Abstract
Based on previous reports, exposure to pesticides could be linked to the prevalence increase of autism spectrum disorders (ASD). Gestational exposure to chlorpyrifos (CPF) has been associated with ASD diagnosis in humans and ASD-like behaviors in rodents. However, ASD severity degree results from the complex relationship between genetic background and environmental factors. Thus, animals with a genetic vulnerability and prenatally exposed to CPF could have a more severe ASD-like phenotype. Fragile X syndrome is one of the most common monogenic causes of ASD, characterized by a mutation in the X chromosome which alters the expression of the fragile X mental retardation protein (FMRP). Based on this, some fmr1 knockout (KO) rodent models have been developed to study the physiological and genetic basis of ASD. Both fmr1-KO and wild-type male rats (F2 generation) were used in the present study. F1 pregnant females were randomly exposed to 1 mg/kg/mL/day of CPF (s.c.) from GD12.5–15.5 or vehicle. Different behavioral, developmental, and molecular variables were analyzed in F2 males. KO rats were heavier, emitted altered USVs, were socially inefficient, reacted more to a novel stimulus, were hyperactive when exploring a new context, but hypoactive when exploring anxiety-inducing environments, and had an upregulated hippocampal expression of the grin2c gene. When exposed to low doses of CPF during gestation, these KO rats showed decreased climbing capacity, dysfunctional social interaction, and increased hippocampal expression for kcc1 and 5ht2c genes. Gestational CPF exposure increased the ASD-like phenotype in those animals with a genetic vulnerability, although its effect was less generalized than expected. It is the first time that this additive effect of CPF exposure and the fmr1-KO genetic vulnerability model is explored concerning social traits or any other behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorders (ASD) are a heterogeneous set of behaviors characterized by decreased sociability, altered communication skills, and a greater appearance of stereotyped behaviors [1]. Along with these core symptoms, diagnosed patients are commonly more impulsive, compulsive, anxious, and show specific alterations in locomotor activity [2,3,4]. Although the genetic root of ASD phenotype is unquestionable [5], the notorious increase in its prevalence observed in recent decades [6, 7] has been associated with several environmental factors, including pesticides. Of the latter, organophosphate (OP) pesticides are gaining special interest regarding their impact on ASD diagnosis in both humans and ASD-like behaviors in rodents [8,9,10].
Chlorpyrifos (CPF) is one of the most widely used OPs in the world [11]. It is also the most studied OP compound concerning ASD-like behaviors in rodents [9]. Based on the literature and our empirical findings, development seems to be particularly sensitive to exposure to low doses of CPF, even at doses that do not significantly inhibit cholinesterase enzymatic activity, regarding multiple behavioral and physiological endpoints, including ASD-like related behaviors in rodent models. For an in-depth review, see Biosca-Brull et al. [9]. In short, the empirical data point to a greater link between gestational exposure to low doses of CPF and increased ASD-like behaviors around gestational days (GD) GD12–15 in mice, e.g., Lan et al. and Lan et al., respectively [12, 13], while other compounds (e.g., valproic acid) have been systematically linked to these types of abnormal behavioral patterns. This ASD-like pattern has also been observed in rats following CPF exposure during this developmental period regarding ultrasound vocalizations [10], and various metabolites related to ASD [14].
Although the empirical evidence of the influence of these types of xenobiotic compounds (and other environmental agents) on ASD-like outcomes is increasing, only a few studies have included both vulnerable genetic background and exposure to CPF during development, focusing on knockout (KO) or transgenic models of BTBR, reeler, and APOE isoforms, showing inconclusive results [15,16,17,18]. This is relevant because, as previously proposed, ASD seems to be the result of a complex relationship between genes, physiology, and environmental factors. Moreover, fmr1-KO models, which represent the animal model of the human fragile X syndrome (FXS), have gained special interest for their use in developing and studying the ASD-like phenotype at the preclinical level [19]. However, to the best of our knowledge, no study has analyzed this interaction between gestational CPF and the fmr1-KO genetic condition in rats, whose social behavior is more similar to humans than mice [20].
The present study set out to analyze the effects of gestational CPF exposure using low doses in rats with genetic vulnerabilities (KO for the fmr1 gene) to understand the relationships between environmental and genetic factors concerning some of the most important behavioral and molecular outcomes associated with ASD. We predict that KO rats will be particularly sensitive to CPF exposure regarding social behaviors (decreased USVs and social interaction). In particular, they could be more anxious and hyperactive than their wild-type (WT) counterparts. Furthermore, we expected alterations in brain gene expression in these vulnerable animals exposed to CPF, particularly for these main components of the GABAergic and glutamatergic system, based on previous evidence concerning the well-known excitatory-inhibitory imbalance found in ASD patients [21].
Materials and Methods
Experimental Animals
A total of 96 male Sprague–Dawley rats (F2 generation) were used in this set of experiments. Briefly, the F0 generation was composed of WT females and KO male rats (from Janvier and SAGE labs INC [102], respectively). The rats were housed in our facilities and left to habituate for 2 weeks. The females’ cycle was monitored daily. Once in proestrus, one female and one male were placed together for mating for 24 h. Females were left alone in one home cage once sperm was confirmed. All male rats of the F1 generation were WT, while all F1 females were heterozygous KO, respectively. Delivery day was defined as GD0. Pups were weaned at PND21, with four animals of the same sex per cage. Mating between males and females from the F1 generation took place once these animals reached > 60 days of age, following the same protocol previously described for the F0 generation. The F2 generation was characterized by around 50% of females and males being heterozygous KO and hemizygous KO, respectively, with the remaining 50% of each sex being WT. This allowed us to complete our experiments with control WT animals whose genetic background was as similar as possible to the KO rats. This mating protocol is visually displayed in Fig. 1a. As half of the animals (approximately) were sacrificed at PND7 following USVs recording, only the remaining rats completed the pre-weaning functional battery and the behavioral tasks during adolescence. The precise number of animals per group is described in the figures and table captions for each experiment. Experimenters were blind to the genotype of the animals during the whole experiment until the statistical analyses were applied. For this reason, the composition (sample size) of the different groups was unbalanced, but enough for proper statistical analysis. Temperature and humidity conditions in the homeroom and all experimental rooms were set at 22 ± 2 °C and 50 ± 10%, respectively, with a 12-h light cycle (lights on 8 pm). The present study is part of the project ES040130002260 and was conducted following the Spanish Royal Decree 53/2013, the European Community Directive (2010/63/EU) for animal research and complies with the ARRIVE guidelines for animal research. The Animal Research Committee of the University of Almeria approved the experiments described here.
Experimental design. Panel a describes how the different groups of rats in the F2 generation were obtained. Briefly, x chromosomes tinted in green represent KO chromosomes, while pink represents WT chromosomes. Red and blue represent CPF and corn oil exposed groups, respectively, at F1 (syringe) and F2 (colored circles) generations. Panel b shows the experimental timeline. A total of 96 males (F2 generation) were used in the current set of experiments, all for USVs and around the half for later tests. Hallmarks such as ocular opening, as well as neuromotor outcomes, are shown here under the title “functional battery.” Behavioral tests during adolescence. Orange arrows indicate the days on which F2 animals were weighed. Orange ellipse indicates the days that maternal behavior (pup collection) was assessed. Red x indicates the day adolescent animals were sacrificed (although half of the animals were sacrificed at PND7, we do not indicate this in the design as we do not present data from these samples in this manuscript)
Toxic Agent
A 1 mg/kg/mL/day of CPF [O, O-diethyl O-(3,5,6-trichloropyridin-2-yl) thiophosphate (Pestanal, Sigma Aldrich)], diluted in corn oil as the vehicle, was randomly administered (subcutaneous) to half of the F1 dams from GD12.5 to 15.5 inclusive. Corn oil was administered to the remaining dams as a control condition. Age and dose were chosen based on previous studies which demonstrated specific alterations in adolescent WT rats [10] and mice with slightly higher doses [12, 13] during this time. This dose was also chosen as it does not significantly inhibit the ChE during development in neither postnatal [22, 23] or gestational stages [15, 24].
Behavioral Tests
Dams’ Testing (PND2 and 9)
Dams’ body weight during exposure and maternal behavior were monitored. Body weight was checked daily from GD12 to 15, immediately before the CPF administration. Maternal behavior was analyzed by using pup collection time. Briefly, animals were driven to another room 30 min before the start. Then, pups were removed and placed in different positions in the home cage. The time that the dams needed to collect every single pup was recorded. As some dams required more time to collect the first pup, we normalized the data to that first latency (the time the dam needed to take the first pup). Thus, data is expressed both in raw seconds and normalized seconds.
Pup Testing
Body Weight Evolution and Functional Battery (PND1-30)
A complete functional battery was done to the pups to understand the possible developmental effects of both CPF exposure and genotype conditions. This functional battery was composed of body weight evolution, ocular opening, and neuromotor development. Testicular descent was also studied at PND21 and 30. Body weight evolution was checked at PND1, 5, 10, 15, 21, and 30. The ocular opening was analyzed from PND12 to 16, with direct scores of 0, 1, or 2, for cases in which the eyes both closed, one open or both open, respectively. This direct score was transformed to a percentage (0, 50, and 100%, respectively). The neuromotor development battery was composed of three different tests: grip capacity, adherence to the inclined plane, and ascending capacity, all of them at PND16, and using an inverted rear.
Briefly, grip capacity was analyzed by placing the pup at the rear and pushing it away by the tail. Depending on the resistance (difficulty to take the rat away from the rear), the animals received a score of 0, 1, or 2 points to indicate resistance, slight resistance, and strong resistance, respectively. Concerning adherence to the inclined plane test, the rear was set with a slope of 60°. Animals were placed in the middle part of the inclined plane. Those rats that immediately fell received a score of 0, while 1 and 2 points were given to those who fell before 15 s or did not fall at all during those 15 s, respectively. Finally, the animal’s capacity to climb in the slope was assessed. Rats were placed in the bottom section of the slope and let free to climb by placing some food in the top section. The slope was divided into three sections, and the rats received 0, 1, or 2 points depending on the region they reached (bottom, middle, or top, respectively). All tests were conducted in the homeroom, from 9 to 12 am, under dim light and the same temperature and humidity conditions previously described.
