C, Schwarzschild MA, Outeiro TF, Kazantsev AG (2015) The sirtuin-2 inhibitor AK7 is neuroprotective in models of Parkinson’s disease but not amyotrophic lateral sclerosis and cerebral ischemia. PLoS ONE 10(1):e0116919
Chopra V, Quinti L, Kim J, Vollor L, Narayanan KL, Edgerly C, Cipicchio PM, Lauver MA, Choi SH, Silverman RB, Ferrante RJ, Hersch S, Kazantsev AG (2012) The sirtuin 2 inhibitor AK7 is neuroprotective in Huntington’s disease mouse models. Cell Rep 2(6):1492–1497
Ferland CL, Hawley WR, Puckett RE, Wineberg K, Lubin FD, Dohanich GP, Schrader LA (2013) Sirtuin activity in dentate gyrus contributes to chronic stress-induced behavior and extracellular signal-regulated protein kinases 1 and 2 cascade changes in the hippocampus. Biol Psychiatry 74:927–935
Kim HD, Hesterman J, Call T, Magazu S, Keeley E, Armenta K, Kronman H, Neve RL, Nestler EJ, Ferguson D (2016) SIRT1 mediates depression-like behaviors in the nucleus accumbens. J Neurosci 36:8441–8452
Erburu M, Muñoz-Cobo I, Diaz-Perdigon T, Mellini P, Suzuki T, Puerta E, Tordera RM (2017) SIRT2 inhibition modulate glutamate and serotonin systems in the prefrontal cortex and induces antidepressant-like action. Neuropharmacology 117:195–208
Duan CM, Zhang JR, Wan TF, Wang Y, Chen HS, Liu L (2020) SRT2104 attenuates chronic unpredictable mild stress-induced depressive-like behaviors and imbalance between microglial M1 and M2 phenotypes in the mice. Behav Brain Res 378:112296
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732
Inan SY, Yalcin I, Aksu F (2004) Dual effects of nitric oxide in the mouse forced swimming test: possible contribution of nitric oxide-mediated serotonin release and potassium channel modulation. Pharmacol Biochem Behav 77(3):457–464
Inan SY, Soner BC, Sahin AS (2016) Behavioural effects of basal ganglia rho-kinase inhibition in the unilateral 6-hydroxydopamine rat model of Parkinson’s disease. Metab Brain Dis 31(4):849–857
Inan SY, Aksu F (2002) Amnesic effects of relative humidity and temperature in mice. Lab Anim 31(2):40–48
Venzala E, García-García AL, Elizalde N, Delagrange P, Tordera RM (2012) Chronic social defeat stress model: behavioral features, antidepressant action, and interaction with biological risk factors. Psychopharmacology 224(2):313–325
Maurissen JP, Marable BR, Andrus AK, Stebbins KE (2003) Factors affecting grip strength testing. Neurotoxicol Teratol 25(5):543–553
Jung HY, Yoo DY, Kim JW, Kim DW, Choi JH, Chung JY, Won MH, Yoon YS, Hwang IK (2016) Sirtuin-2 inhibition affects hippocampal functions and sodium butyrate ameliorates the reduction in novel object memory, cell proliferation, and neuroblast differentiation. Lab Anim Res 32(4):224–230
Yuan F, Xu ZM, Lu LY, Nie H, Ding J, Ying WH, Tian HL (2016) SIRT2 inhibition exacerbates neuroinflammation and blood-brain barrier disruption in experimental traumatic brain injury by enhancing NF-κB p65 acetylation and activation. J Neurochem 136(3):581–593
Li D, Wang Y, Jin X, Hu D, Xia C, Xu H, Hu J (2020) NK cell-derived exosomes carry miR-207 and alleviate depression-like symptoms in mice. J Neuroinflammation 17(1):126
Du X, Yin M, Yuan L, Zhang G, Fan Y, Li Z, Yuan N, Lv X, Zhao X, Zou S, Deng W, Kosten TR, Zhang XY (2020) Reduction of depression-like behavior in rat model induced by ShRNA targeting norepinephrine transporter in locus coeruleus. Transl Psychiatry 10(1):130
Erburu M, Muñoz-Cobo I, Domínguez-Andrés J, Beltran E, Suzuki T, Mai A, Valente S, Puerta E, Tordera RM (2015) Chronic stress and antidepressant induced changes in Hdac5 and Sirt2 affect synaptic plasticity. Eur Neuropsychopharmacol 25(11):2036–2048
Malhi GS, Morris G, Bell E, Hamilton A (2020) A new paradigm for achieving a rapid antidepressant response. Drugs 80(8):755–764