Ultrasound Vocalizations (PND7)
Rats were driven to a “waiting room"” relatively close to the “experimental room,” where pups stayed with their respective litters. Fifteen minutes before starting, pups were separated from their mothers and placed in the “pre-test room.” Pups (PND7) were placed in a small plastic box covered with cotton, always with at least three pups simultaneously to avoid a significant temperature drop following separation. Once each pup had spent 15 min in such conditions, it was ready to start the recording. For this, the pup was placed in another small plastic box into a sound-attenuated chamber (80 × 60 × 70 cm). USVs were recorded with an ultrasonic microphone (Dodotronic ultramic 250 K), placed 10 cm from the rat. This recording was carried out for 5 min, and USVs were processed using the software SeaWave v2.0 (CIBRA), at 250 kHz and 16 bits format. Recording order was counter-balanced concerning treatment condition (firstly, all pups from a control dam were tested, then all pups of a CPF-exposed dam, etc.) to avoid an hour of the day bias. Dependent variables were total calls (number), calls each minute (number), latency to first call (seconds), maximum frequency peak, and the average of principal frequency. Room temperature and humidity were set to the homeroom conditions previously described. USV tests were conducted between 9 and 12 am. The lighting of the room was set as dim light, while the experimental cage had no light source during the test.
Three Chambers Crawley Test (PND33-34)
A standard three chambers Crawley test (3CT) analyzed sociability and reaction to social novelty. Briefly, the apparatus was composed of one central chamber and two other equally sized chambers at each side (the size of each chamber was 30 × 98 × 50 cm). These chambers were separated by two walls of 50 cm made of plexiglass, with one gate (10 × 10 cm) in each wall to facilitate the free movement of the animals. This test was used during adolescence (PND33). Rats were driven to the experimental room 1 h before the experiment started. During the first phase (habituation phase), experimental rats were placed in the central chamber, and their motor behavior was monitored for 5 min. Following this, one unknown animal (namely Stranger 1, or S1) was introduced in a grid cage (23 × 15 × 23 cm) placed in one of the lateral chambers (second phase or social phase). Gates that were previously closed during the habituation phase were then opened, and the experimental rat’s exploratory behavior was recorded and analyzed for 10 min. After this period, another unknown rat (Stranger 2, or S2) was also introduced in another grid cage placed in the opposite lateral chamber, while S1 was maintained in its place (third phase or reactions to social novelty phase). The experimental rat was allowed to freely explore the apparatus for 10 min. Locomotor activity was monitored during all the phases. Animals’ behavior was tracked with Ethovision v3.1 (Noldus). Order of testing was counter-balanced concerning treatment condition (CPF-exposed rats completed the test during even sessions, while control rats completed it during odd sessions).
Regarding motor behavior, total distance (cm), time in movement (sec), velocity (cm/sec), rearing (frequency), immobility (frequency), and strong mobility (frequency) were included for analysis. Rats are considered immobile by Ethovision when they move at a very low rate or do not move at all, falling below the threshold of 5% of total mobility (that means, pixels detection regarding the experimental object cannot vary more than 5% between the current vs. previous sample), whereas strong mobility is defined by those episodes when the animals move so fast that they surpass the threshold of 95% of total mobility (that means, pixels detection regarding the experimental object cannot vary less than 95% between the current vs. previous sample). Although strong mobility is a simpler variable regarding its meaning (sudden and momentary heavy increase in mobility), immobility could be composed of different behaviors with different functions such as freezing (fear) or grooming (self-maintenance, stereotypy). Concerning social behavior, time in each chamber (sec) and sniffing time for each grid cage (sec) for each phase were included. Added to this, both Social [SI (\(\frac{(S1-\mathrm{empty})}{(S1+\mathrm{empty})}\))] and reaction to social novelty indexes [SNI (\(\frac{(S2-S1)}{(S2+S1)}\))] were included, where we analyzed the time that experimental animals spent in the social room/sniffing Stranger 1 vs. the time spent in the empty chamber/sniffing the empty box during the second phase (SI). In addition, the time these rats spent exploring the novelty chamber/sniffing Stranger 2 vs. the time in the social chamber/sniffing Stranger 1 during phase 3 (SNI) was also analyzed. Chambers and grid cages were cleaned with ethanol 70% between experimental rats. This experimental procedure was completed between 9.00 and 13.00 am under dim light conditions.
Novel Object Recognition (PND35)
A novel object recognition test (NOR) was conducted to discard any possible effects on the third phase of the 3-CT that resulted from a general alteration regarding reaction to novel stimulus rather than a reaction to social novelty. The NOR test was conducted in four open fields (75 × 75 × 75 cm each open field) and was composed of three different phases, as those described for 3-CT, one day after 3-CT was completed. The first phase was also used for habitation purposes, where the experimental rat could freely explore the open field for 5 min. Following this, the experimental rat was returned to its home cage, and two equally sized objects (small plastic glasses full of dark sand with a red cap) were then placed in two opposite corners in the previously explored apparatus. The experimental rat was then re-introduced to the apparatus, and its exploratory behavior was monitored for 5 min (phase 2). Once this period was completed, the animal was placed into its home cage again and remained there for 150 min. After this period, both objects were cleaned with ethanol, and only one was put back in its place, along with a novel object (red square). The experimental animal was then introduced again in the open field, and its exploratory behavior was analyzed for 5 min (phase 3 or novelty phase). Motor behavior was analyzed in all phases using Ethovision. The open field was cleaned between animals (and between phases 2 and 3 for the same rat) with 70% ethanol. The order of testing was counter-balanced regarding treatment condition. Motor outcomes were the same as those previously described for the 3-CT. Reaction to novelty was studied by analyzing the total time (sec) and episodes (frequency) of rats’ sniffing behavior towards the novel stimulus in phase 3. Illumination was the same as that previously described for the 3-CT.
Plus maze (PND37)
Plus maze (PM) was studied as described in previous studies [25]. One day after NOR testing, the rats’ anxiety level was analyzed. Briefly, this apparatus is composed of 4 arms, placed 90 cm from the floor. Two of these arms were enclosed within a wall (closed arms), while the remaining two had no walls (open arms), all of them connected by a central square. This test creates a conflict situation for rodents, as their natural tendency is to explore, but they also require some degree of physical protection when exploring. Therefore, rats will preferably explore closed arms rather than open arms, as they can protect their back by using the walls. In addition, longer periods spent at these open arms generally reflect lower anxiety levels, while the opposite is true concerning closed arms. Rats were driven to the experimental room 1 h before the start. Following this, one rat was placed into the center of the apparatus and allowed to freely explore for 5 min. The animals’ behavior was tracked with Ethovision v3.1 (Noldus). All those motor outcomes previously described for the 3-CT and NOR tests were also analyzed for PM, and entries to closed arms (frequency) were included as a motor-like variable. Anxiety-related analyzed behaviors were the time spent at the closed arms (CA, sec) and time spent at the open arms (OA, sec). Furthermore, an anxiety index (AI), similar to those created for the 3-CT concerning social traits, was also analyzed as the ratio between these two sections (AI (\(\frac{(\mathrm{CA}-\mathrm{OA})}{(\mathrm{CA}+\mathrm{OA})}\))]). The apparatus was cleaned with 70% ethanol between animals, and the order was counter-balanced regarding treatment condition. Illumination was set as previously described for the other behavioral tests.
Biochemical Analyses (pups)
Sacrifices (PND7 and 41)
Half of the rats were sacrificed at PND7 (pups) at least 2 h after USV testing. The remaining rats completed the behavioral tests previously described and were sacrificed at PND41 (adolescents) 1 day after the last behavioral test had been completed. Rats were anesthetized with Isoflurane and sacrificed by fast decapitation. While one of the researchers processed stool samples, another proceeded with the brain. For pups, each brain hemisphere was separately collected in two different fresh, RNAse free tubes (1.5 mL). In addition, the total content of the gut was collected in one single fresh, RNAse free tube (1.5 mL). Concerning adolescent samples, the process with the stool was the same, but the brain was dissected at the frontal cortex, hippocampus, hypothalamus, and cerebellum. All samples were immediately flash-frozen when removed from the animal to avoid RNA and protein degradation. Accordingly, all materials and spaces used during sacrifice were autoclaved or treated with RNAse ZAP (Invitrogen). All samples were stored at − 80 °C until use.
Genotyping by Polymerase Chain Reaction
gDNA was isolated from ear samples at these two time periods of sacrifice using a column-based method (GeneJET, Fisher Scientific). Isolated and purified gDNA quantity and quality (260/280 ratio) were checked with a nanodrop. Of gDNA, 10 ng/uL were finally included in the PCR reaction along with Taq Nzytech Master Mix (Nzytech), nuclease-free water, and primers. The sequences of the primers were Fwd:5′-tggcatagaccttcagtagcc-3′, Rev: 5′-tatttgcttctctgaggggg-3′ (Hamilton et al., 2014), and PCR conditions were as follows: 10 min at 94 °C, 35 cycles of 1 min at 94 °C, 1 min at 59 °C and another minute at 72 °C, and 10 min at 72 °C. Following the PCR, samples were charged in an agarose gel, and electrophoresis began. WT samples expressed at 400 bp while for the KO animals this was around < 300 bp. One example from the genotyping results can be seen in Supplementary image 1.
Brain Gene Expression by Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
Only samples from the hippocampus of adolescent rats were used for gene expression analyses. Briefly, RNA was isolated using the Trizol method (Ambion). RNA quality (integrity and purity) was checked using gel electrophoresis and the absorbance 260/280 nm ratio with a nanodrop. RNA total concentration was quantified by fluorometry with a Qubit (Invitrogen). Samples were then diluted to 100 ng/uL, using this concentration for the cDNA synthesis (20uL). Twenty microliters of that cDNA was then diluted in RNAse free water (1:4 factor), and this dilution was finally used for the qPCR reaction. qPCR was conducted in microplates, composed of SYBR green master mix, nuclease-free water, primers, and the cDNA (total reaction 10uL per well). Samples were added in duplicates, run, and analyzed in a thermocycler (Step One v2.2.2, Applied Biosystems). Ct values and melting curves were carefully analyzed to detect any abnormal patterns in each reaction. Primers’ performance was analyzed using a dilution factor of 1:10, checking the slope and efficacy rates. Primers designed in exon sections of the gapdh gene were used as a housekeeping gene, while primers designed in intron sections of this same gene were used to check gDNA contamination of the samples. Primers’ associated information is described in Supplementary Table 1. Only those samples that expressed no gDNA contamination, early expression rates for the remaining genes (< Ct30), and no abnormalities in their melting curves (e.g., absence of double/multiple peaks) were accepted for statistical analyses. Briefly, Ct values for every single gene were normalized to those observed in the housekeeping gene (ΔCt) and then normalized to internal control (ΔΔCt), which was the averaged ΔCt of the control, wild-type group. This ΔΔCt was then transformed to obtain the fold change (2^ ΔΔCt), displayed in the results section.
Statistical Analyses
Both treatment (two levels, control, and CPF) and genotype (two levels, WT and KO) were used as main factors for all analyses, except for those carried out for F1 dams, where only treatment was analyzed. Repeated measures analyses of variance (rmANOVA) were applied for all maternal behavior-related variables, using days (GD or PND) or pups as within-subject variables. rmANOVAs were also used for the pups’ emitted vocalizations (minute as the within-subject factor) and body weight evolution. Multiple two-way ANOVAs were used for all the remaining outcomes associated with the F2 generation, including behavioral and molecular variables, always with treatment and genotype as main factors. Extreme outlier data were removed from the gene expression analyses following Grubb’s test/ESD method. Data were considered statistically significant at p < 0.05. All analyses were carried out using SPSS v26 (IBM), except for the correlation matrices, carried out with JASP v0.13. Figures were designed with GraphPad Prism v9 and tables were designed with Microsoft Excel office 365®. Figure 1a was designed with Biorender®. Statistics for every significant analysis are described in the Supplementary Table 2, and indicated in its appropriate figure/table. Partial eta squared (ηp2, for ANOVAs main effects) and Cohen’s d (d, for pair-wise comparisons) were analyzed to study the effect size of each significant data except for correlations’ section. Adjusted (Sidak’s correction) interval confidences of the differences (dCI95%) were also included along with the effect size calculations. P values are also included in the main text.
Results
Figure 1a and b summarizes the experimental design and timeline for this set of experiments. Only F2 males were used in this set of experiments, as ASD diagnosis is more common in boys than girls, as well as fmr1 gene is located at the X chromosome, facilitating the work with males.
Dams’ Functional Battery and Maternal Behavior
There were no differences between CPF and control dams concerning body weight at the beginning of the exposure. CPF exposure did not influence the dams’ body weight from GD12.5 to GD15.5. Furthermore, CPF exposure did not alter maternal behavior as observed in the pup collection test, shown by the latency to collect the first pup and evolution of collection time at both PND2 and 9 (Supplementary Fig. 1). Normalized time (total collection time minus latency to collect the first pup) was similar between both treatment groups (data not shown).
Pup’s Testing
Functional battery
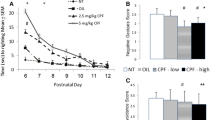
Regarding body weight, there were no differences between groups at PND1 or PND5 when analyzing all pups (Supplementary Fig. 2). However, for those animals that were not sacrificed at PND7 following USVs, a significant interaction was found for PND × genotype (p = 0.010) at PND30, where KO animals weighed more than their WT counterparts (p = 0.019) (Fig. 2a). Regarding neuromotor outcomes, no differences were found for ocular opening, grip capacity, or adherence to the inclined plane. However, a non-significant trend was observed for grip capacity, where KO-exposed rats had the lowest performance (Supplementary Fig. 2). Moreover, a significant interaction treatment × genotype was found for climbing capacity (p = 0.046), where KO rats exposed to CPF had more difficulties climbing the inverted slope compared to exposed WT animals (p = 0.023) (Fig. 2b). Finally, no statistical analysis was applied to testicular descent as all animals showed the same pattern of absence and presence of testicular descent at PND21 and 30, respectively.
Pup’s body weight evolution (panel a, left) and climbing capacity (panel b, right) from the functional battery. n = 52 divided into 13, 8, 12, and 19 for WT-Control, WT-CPF, KO-Control, and KO-CPF, respectively, for both outcomes. A single asterisk (*) indicates significant differences between KO and WT rats at PND30 regarding body weight. A single number sign (#) indicates significant differences between WT and KO rats exposed to CPF during gestation regarding climbing capacity. Data are expressed as means and SEMs. p < 0.05
Communication (USVs)
Genotype and exposure conditions had no effects on USVs at PND7 in latency to the first call, total calls (Both in Supplementary Fig. 3a and b), or the evolution of the number of calls throughout the 5 min of recording. However, it is noteworthy that KO animals made fewer total calls, particularly for the first 3 min of testing, although this effect was not statistically significant (Fig. 3). KO rats also made longer calls on average, although this effect did not reach significance (Supplementary Fig. 3c). To our particular interest, all groups showed a lower principal frequency than control WT animals (Fig. 3b). For this, a significant effect was found regarding genotype (p = 0.020) and treatment (p = 0.030), but no interaction between these two factors, where KO rats had a lower principal frequency compared with their WT counterparts (p = 0.020), and the rats exposed to CPF during gestation also showed this pattern in comparison with control rats (p = 0.030). Finally, peak frequencies also showed this pattern (Fig. 3c). WT animals exposed to the vehicle had the highest frequency compared to the other groups. A significant statistical effect was found for treatment condition (p = 0.029), while the effects of the genotype only approached significance (p = 0.059). Rats exposed to CPF clearly showed a generally lower peak frequency when compared to control animals (p = 0.029).
Pup’s number of vocalizations for 5 min (panel a, upper), USV principal frequency (panel b, lower left), and peak frequency (panel c, lower-right) from the USV test at PND7. n = 92 divided into 21, 15, 23, and 33 for WT-Control, WT-CPF, KO-Control, and KO-CPF, respectively. A single asterisk (*) indicates significant differences between WT and KO animals regarding principal frequency. A single number sign (#) indicates significant differences between control and CPF-exposed rats concerning both principal and peak frequencies. A single astrophe (') indicates a non-significant trend towards an effect of genotype condition. Data are expressed as means and SEMs. p < 0.05
Sociability (3CT)
During the social phase (S1 vs. empty cage), the KO condition did not affect social rates compared to WT when both were exposed to the vehicle. However, analyses showed a significant simple interaction treatment × genotype (p = 0.049), where CPF exposure significantly decreased the total time of social exploration in the S1 chamber exclusively in those animals with the genetic vulnerability in comparison with WT (p = 0.039) (Fig. 4a). Otherwise, no effects were observed regarding the third phase of the test, where all animals showed similar reactions to social novelty in terms of total S2 chamber exploration time (Fig. 4d). Focusing on more direct social behaviors, KO animals spent less time sniffing the cage that contained the S1 animal than WT animals (p = 0.15) (Fig. 4b). This behavior is particularly evident in those KO animals exposed to CPF during gestation. However, statistical analyses only showed a significant main effect of genotype (p = 0.015). Furthermore, neither genotype nor treatment conditions were significant regarding sniffing time in the reaction to social novelty phase (Fig. 4e). Concerning social efficiency (time spent engaged in direct social behavior concerning total time spent in the chamber), the genotype condition showed that KO rats were significantly less efficient than WT animals (p = 0.021), showing a lower efficiency ratio during phase two (p = 0.021), as observed in Fig. 4c. This effect was, once again, not observed during the latter phase of the test (Fig. 4f). Finally, direct comparisons S1 vs. empty chamber, S2 vs. S1, and their respective SI and SNI indexes can be found in Supplementary Fig. 4. Briefly, no significant effects were found when both S1 or S2 were compared with the empty cage or the S1 in phases 2 and 3, respectively. However, a significant genotype effect was found in the SNI associated with sniffing time during the third phase of the test (p = 0.033), where, surprisingly, KO animals showed a more positive ratio (higher reaction to novelty) than their WT counterparts (p = 0.033) (Supplementary Fig. 4 h).
Sociability and reaction to social novelty were assessed with the 3-CT. Left-hand figures (from top to bottom, panels a, b, and c, respectively) represent the second testing phase (social phase). Right-hand figures (from top to bottom, panels d, e, and f, respectively) represent the third phase of testing (reaction to social novelty phase). n = 49, divided into 11, 8, 11, and 19 for WT-Control, WT-CPF, KO-Control, and KO-CPF, respectively. A single asterisk (*) indicates significant differences between the exposed animals of both genotypes. A single number sign (#) indicates significant differences regarding genotype condition. Data are expressed as means and SEMs. p < 0.05
Novelty Recognition (NOR, PND35) and Anxiety (PM)
No significant differences were found between groups during the second phase of the test, where two similar objects were placed in opposite corners of the apparatus (Supplementary Fig. 5a). This lack of significant effects was also found during the third phase once the novel stimulus was introduced (Supplementary Fig. 5b). However, the novelty index showed that the WT rats (particularly controls) did not react to the novel stimulus compared with the familiar object, while KO rats did show such a preference (Fig. 5a). This effect was statistically significant regarding genotype condition (p = 0.035). Concerning anxiety, no significant differences between groups were found, as assessed with the PM test. However, an interesting tendency was found for genotype regarding the time spent by the animals in the open arms (p = 0.083), with higher anxiety levels in KO rats in comparison with their WT counterparts, particularly in those animals that were exposed to the vehicle during gestation (Fig. 5b). The non-significant effects regarding time spent in closed arms and anxiety index can be found in Supplementary material (Supplementary Fig. 6).
Novelty recognition (panel a, left) and anxiety (panel b, right) were assessed with NOR (n = 41, divided into 9, 6, 8, and 18 for WT-Control, WT-CPF, KO-Control, and KO-CPF, respectively) and PM (n = 49, divided into 11, 8, 11 and 19 for WT-Control, WT-CPF, KO-Control, and KO-CPF, respectively). A single number sign (#) indicates significant differences regarding genotype condition. A single astrophe (') indicates statistical tendency to significance. Data are expressed as means and SEMs. p < 0.05
Locomotor Activity (3-CT, NOR, and PM)
Both genotype and treatment conditions also affected various motor outcomes during the behavioral paradigms previously described. The influence of both factors on each motor-related variable is described in Tables 1, 2, and 3 for 3-CT, NOR, and PM tests, respectively. Those behaviors significantly influenced by genotype and treatment conditions are also displayed in figures to facilitate visual comprehension. Concerning the 3-CT, those behaviors significantly affected by any of our factors are displayed in Fig. 6. Briefly, KO rats were faster (p = 0.005), covered more distance (p = 0.007), and spent more time engaged in strong mobility (p = 0.037) in comparison with WT animals during the habituation phase of the 3-CT. Control animals exposed to the vehicle during gestation also showed this pattern compared with their exposed counterparts for velocity (p = 0.041), total distance (p = 0.032), and strong mobility (p = 0.038) (Fig. 6b, a, and c, respectively). However, no effects were found regarding the interaction between treatment and genotype factors.