Jesulola E, Micalos P, Baguley IJ (2018) Understanding the pathophysiology of depression: from monoamines to the neurogenesis hypothesis model—are we there yet? Behav Brain Res 341:79–90
Levy MJF, Boulle F, Steinbusch HW, van den Hove DLA, Kenis G, Lanfumey L (2018) Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 235(8):2195–2220
Liu B, Liu J, Wang M, Zhang Y, Li L (2017) From serotonin to neuroplasticity: evolvement of theories for major depressive disorder. Front Cell Neurosci 11:305
Serafini G (2012) Neuroplasticity and major depression, the role of modern antidepressant drugs. World J Psychiatry 2(3):49–57
Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM (2014) Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol Psychiatry 19(7):791–800
Yu H, Chen ZY (2011) The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol Sin 32(1):3–11
Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, Newton SS, Duman RS (2010) A negative regulator of MAP kinase causes depressive behavior. Nat Med 16(11):1328–1332
Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS (2002) Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22(8):3251–3261
Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, Pandey GN (2001) Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J Neurochem 77(3):916–928
Neve RL, Finch EA, Bird ED, Benowitz LI (1988) Growth-associated protein GAP-43 is expressed selectively in associative regions of the adult human brain. Proc Natl Acad Sci USA 85(10):3638–3642
De la Monte SM, Federoff HJ, Ng SC, Grabczyk E, Fishman MC (1989) GAP-43 gene expression during development: persistence in a distinctive set of neurons in the mature central nervous system. Brain Res Dev Brain Res 46(2):161–168
Han MH, Jiao S, Jia JM, Chen Y, Chen CY, Gucek M, Markey SP, Li Z (2013) The novel caspase-3 substrate Gap43 is involved in AMPA receptor endocytosis and long-term depression. Mol Cell Proteomics 12(12):3719–3731
Nibuya M, Nestler EJ, Duman RS (1996) Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci 16(7):2365–2372
Blendy JA (2006) The role of CREB in depression and antidepressant treatment. Biol Psychiatry 59(12):1144–1150
Björkholm C, Monteggia LM (2016) BDNF—a key transducer of antidepressant effects. Neuropharmacology 102:72–79
Trautmann C, Bock A, Urbach A, Hübner CA, Engmann O (2020) Acute vitamin B12 supplementation evokes antidepressant response and alters Ntrk-2. Neuropharmacology 171:108112
Acknowledgements
We would like to thank SUDAM staff (especially Salih Metin Gokyaprak, DVM and Mehmet Kosen) for their full support.
Funding
This study was supported by the Scientific and Research Projects Department at the University of Konya-NE (Project Number 191218013).
Author information
Authors and Affiliations
Contributions
The main idea of the present study was from Ebru Guclu and Salim Yalcin Inan. Salim Yalcin Inan and Ebru Guclu designed the protocol of the study. Hasibe Cingilli Vural provided budget for this study. While data collection for behavioral experiments has been done by Salim Yalcin Inan and Ebru Guclu, molecular studies have been performed by Ebru Guclu and Hasibe Cingilli Vural. Writing the manuscript has been done by Ebru Guclu and Salim Yalcin Inan. All authors contributed to and have approved the final manuscript before submission.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All procedures conformed to NIH guidelines and were approved by the University of Selcuk Animal Care and Use Committee (Protocol 2019–28).
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guclu, E., Inan, S.Y. & Vural, H.C. The Sirtuin 2 Inhibitor AK-7 Leads to an Antidepressant-Like Effect in Mice via Upregulation of CREB1, BDNF, and NTRK2 Pathways. Mol Neurobiol 59, 7036–7044 (2022). https://doi.org/10.1007/s12035-022-03026-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03026-8