Motor behavior rates during the three phases of the 3-CT. Total distance (cm), velocity (cm/sec), time in movement (sec), rearing (frequency), immobility (frequency) and strong mobility (sec) are described in the table. For the habituation phase, total distance (upper), velocity (center), and strong mobility (lower) are also displayed in panels a, b, and c, respectively. The number of animals per group is the same as previously indicated in Fig. 4. A single asterisk (*) indicates significant differences regarding treatment. A single number sign (#) indicates significant differences regarding genotype. Data are expressed as adjusted (Sidak) means and SEMs. p < 0.05
Both factors also influenced locomotor activity during NOR testing. Regarding treatment, CPF-exposed rats spent less time engaged in strong mobility during the habituation phase than their control counterparts (p = 0.040). At the same time, genotype influenced vertical activity with a significant decrease in rearing episodes in KO animals compared with their WT counterparts during the novelty phase (p = 0.022) (Fig. 7a and b, respectively). Although this last outcome was focused on control (exposed to vehicle) rats, there were no significant treatment × genotype interactions for this, or the remaining variables analyzed.
Motor behavior rates during the three phases of the NOR. Total distance (cm), velocity (cm/sec), time in movement (sec), rearing (frequency), immobility (frequency) and strong mobility (sec) are described in the Table. strong mobility duration during the habituation phase and rearing episodes during the novelty phase are displayed in panels a and b, respectively (n = 45, divided into 10, 8, 8, and 19 for WT-Control, WT-CPF, KO-Control, and KO-CPF, respectively). A single asterisk (*) indicates significant differences regarding treatment. A single number sign (#) indicates significant differences regarding genotype. Data are expressed as adjusted (Sidak) means and SEMs. p < 0.05
Finally, both factors also affected certain motor-related behaviors during the 5 min of the PM test. Once again, the treatment condition significantly decreased strong mobility in the exposed rats compared with the non-exposed animals (p = 0.019), as observed in Fig. 8c. Regarding genotype, KO rats generally had lower mobility rates in comparison with their WT counterparts, particularly for total distance covered (Fig. 8a) and velocity (Fig. 8b) (p = 0.030), and (p = 0.040), respectively.
Motor behavior rates during the PM test. Total distance (cm), velocity (cm/sec), time in movement (sec), rearing (frequency), immobility (frequency) and strong mobility (sec) are described in the Table. Total distance covered, velocity, and strong mobility time are also displayed in panels a (up) and b (middle), and c (down), respectively. The number of animals per group is the same as previously indicated in Fig. 5b. A single asterisk (*) indicates significant differences regarding treatment. A single number sign (#) indicates significant differences regarding genotype. Data are expressed as adjusted (Sidak) means and SEMs. p < 0.05
Gene Expression at the Hippocampus
Exposure, genetic background, or an interaction between these two factors did not influence the expression of most of the hippocampal genes studied in the present experiment (Supplementary Fig. 7), except for 5-ht2c and kcc1 genes concerning the significant interaction treatment × genotype (p = 0.014) and (p = 0.042), respectively, and grin 2c regarding genotype, where KO rats had higher expression levels in comparison with WT (p = 0.003) (Fig. 9c). CPF exposure increased the relative expression of both 5-ht2c and kcc1 genes in those animals with the genetic vulnerability to ASD compared to their exposed WT counterparts (p = 0.046 and p = 0.037) (Fig. 9a and b, respectively).
Relative gene expression levels at the hippocampus for 5-ht2c (a, left), kcc1 (b, center), and grin 2c (c, right) genes. Number of animals per group is represented with individual dots. * indicates significant differences between both exposed groups. # indicates significant differences regarding genotype condition. Data are expressed with means and SEMs. p < 0.05
Correlation Matrix Between Behavioral Outcomes and Gene Expression
Concerning behavior and gene expression correlations, only those behaviors analyzed during adolescence were included in the present analysis. For the complete correlation matrices, please see Supplementary Figs. 8–13. Reduced matrices of the significant interactions found are displayed here to increase data visualization and interpretation (Fig. 10). Reaction to novelty (NOR)-related data are not shown in the main text. There were no significant correlations with the expression level of the various genes analyzed in the present study.
Simplified view of the main behavioral outcomes analyzed in the present study (only significant correlations are included) showing correlation matrices between gene expression values and the main behavioral outcomes. a Correlation between gene values and the main social outcomes from the 3-CT. b Correlation between gene values and PM test. c Correlation between gene values and locomotor outcomes from the PM test. d Correlation between gene values and locomotor outcomes from the habituation phase (first phase) of the 3-CT. SOCS1 = Time spent in the social chamber during phase 2. SI, social index from chamber data. OBSF3TIMES2, time spent sniffing Stranger two during phase 3. NOVEFFRATIO, social novelty efficiency ratio during phase 3. AI, anxiety ratio. TDIST, total distance covered. VELOC, velocity. TOTDURMOV, time in movement. REARINGFREQ, number of rearing episodes. IMMOBFREQ, immobility episodes. STRONGSEC, strong mobility episodes. Data are expressed as Pearson’s r. A single asterisk (*) and double asterisks (**) indicate p < 0.05 and p < 0.01, respectively. p values of these significant correlations are described in the main text
Regarding the 3-CT social-related dependent variables, we can observe that only a few genes correlated with some of the most important behavioral outcomes (Fig. 10a). Briefly, both gad1 and nicotinic α7 gene expression values negatively correlated (r values displayed in the figure) with both time in the social chamber and SI ratio during the social phase of the 3-CT (p = 0.013 and 0.004 for gad1, and p = 0.030 and 0.022 for nicotinic α7), while GABA-a-α2 also showed this pattern but with sniffing time with stranger two during the novelty phase. Oxytocin receptor expression levels are also negatively correlated with this latter behavior, along with the efficiency ratio for social novelty (p = 0.024 and p = 0.040, respectively). Figure 10b displays significant correlations in the PM test, where GABA-a-α2 expression negatively correlated with time spent in the open arms, while a positive correlation was found regarding the anxiety index (p = 0.003 and p = 0.004, respectively).
PM data were analyzed concerning motor behavior, while only locomotor values from the first phase of both 3-CT and NOR were included to simplify the analyses. NOR motor behavior data were not included in this simplified figure as no significant correlations were found. Regarding 3-CT, both total distance covered, and velocity positive correlated with oxytocin expression levels (p = 0.035 and p = 0.038, respectively), while vertical activity (rearing frequency) showed a negative correlation with GABA-a-α2 and nicotinic α7 genes (p = 0.028 and p = 0.038, respectively). Expression levels of this last gene, along with grin2a, grin2b, and grin1, also negatively correlated with immobility episodes (p = 0.029, p = 0.013, p = 0.036, and p = 0.031, respectively), and both gad1 and gad2 negatively correlated with strong mobility time (p = 0.040 and p = 0.041, respectively) (Fig. 10d). Finally, focusing our attention on PM test locomotor outcomes, total distance covered negatively correlated with several genes as in the case of GABA-a-α2 (p = 0.018), kcc1 (p = 0.006), chat (p = 0.014), vacht (p = 0.012), nicotinic a7r (p = 0.023), ht2c (p = 0.036) and ht2a (p = 0.003). Moreover, expression levels of GABA-a-α2, kcc1, muscarinic 2 receptor, chat, vacht, and ht2a also negatively correlated with velocity rates (p = 0.015, p = 0.019, p = 0.027, p = 0.006, p = 0.002 and, p = 0.027 respectively) (Fig. 10c).
Discussion
The present study shows that fmr1-KO rats had a larger body mass (adolescence), showed vocalizations that were characterized by decreased peak and principal frequencies (pups), were socially inefficient (adolescence) and reacted more to a novel stimulus (both social and inanimate stimulus in adolescence), their locomotor rates were context-dependent, and had an upregulated hippocampal expression of the grin2c gene when compared with WT rats. In addition, these vulnerable animals—when exposed to low doses of CPF during gestation—showed altered developmental patterns including decreased climbing capacity (pups), dysfunctional social interaction rates (adolescence), and increased hippocampal expression for kcc1 and 5ht2c genes.
Both WT and KO animals showed similar body weights until adolescence (PND30), when KO rats became heavier than their WT counterparts. Some previous studies carried out with fmr1-KO mice found this increase in body weight [26,27,28], with this difference starting to emerge around this age in some cases [29]. This is unsurprising given that some variants of the fragile × syndrome in humans include severe obesity in their prototypical phenotype [30, 31], while people diagnosed with this syndrome present higher obesity rates compared with age-matched controls [32]. However, these effects have not been systematically reported in mice [33,34,35], and studies conducted with rats show contradictory findings, with some authors reporting a decrease [36] and others no effect [37,38,39] on body weight concerning this genotype.
The present study has revealed neuromotor developmental delay in preweaning fmr1-KO rats when exposed to CPF during gestation, reaching statistical significance for climbing capacity. Exposed KO animals showed a lower score concerning this ability at PND16 than their WT counterparts. This is the first time that this genotype has been associated with this specific alteration. This is relevant as delayed development of basal locomotor activity is generally observed in ASD and FRX patients. Surprisingly, this sort of functional battery has been little explored regarding the fmr1-KO model based on the published literature. One of the few studies that analyzed these variables found no differences between KO and WT young adult mice regarding climbing or grip capacity and motor coordination [35]. However, the difference in methodology and age of assessment makes it difficult to compare both results. Other studies found altered sensorimotor state and motor coordination in fmr1-KO mice when compared with their control counterparts [40,41,42,43,44,45]. Despite all these mice-based studies, we could not find another study using rats apart from the present manuscript. Our results, along with all these published by other researchers, support the idea that specific alterations in the fmr1 gene lead to abnormalities in essential components of the development of motor and sensorimotor abilities in rodents, imitating those behaviors observed in ASD patients.
Regarding the effects of developmental CPF exposure on social interaction/exploration and communication, we have previously demonstrated the lack of effects of postnatal exposure [46] and the decrease of USV following gestational exposure [10], as well as the selective alteration of multiple metabolic pathways at different ages and structures that are also linked to ASD [14]. Interestingly, we found that this gestational exposure decreased social interaction rates during adolescence (unpublished results). In this work, we observed that fmr1-KO rats showed a tendency to emit fewer USVs during the initial minutes of recording at PND7, but this apparent phenomenon was not statistically significant. However, these animals showed a decrease in peak and mean principal frequencies compared to WT rats. Contradictory findings can be observed in previous studies concerning this set of behaviors, with some examples of decreased [47, 48] or increased [49] USVs. Other studies have also found that, rather than the total count, fmr1-KO mice emit longer vocalizations [50] and different call types [51, 52]. Regarding peak and average principal frequencies, our results agree with previous studies using mice [48] and contrast with those found by Hodges et al. [52], where their fmr1-KO mice emitted at higher frequencies than their WT counterparts. Other authors have proposed that while this genetic vulnerability can be considered a good model for ASD, it better reproduces the core abnormal social behaviors observed in human ASD than communication deficits [53], as found in the current set of experiments where fmr1-KO showed a tendency towards a significantly decreased number of vocalizations in the early minutes of USV recording.
Concerning social exploration, fmr1-KO rats were less prone towards socially interacting with unknown animals during adolescence, showing a decreased level of direct exploration (sniffing) and less efficiency in terms of social interactions during the social phase of the 3-CT. Gestational CPF exposure induced a more severe ASD-like profile in those KO animals regarding this behavior. This additive effect of this vulnerable genetic background and environmental exposure to a well-known neurotoxic agent as CPF has not been previously studied. Thus, the lack of previous scientific evidence makes it difficult to contextualize and explain the current findings. The only study we have found that theoretically related both factors was that of Saldarriaga et al. [54]. The authors suggested that fmr1 premutation carriers could increase the susceptibility to neurological alterations associated with exposure to different toxins, including CPF. While there is still a lack of empirical evidence regarding this sort of hypothesis, the analysis of how environmental exposure to these types of pesticides influences social behaviors in animals with genetic vulnerabilities is not new. Biosca-Brull et al. [9] recently published a systematic review that included the evaluation of some studies that used different genetic mice models such as BTBR [15, 16], Reeler [18], and APOE [17], where mice were also exposed to CPF or its -oxon metabolite, with inconclusive and often contradictory results in all cases.
Although the lower pro-social profile of the fmr1-KO was evident during the second phase of the 3-CT, an unexpected finding emerged during the third phase. These animals showed the highest rates of reaction to social novelty during phase 3 of the test (Supplementary Fig. 4 h). Although at first glance, this finding might be taken to suggest that these animals had a better social memory or did not develop something akin to an “attachment relationship” with stranger 1, we consider that there is a more feasible explanation for this behavior. As described, NOR testing was also included in the present set of experiments as a control to determine whether the results obtained during phase 3 of the 3-CT were due to the social nature of the test. Fmr1-KO rats showed better recognition capacity during the recognition phase of the NOR in comparison with their WT counterparts. Taking both the results of the third phase of the 3-CT and the recognition phase of the NOR test, we conclude that those animals with the genetic vulnerability simply had increased or improved their reaction to novelty during a conflict situation with a novel stimulus, regardless of the nature of that stimulus (social or inanimate). Our NOR results directly contrast with most previous studies using fmr1-KO/Ki mice regarding recognition tasks [38, 55, 56]. The discrepancies between our results and most of these previously published concerning NOR could be due to several factors, such as the animal model (mice vs. rat model), the age of testing (adult vs. adolescent animals), or the fmr1- KO model itself.
Along with communicative and social domains, motor function is also generally altered in patients with FXS [57] and ASD [58], something also observed in preclinical models. Here, we found an interesting context-dependent effect, where KO animals were hyperactive in the open field test and hypoactive in more anxiety-inducing environments such as the PM test. This set of results is completely novel regarding this genetic model. Regarding the preclinical field, increased gross locomotor activity has mostly been found in fmr1-KO animals compared to their WT counterparts [59,60,61,62,63,64,65,66,67,68], but see [69, 70]. Of all these cited studies, only Wong et al. [69] and Kozono et al. [68] used rats. Interestingly, Wong et al. [69] found a general hypoactivity in KO rats in the open field compared to WT animals. Still, this effect was particularly marked in the central quadrant of the paradigm, confirming our results that anxiety-inducing contexts trigger this motor reduction in fmr1-KO animals. Finally, other authors also evaluated motor behavior in both open field and PM tests but found general hyperactivity in fmr1-KO mice in both paradigms [67].
Only the expression of three genes differed in the fmr1-KO rat compared to their WT counterparts (5ht2c, kcc1, and grin2c) in the adolescent hippocampus, where two of them were specifically modulated in these KO rats following CPF exposure (5ht2c, kcc1). Fmr1-KO animals had higher expression mRNA levels of the grin2c gene compared to WT, a gene marginally linked to ASD [71]. Despite the well-known alterations associated with the glutamatergic system in fmr1-KO models, particularly regarding metabotropic receptors, e.g., Dölen G and Bear MF [72], different studies have shed some light on the relationship between glutamatergic ionotropic receptors and the etiopathogenesis of fmr1-KO phenotype. Previous works found NMDA-associated hypo functionality in FSX models, while this hypofunction also seems to be linked to decreased dendritic complexity [73]. Other authors observed an increase in AMPA/NMDAr ratio during early development, pointing out the increase in AMPA components expression and decreased NMDA activation [74]. The alteration of some ionotropic NMDA subunits, such as grin1, 2a, and 2b, could be at the basis of these alterations, as these are known to be modulated by FMRP [75]. This is the first time that NMDA 2c subunit isoform has also been linked to fmr1-KO animals (at least at gene expression level), adding stronger support for the notion that the FMR protein is able to modulate a large set of ionotropic glutamatergic receptor subunits, essential for the proper functioning and development of the whole neural system, both in neurons (gin1, 2a and b), and interneurons and glial cells (grin2c and d) [76].
CPF exposure during gestation upregulated the expression of both 5ht2c and kcc1 genes at the hippocampus only in those animals with genetic vulnerability. 5-HT2c is one of the many binding sites for serotonin neurotransmitters in the central nervous system, which seems to play an important role in multiple behaviors such as depression and anxiety. Its hyperactivation has been linked to mismatches in inhibitory control of feeding behavior [77]. Only a couple of studies focused their attention on this serotonergic receptor in fmr1-KO rodents. For example, Prophitt et al. [78] found that fmr1-KO mice had similar protein expression levels for different serotonergic components, including 5-HT2c, compared to WT rodents. Saraf et al. [79] used similar techniques and found that adult fmr1-KO mice expressed lower levels of the 5-HT2c receptor at the anterior olfactory nucleus and nucleus accumbens. Although these results run counter to our findings, we must point out that our results were observed in CPF-exposed animals. We used a different preclinical model (mice vs. rats), obtained the tissue at a different age (adulthood vs. adolescence), and analyzed different brain areas (nucleus accumbens and olfactory nucleus vs. hippocampus) by using different molecular techniques by analyzing gene instead of protein expression levels (binding vs. RTqPCR). Concerning kcc1, this is the first time that the expression of this gene has been found to increase in fmr1-KO animals, more specifically in those gestationally exposed to CPF. KCC1 (SCLC12A4) is a ubiquitous cation chloride cotransporter essential for the proper Cl-efflux of the cell [80]. This specific modulation of kcc1 mRNA expression in fmr1-KO rats exposed to CPF could be associated with an altered transmembrane ionic homeostatic state (e.g., depolarization) in those animals, a mechanism that has previously been linked to ASD. However, we do not have enough information to know whether this relates to the ASD-like phenotype observed in that group. Moreover, there is little published research relating this gene or its protein with fmr1-KO models or ASD, especially when scientists have focused their efforts on the developmental dynamics between KCC2 (SCLC12A5) and NKCC1 (SLC12A2), which are more strongly expressed in the CNS, essential for establishing and maintaining the intra/extracellular ion balance required for the proper functioning of the GABAergic system [81].
Although our results on gene expression are of interest and novel, these must be taken with caution. We did not study this at the protein expression level. It is relevant as we cannot conclude, based on the empirical information obtained in the present set of experiments, that the 5HT2C, the GRIN2C, or the KCC1 proteins are affected by genotype, exposure, or both factors. Protein is the molecule that exerts the function within the cell, while mRNA is the precursor of the protein, containing most of the information for its synthesis. Although a similar effect between gene and protein expression could be expected, there are several post-translational mechanisms, proteasomal changes, autophagy degradation, or simply changes in protein expression which could lead to different effects at both levels (gene and protein expression).
Finally, we conducted correlation analyses between gene expression levels and the various behaviors of interest. Concerning GABAergic genes, gad1 was negatively correlated with social exploration and marginally with secondary locomotor behaviors. Moreover, gaba-a-α2 negatively correlated with reaction to social novelty, locomotor activity (vertical activity and velocity, as gad2), and anxiety levels, and positively correlated with anxiety index (animals spent more time in the closed than open arms). These results point to an inverse relationship between the activity of the GABAergic system and anxiety, social, and motor behaviors, something that is unsurprising based on the preclinical literature and current treatments in the clinical field [82,83,84]. For the cholinergic genes, the nicotinic α7 receptor negatively correlated with social exploration, and this gene, along with the muscarinic 2 receptors, chat and vacht, negatively correlated with different locomotor behaviors. Although the motor regulation mediated by the cholinergic system is known for the role played by both muscarinic and nicotinic receptors [85], its implication in regulating social behavior is not clear. In this regard, KO animals for the M4 presynaptic receptor, a receptor with similar functions to those attributed to M2, have been linked to decreased social interaction episodes [86], while the nicotinic α7 receptors seem to play a relevant role in social regulation [87]. Thus, the correlation data presented here could be taken to indicate that M2 receptors could play a relevant role in sociability regulation, as previously found for M4 receptors.
Regarding serotonergic genes, 5ht2a, and c, these negatively correlated with locomotor activity outcomes. While the role of 5-HT2a in regulating locomotor activity is well-known, previous pharmacological findings suggest a positive relationship between the activation of this receptor and hyperactivity [88]. However, these same authors previously demonstrated that 5-HT2c—but not 5-HT2a—drugs decrease motor behavior rates [89], which is consistent with our correlation results regarding the 5ht2c gene expression levels and locomotor activity. Furthermore, glutamatergic gene grin2b negatively correlated with immobile episodes during the habituation phase of the 3-CT. Interestingly, GRIN2B-related neurodevelopmental disorder is a rare syndrome characterized by intellectual, developmental, and muscle tone alterations, while around 1 out of 4 patients show ASD phenotype and epilepsy [90]. Finally, oxytocin receptor negatively correlated with reaction to social novelty but positively correlated with the most important locomotor outcomes during the habituation phase of the 3-CT. However, and although the locomotor correlations agree with those reported in the literature, e.g., Narita et al. [91], most of the previous empirical data suggest an opposite role for the oxytocin system regarding sociability and social memory, including specific analyses on the hippocampus, e.g., Raam et al. [92].
Conclusions and Future Guidelines
This article summarizes a set of experiments that demonstrate specific ASD-like behaviors associated with fmr1-KO rats, ranging from alterations during development to diminished sociability and context-dependent locomotor dysregulation and other secondary variables such as higher body weight and better reaction to a novel stimulus. The profile of some of these behaviors (climbing capacity and social exploration) deteriorated in those animals exposed to low doses of CPF during gestation. Similarly, we found that, during adolescence, fmr1-KO animals had an upregulation of one glutamatergic receptor isoform (grin2c, at mRNA expression level). These vulnerable rats also increased the expression levels of both 5ht2c and kcc1 at the hippocampus when exposed to CPF during gestation. These molecular experiments were exclusively analyzed at the gene expression level; thus, the lack of protein expression analyses limits our capacity to conclude the functional effects that both genotype and exposure have on the neurons of the hippocampus regarding these three altered genes. The fmr1-KO rat model, along with gestational exposure to low doses of CPF, seems to provide a good interactive model between the genetic background of vulnerability and environmental facilitators concerning ASD-like behaviors, at least for social exploration. However, the findings of this study should be understood as preliminary—rather than conclusive—evidence of this relationship, requiring more in-depth research in the future. New lines of investigation could focus on more ethologic measures of social interaction as well as new molecular systems (e.g., more receptors, other neurotransmitter systems, and intracell signaling pathways), physiological structures (other than the hippocampus), different ages (pups and adults), and lower doses of CPF (more similar to the doses to which are humans are exposed daily). Analysis of the protein expression levels of 5HT2C, KCC1, and grin2c (NMDA2C) by immunoblotting would be also essential to reinforce what we have found here at the mRNA expression level.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AI:
-
Anxiety index
- ANOVA:
-
Analysis of the variance
- APOE:
-
Apolipoprotein E
- ASD:
-
Autism spectrum disorders
- CA:
-
Closed arm
- CPF:
-
Chlorpyrifos
- FSX:
-
X fragile syndrome
- GD:
-
Gestational day
- KO:
-
Knockout
- NOR:
-
Novel object recognition
- OA:
-
Open arm
- OP:
-
Organophosphate
- PM:
-
Plus maze
- PND:
-
Postnatal day
- RT-qPCR:
-
Retro-transcription-quantitative polymerase chain reaction
- S1:
-
Stranger 1
- S2:
-
Stranger 2
- SI:
-
Social index
- SNI:
-
Social novelty index
- USV:
-
Ultrasound vocalization
- WT:
-
Wild-type
- 3-CT:
-
3 Chambers test
References
Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J (2018) Autism spectrum disorder. Lancet 392(10146):508–520. https://doi.org/10.1016/S0140-6736(18)31129-2
Christ SE, Holt DD, White DA, Green L (2007) Inhibitory control in children with autism spectrum disorder. J Autism Dev Disord 37(6):1155–1165. https://doi.org/10.1007/s10803-006-0259-y
Whyatt C, Craig C (2013) Sensory-motor problems in autism. Front Integr Neurosci 7 https://doi.org/10.3389/fnint201300051
Vasa RA, Mazurek MO (2015) An update on anxiety in youth with autism spectrum disorders. Curr Opin Psychiatry 28(2):83–90. https://doi.org/10.1097/YCO0000000000000133
Choi L, An JY (2021) Genetic architecture of autism spectrum disorder: lessons from large-scale genomic studies. Neurosci Biobehav Rev 128:244–257. https://doi.org/10.1016/J.NEUBIOREV.2021.06.028
Chaste P, Leboyer M (2012) Autism risk factors: genes environment and gene-environment interactions. Dialogues Clin Neurosci 2012,14(3) 281. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3513682/
Karimi P, Kamali E, Mousavi SM, Karahmadi M (2017) Environmental factors influencing the risk of autism In. J Res Med Sci 22(1) Isfahan University of Medical Sciences (IUMS) https://doi.org/10.4103/1735–1995200272
Tessari L, Angriman M, Díaz-Román A, Zhang J, Conca A, Cortese S (2020) Association between exposure to pesticides and ADHD or autism spectrum disorder: a systematic review of the literature. J Atten Disord https://doi.org/10.1177/1087054720940402
Biosca-Brull J, Pérez-Fernández C, Mora S, Carrillo B, Pinos H, Conejo NM, Collado P, Arias JL, Martín-Sánchez F, Sánchez-Santed F, Colomina MT (2021) Relationship between autism spectrum disorder and pesticides: a systematic review of human and preclinical models. Int J Environ Res Public Health 18(10):5190. https://doi.org/10.3390/IJERPH18105190
Morales-Navas M, Castaño-Castaño S, Pérez-Fernández C, Sánchez-Gil A, Colomina MT, Leinekugel X, Sánchez-Santed F (2020) Similarities between the effects of prenatal chlorpyrifos and valproic acid on ultrasonic vocalization in infant Wistar rats. Int J Environ Res Public Health 17(17):6376. https://doi.org/10.3390/IJERPH17176376
Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, Coyle J, McKhann G, Mobley WC, Nadel L, Neubert D, Schulte-Hermann R, Spencer PS (2008) Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol 38(sup2):1–125. https://doi.org/10.1080/10408440802272158
Lan A, Stein D, Portillo M, Toiber D, Kofman O (2019) Impaired innate and conditioned social behavior in adult C57Bl6/J mice prenatally exposed to chlorpyrifos. Behav Brain Funct 15(1):2. https://doi.org/101186/s12993-019-0153-3
Lan A, Kalimian M, Amram B, Kofman O (2017) Prenatal chlorpyrifos leads to autism-like deficits in C57Bl6/J mice. Environ Health 16(1):43. https://doi.org/10.1186/s12940-017-0251-3
Abreu AC, Navas MM, Fernández CP, Sánchez-Santed F, Fernández I (2021) NMR-based metabolomics approach to explore brain metabolic changes induced by prenatal exposure to autism-inducing chemicals. ACS Chem Biol 16(4):753–765. https://doi.org/10.1021/ACSCHEMBIO.1C00053
De Felice A, Scattoni ML, Ricceri L, Calamandrei G (2015) Prenatal exposure to a common organophosphate insecticide delays motor development in a mouse model of idiopathic autism. PLoS One 10(3):e0121663. https://doi.org/10.1371/journal.pone.0121663
De Felice A, Anita G, Calamandrei G, Minghetti L (2016) Prenatal exposure to the organophosphate insecticide chlorpyrifos enhances brain oxidative stress and prostaglandin E2 synthesis in a mouse model of idiopathic autism. J Neuroinflammation 13(1):149. https://doi.org/10.1186/s12974-016-0617-4
Basaure P, Guardia-Escote L, Biosca-Brull J, Blanco J, Cabré M, Peris-Sampedro F, Sánchez-Santed F, Domingo JL, Colomina MT (2019) Exposure to chlorpyrifos at different ages triggers APOE genotype-specific responses in social behavior body weight and hypothalamic gene expression. Environ Res 178:108684. https://doi.org/10.1016/J.ENVRES.2019.108684
Mullen BR, Khialeeva E, Hoffman DB, Ghiani CA, Carpenter EM (2013) Decreased reelin expression and organophosphate pesticide exposure alters mouse behaviour and brain morphology. ASN Neuro 5(1):AN20120060. https://doi.org/10.1042/AN20120060
Fyke W, Velinov M (2021) FMR1 and autism an intriguing connection revisited. Genes 12(8). https://doi.org/10.3390/GENES12081218
Ellenbroek B, Youn J (2016) Rodent models in neuroscience research: Is it a rat race? DMM Dis Models Mech 9(10):1079–1087. https://doi.org/10.1242/dmm.026120
Siegel-Ramsay JE, LRomaniuk L, Whalley HC, Roberts N, Holly Branigan H, Stanfield AC, Lawrie SM, Dauvermann MR (2021) Glutamate and functional connectivity - support for the excitatory-inhibitory imbalance hypothesis in autism spectrum disorders. Psychiatry Res Neuroimaging 313 https://doi.org/10.1016/JPSCYCHRESNS2021111302
Perez-Fernandez C, Morales-Navas M, Guardia-Escote L, Garrido-Cárdenas JA, Colomina MT, Giménez E, Sánchez-Santed F (2020) Long-term effects of low doses of Chlorpyrifos exposure at the preweaning developmental stage: a locomotor pharmacological brain gene expression and gut microbiome analysis. Food Chem Toxicol 135 https://doi.org/10.1016/jfct2019110865
Perez-Fernandez C, Morales-Navas M, Guardia-Escote L, Colomina MT, Giménez E, Sánchez-Santed F (2020) Postnatal exposure to low doses of chlorpyrifos induces long-term effects on 5C-SRTT learning and performance cholinergic and GABAergic systems and BDNF expression. Exp Neurol 202:113356. https://doi.org/10.1016/jexpneurol2020113356
Venerosi A, Ricceri L, Scattoni ML, Calamandrei G (2009) Prenatal chlorpyrifos exposure alters motor behavior and ultrasonic vocalization in CD-1 mouse pups. Environ Health Glob Access Sci Source 8(1):12. https://doi.org/10.1186/1476-069X-8-12
Perez-Fernandez C, Morales-Navas M, Guardia-Escote L, Colomina MT, Giménez E, Sánchez-Santed F (2021) Pesticides and aging: preweaning exposure to chlorpyrifos induces a general hypomotricity state in late-adult rats. NeuroToxicol 86:69–77. https://doi.org/10.1016/JNEURO202107002
Liu ZH, Chuang DM, Smith CB (2011) Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome. Int J Neuropsychopharmacol 14(5):618–630. https://doi.org/10.1017/S1461145710000520
Leboucher A, Bermudez-Martin P, Mouska X, Amri E-Z, Pisani DF, Davidovic L (2019) Fmr1-deficiency impacts body composition skeleton and bone microstructure in a mouse model of fragile X syndrome. Front Endocrinol 10:678. https://doi.org/10.3389/fendo201900678
Qin M, Xia Z, Huang T, Smith CB (2011) Effects of chronic immobilization stress on anxiety-like behavior and basolateral amygdala morphology in fmr1 knockout mice. Neurosci 194:282. https://doi.org/10.1016/JNEUROSCIENCE201106047
Dölen G, Osterweil E, Rao BSS, Smith GB, Auerbach BD, Chattarji S, Bear MF (2007) Correction of fragile X syndrome in mice. Neuron 56(6):955. https://doi.org/10.1016/J.NEURON.2007.12.001
de Vries BBA, Fryns JP, Butler MG, Canziani F, Wesby-van Swaay E, van Hemel JO, Oostra BA, Halley DJJ, Niermeijer MF (1993) Clinical and molecular studies in fragile X patients with a Prader-Willi-like phenotype. J Med Genet 30(9):761–766. https://doi.org/10.1136/jmg.30.9.761
Schrander-Stumpel C, Gerver W‐J, Engelen J, Mulder H, Fryns J‐P (1994) Prader-Willi-like phenotype in fragile X syndrome. Clin Genet 45(4):175–180. https://doi.org/10.1111/j1399-00041994tb04018x
McLennan Y, Polussa J, Tassone F, Hagerman R (2011) Fragile X Syndrome. Curr Genomics 12(3):216–224. https://doi.org/10.2174/138920211795677886
Moon J, Beaudin AE, Verosky S, Driscoll LL, Weiskopf M, Levitsky DA, Crnic LS, Strupp BJ (2006) Attentional dysfunction impulsivity and resistance to change in a mouse model of fragile X syndrome. Behav Neurosci 120(6):1367–1379. https://doi.org/10.1037/0735-704412061367
Bausch AE, Ehinger R, Straubinger J, Zerfass P, Nann Y, Lukowski R (2018) Loss of sodium-activated potassium channel slack and FMRP differentially affect social behavior in mice. Neuroscience 384:361–374. https://doi.org/10.1016/j.neuroscience.2018.05.040
Roy S, Zhao Y, Allensworth M, Farook MF, LeDoux MS, Reiter LT, Heck DH (2011) Comprehensive motor testing in fmr1-KO mice exposes temporal defects in oromotor coordination. Behav Neurosci 125(6):962–969. https://doi.org/10.1037/a0025920
Schiavi S, Carbone E, Melancia F, Buzzelli V, Manduca A, Campolongo P, Pallottini V, Trezza V (2020) Perinatal supplementation with omega-3 fatty acids corrects the aberrant social and cognitive traits observed in a genetic model of autism based on FMR1 deletion in rats. Nutr Neurosci https://doi.org/10.1080/1028415X20201819107
Tian Y, Yang C, Shang S, Cai Y, Deng X, Zhang J, Shao F, Zhu D, Liu Y, Chen G, Liang J, Sun Q, Qiu Z, Zhang C (2017) Loss of FMRP impaired hippocampal long-term plasticity and spatial learning in rats. Front Mol Neurosci 10 https://doi.org/10.3389/fnmol201700269
Asiminas A, Jackson AD, Louros SR, Till SM, Spano T, Dando O, Bear MF, Chattarji S, Hardingham GE, Osterweil EK, Wyllie DJA, Wood ER, Kind PC (2019) Sustained correction of associative learning deficits after brief early treatment in a rat model of fragile X syndrome. Sci Transl Med 11(494). https://doi.org/10.1126/scitranslmed.aao0498
Saxena K, Webster J, Hallas-Potts A, Mackenzie R, Spooner PA, Thomson D, Kind P, Chatterji S, Morris RGM (2018) Experiential contributions to social dominance in a rat model of fragile-X syndrome. Proc R Soc B Biol Sci 285:1880. https://doi.org/10.1098/rspb20180294
Thomas AM, Bui N, Perkins JR, Yuva-Paylor LA, Paylor R (2012) Group i metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for fragile X syndrome. Psychopharmacology 219(1):47–58. https://doi.org/10.1007/s00213-011-2375-4
Baker KB, Wray SP, Ritter R, Mason S, Lanthorn TH, Savelieva KV (2010) Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes Brain Behav 9(6):562–574. https://doi.org/10.1111/J.1601-183X.2010.00585.X
Renoux AJ, Sala-Hamrick KJ, Carducci NM, Frazer M, Halsey KE, Sutton MA, Dolan DF, Murphy GG, Todd PK (2014) Impaired sensorimotor gating in Fmr1 knock out and fragile X premutation model mice. Behav Brain Res 267:42–45. https://doi.org/10.1016/jbbr201403013
de Vrij FMS, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R (2008) Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis 31(1):127–132. https://doi.org/10.1016/j.nbd.2008.04.002
Spencer CM, Serysheva E, Yuva-Paylor LA, Oostra BA, Nelson DL, Paylor R (2006) Exaggerated behavioral phenotypes in Fmr1/Fxr2 double knockout mice reveal a functional genetic interaction between fragile X-related proteins. Human Mol Genet 15(12):1984–1994. https://doi.org/10.1093/hmg/ddl121
Kokash J, Alderson EM, Reinhard SM, Crawford CA, Binder DK, Ethell IM, Razak KA (2019) Genetic reduction of MMP-9 in the Fmr1 KO mouse partially rescues prepulse inhibition of acoustic startle response. Brain Res 1719:24–29. https://doi.org/10.1016/jbrainres201905029
Perez-Fernandez C, Morales-Navas M, Aguilera-Sáez LM, Abreu AC, Guardia-Escote L, Fernández I, Garrido-Cárdenas JA, Colomina MT, Giménez E, Sánchez-Santed F (2020) Medium and long-term effects of low doses of chlorpyrifos during the postnatal preweaning developmental stage on sociability dominance gut microbiota and plasma metabolites. Environ Res 184:109341. https://doi.org/10.1016/jenvres2020109341
Rotschafer SE, Trujillo MS, Dansie LE, Ethell IM, Razak KA (2012) Minocycline treatment reverses ultrasonic vocalization production deficit in a mouse model of fragile X Syndrome. Brain Res 1439:7–14. https://doi.org/10.1016/jbrainres201112041
Toledo MA, Wen TH, Binder DK, Ethell IM, Razak KA (2019) Reversal of ultrasonic vocalization deficits in a mouse model of fragile X syndrome with minocycline treatment or genetic reduction of MMP-9. Behav Brain Res 372:112068. https://doi.org/10.1016/jbbr2019112068
Lai JKY, Sobala-Drozdowski M, Zhou L, Doering LC, Faure PA, Foster JA (2014) Temporal and spectral differences in the ultrasonic vocalizations of fragile X knock out mice during postnatal development. Behav Brain Res 259:119–130. https://doi.org/10.1016/jbbr201310049
Belagodu AP, Johnson AM, Galvez R (2016) Characterization of ultrasonic vocalizations of fragile X mice. Behav Brain Res 310:76–83. https://doi.org/10.1016/j.bbr.2016.04.016
Nolan SO, Hodges SL, Lugo JN (2020) High-throughput analysis of vocalizations reveals sex-specific changes in Fmr1 mutant pups. Genes Brain Behav 19(2) https://doi.org/10.1111/gbb12611
Hodges SL, Nolan SO, Reynolds CD, Lugo JN (2017) Spectral and temporal properties of calls reveal deficits in ultrasonic vocalizations of adult Fmr1 knockout mice. Behav Brain Res 332:50–58. https://doi.org/10.1016/jbbr201705052
Pietropaolo S, Guilleminot A, Martin B, D’Amato FR, Crusio WE (2011) Genetic-background modulation of core and variable autistic-like symptoms in Fmr1 knock-out mice. PLoS One 6(2) https://doi.org/10.1371/journalpone0017073
Saldarriaga W, Lein P, Teshima LYG, Isaza C, Rosa L, Polyak A, Hagerman R, Girirajan S, Silva M, Tassone F (2016) Phenobarbital use and neurological problems in FMR1 premutation carriers. Neurotoxicology 53:141. https://doi.org/10.1016/JNEURO201601008
Costa L, Sardone LM, Bonaccorso CM, D’Antoni S, Spatuzza M, Gulisano W, Tropea MR, Puzzo D, Leopoldo M, Lacivita E, Catania MV, Ciranna L (2018) Activation of serotonin 5-HT7 receptors modulates hippocampal synaptic plasticity by stimulation of adenylate cyclases and rescues learning and behavior in a mouse model of fragile X syndrome. Front Mol Neurosci 0:353. https://doi.org/10.3389/FNMOL.2018.00353
Prieto M, Folci A, Poupon G, Schiavi S, Buzzelli V, Pronot M, François U, Pousinha P, Lattuada N, Abelanet S, Castagnola S, Chafai M, Khayachi A, Gwizdek C, Brau F, Deval E, Francolini M, Bardoni B, Humeau Y, Martin S (2021) Missense mutation of Fmr1 results in impaired AMPAR-mediated plasticity and socio-cognitive deficits in mice. Nat Commun 12(1):1–15. https://doi.org/10.1038/s41467-021-21820-1
Lieb-Lundell C (2016) Three faces of fragile X. Physical Therapy 96(11):1782–1790. https://doi.org/10.2522/PTJ20140430
Garrido D, Petrova D, Watson LR, Garcia-Retamero R, Carballo G (2017) Language and motor skills in siblings of children with autism spectrum disorder: a meta-analytic review. Autism Res 10(11):1737–1750. https://doi.org/10.1002/AUR.1829
Hayashi ML, Rao BSS, Seo J-S, Choi H-S, Dolan BM, Choi S-Y, Chattarji S, Tonegawa S (2007) Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci U S A 104(27):11489. https://doi.org/10.1073/PNAS0705003104
Uutela M, Lindholm J, Louhivuori V, Wei H, Louhivuori LM, Pertovaara A, Åkerman K, Castrén E, Castrén ML (2012) Reduction of BDNF expression in Fmr1 knockout mice worsens cognitive deficits but improves hyperactivity and sensorimotor deficits. Genes Brain Behav 11(5):513–523. https://doi.org/10.1111/J1601-183X201200784X
Uutela M, Lindholm J, Rantamäki T, Umemori J, Hunter K, Võikar V, Castrén ML (2014) Distinctive behavioral and cellular responses to fluoxetine in the mouse model for fragile X syndrome. Front Cell Neurosci 8 https://doi.org/10.3389/FNCEL201400150
Dansie LE, Phommahaxay K, Okusanya AG, Uwadia J, Huang M, Rotschafer SE, Razak KA, Ethell DW, Ethell IM (2013) Long-lasting effects of minocycline on behavior in young but not adult fragile X mice. Neuroscience 246:186–198. https://doi.org/10.1016/J.NEUROSCIENCE.2013.04.058
Oddi D, Subashi E, Middei S, Bellocchio L, Lemaire-Mayo V, Guzmán M, Crusio WE, D’Amato FR, Pietropaolo S (2015) Early social enrichment rescues adult behavioral and brain abnormalities in a mouse model of fragile X syndrome. Neuropsychopharmacology 40(5):1113. https://doi.org/10.1038/NPP2014291
Qiu G, Chen S, Guo J, Wu J, Yi YH (2016) Alpha-asarone improves striatal cholinergic function and locomotor hyperactivity in Fmr1 knockout mice. Behav Brain Res 312:212–218. https://doi.org/10.1016/JBBR201606024
Schaefer TL, Davenport MH, Grainger LM, Robinson CK, Earnheart AT, Stegman MS, Lang AL, Ashworth AA, Molinaro G, Huber KM, Erickson CA (2017) Acamprosate in a mouse model of fragile X syndrome: modulation of spontaneous cortical activity ERK1/2 activation locomotor behavior and anxiety. J Neurodevelopmental Disord 9(1) https://doi.org/10.1186/S11689-017-9184-Y
Chatterjee M, Kurup PK, Lundbye CJ, Hugger Toft AK, Kwon J, Benedict J, Kamceva M, Banke TG, Lombroso PJ (2018) STEP inhibition reverses behavioral electrophysiologic and synaptic abnormalities in Fmr1 KO mice. Neuropharmacology 128:43–53. https://doi.org/10.1016/J.NEUROPHARM.2017.09.026
Pirbhoy PS, Rais M, Lovelace JW, Woodard W, Razak KA, Binder DK, Ethell IM (2020) Acute pharmacological inhibition of matrix metalloproteinase-9 activity during development restores perineuronal net formation and normalizes auditory processing in Fmr1 KO mice. J Neurochem 155(5):538–558. https://doi.org/10.1111/JNC15037
Kozono NO, Kamura A, Honda S, Matsumoto M, Mihara T (2020) Gamma power abnormalities in a Fmr1-targeted transgenic rat model of fragile X syndrome. Sci Rep 10(1) https://doi.org/10.1038/S41598-020-75893-X
Wong H, Hooper AWM, Niibori Y, Lee SJ, Hategan LA, Zhang LZ, Karumuthil-Melethil S, Sally M, Till SM, Peter C, Kind PC, Olivier Danos O, Bruder JT, Hampson DR (2020) Sexually dimorphic patterns in electroencephalography power spectrum and autism-related behaviors in a rat model of fragile X syndrome. Neurobiol Dis 146 https://doi.org/10.1016/JNBD2020105118
Fish EW, Krouse MC, Stringfield SJ, Diberto JF, Robinson JE, Malanga CJ (2013) Changes in sensitivity of reward and motor behavior to dopaminergic glutamatergic and cholinergic drugs in a mouse model of fragile X syndrome. PloS One 8(10). https://doi.org/10.1371/JOURNAL.PONE.0077896
Yu Y, Lin Y, Takasaki Y, Wang C, Kimura H, Xing J, Ishizuka K, Toyama M, Kushima I, Mori D, Arioka Y, Uno Y, Shiino T, Nakamura Y, Okada T, Morikawa M, Ikeda M, Iwata N, Okahisa Y, Ozaki N (2018) Rare loss of function mutations in N-methyl-d-aspartate glutamate receptors and their contributions to schizophrenia susceptibility Translational. Psychiatry 8(1):12. https://doi.org/10.1038/S41398-017-0061-Y
Dölen G, Bear MF (2008) Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol 586(6):1503–1508. https://doi.org/10.1113/JPHYSIOL.2008.150722
Yau S-Y, Bettio L, Chiu J, Chiu C, Christie BR (2019) Fragile-X syndrome is associated with NMDA receptor hypofunction and reduced dendritic complexity in mature dentate granule cells. Front Mol Neurosci 11 https://doi.org/10.3389/FNMOL201800495
Banke TG, Barria A (2020) Transient enhanced GluA2 expression in young hippocampal neurons of a fragile X mouse model. Front Synaptic Neurosci 12. https://doi.org/10.3389/FNSYN.2020.588295
Bostrom CA, Majaess N-M, Morch K, White E, Eadie BD, Christie BR (2015) Rescue of NMDAR-dependent synaptic plasticity in Fmr1 knock-out mice. Cereb Cortex 25(1):271–279. https://doi.org/10.1093/CERCOR/BHT237
Vieira M, Yong XLH, Roche KW, Anggono V (2020) Regulation of NMDA glutamate receptor functions by the GluN2 subunits. J Neurochem 154(2):121–143. https://doi.org/10.1111/JNC14970
Wold EA, Wild CT, Cunningham KA, Zhou J (2019) Targeting the 5-HT2C receptor in biological context and the current state of 5-HT2C receptor ligand development. Curr Topics Med Chem 19(16):1381. https://doi.org/10.2174/1568026619666190709101449
Prophitt J, Armstrong J, Chen Y, Canal C (2019) Assessment of brain serotonin receptors in an Fmr1 knockout mouse model of fragile X syndrome. FASEB J 33(S1):6671–6671. https://doi.org/10.1096/FASEBJ2019331_SUPPLEMENT6671
Saraf T, Chen Y, Armstrong JL, Prophitt J, Canal C (2021) Evaluation of Serotonin 5-HT1A 5-HT2A and 5-HT2C receptors and the serotonin transporter in an Fmr1 knockout mouse model of fragile X syndrome. FASEB J 35(S1) https://doi.org/10.1096/FASEBJ202135S103781
Garneau AP, Slimani S, Tremblay LE, Fiola MJ, Marcoux AA, Isenring P (2019) K+-Cl- cotransporter 1 (KCC1): A housekeeping membrane protein that plays key supplemental roles in hematopoietic and cancer cells. J Hematol Oncol 12(1). https://doi.org/10.1186/S13045-019-0766-X
Wang C, Shimizu-Okabe C, Watanabe K, Okabe A, Matsuzaki H, Ogawa T, Mori N, Fukuda A, Sato K (2002) Developmental changes in KCC1 KCC2 and NKCC1 mRNA expressions in the rat brain. Dev Brain Res 139(1):59–66. https://doi.org/10.1016/S0165-3806(02)00536-9
Lydiard RB (2003) The role of GABA in anxiety disorders. J Clin Psychiatry 2003 64(suppl 3).
Hettema JM, An SS, Neale MC, Bukszar J, van den Oord EJC, Kendler G, KS, Chen X, (2006) Association between glutamic acid decarboxylase genes and anxiety disorders major depression and neuroticism. Mol Psychiatry 11(8):752–762. https://doi.org/10.1038/sjmp4001845
Engin E, Liu J, Rudolph U (2012) α2-containing GABAA receptors: a target for the development of novel treatment strategies for CNS disorders. Pharmacol Ther 136(2):142. https://doi.org/10.1016/J.PHARMTHERA.2012.08.006
Myslivecek J, Farar V, Valuskova P (2017) M(4) muscarinic receptors and locomotor activity regulation. Physiol Res 66(Suppl 4):S443–S455. https://doi.org/10.33549/PHYSIOLRES933796
Koshimizu H, Leiter LM, Miyakawa T (2012) M 4 muscarinic receptor knockout mice display abnormal social behavior and decreased prepulse inhibition. Mol Brain 5(1):1–10. https://doi.org/10.1186/1756-6606-5-10
Unal G, Bekci H, Cumaoglu A, Yerer MB, Aricioglu F (2020) Alpha 7 nicotinic receptor agonist and positive allosteric modulators improved social and molecular deficits of MK-801 model of schizophrenia in rats. Pharmacol Biochem Behav 193:172916. https://doi.org/10.1016/JPBB2020172916
Halberstadt AL, Powell SB, Geyer MA (2013) Role of the 5-HT2A receptor in the locomotor hyperactivity produced by phenylalkylamine hallucinogens in mice. Neuropharmacology 70:218. https://doi.org/10.1016/JNEUROPHARM201301014
Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB (2009) 5-HT2A and 5-HT2C receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology 34(8):1958–1967. https://doi.org/10.1038/npp200929
Platzer K, Yuan H, Schütz H, Winschel A, Chen W, Hu C, Kusumoto H, Heyne HO, Helbig KL, Tang S, Willing MC, Tinkle BT, Adams DJ, Depienne C, Keren B, Mignot C, Frengen E, Strømme P, Biskup S, Lemke JR (2017) GRIN2B encephalopathy: novel findings on phenotype variant clustering functional consequences and treatment aspects. J Med Genet 54(7):460–470. https://doi.org/10.1136/JMEDGENET-2016-104509
Narita K, Murata T, Matsuoka S (2016) The ventromedial hypothalamus oxytocin induces locomotor behavior regulated by estrogen. Physiol Behav 164:107–112. https://doi.org/10.1016/JPHYSBEH201605047
Raam T, McAvoy KM, Besnard A, Veenema AH, Sahay A (2017) Hippocampal oxytocin receptors are necessary for discrimination of social stimuli Nature. Communications 8(1):1–14. https://doi.org/10.1038/s41467-017-02173-0
Chamoun M, Groleau M, Bhat M (2016) Dose-dependent effect of donepezil administration on long-term enhancement of visually evoked potentials and cholinergic receptor overexpression in rat visual cortex. J Phys Paris 110(1–2):65–74. https://doi.org/10.1016/J.JPHYSPARIS.2016.11.010
Lips KS, Lührmann A, Tschernig T, Stoeger T, Alessandrini F, Grau V, Haberberger RV, Koepsell H, Pabst R, Kummer W (2007) Down-regulation of the non-neuronal acetylcholine synthesis and release machinery in acute allergic airway inflammation of rat and mouse. Life Sci 80(24–25):2263–2269. https://doi.org/10.1016/jlfs200701026
Jameson RR, Seidler FJ, Slotkin TA (2007) Nonenzymatic functions of acetylcholinesterase splice variants in the developmental neurotoxicity of organophosphates: chlorpyrifos, chlorpyrifos oxon, and diazinon. Environ Health Perspect 115(1):65–70. https://doi.org/10.1289/ehp9487
Fujimura J, Nagano M, Suzuki H (2005) Differential expression of GABAA receptor subunits in the distinct nuclei of the rat amygdala. Mol Brain Res 138(1):17–23. https://doi.org/10.1016/j.molbrainres.2005.03.013
Jaenisch N, Witte OW, Frahm C (2010) Downregulation of potassium chloride cotransporter KCC2 after transient focal cerebral ischemia. Stroke 41(3):e151-9. https://doi.org/10.1161/STROKEAHA109570424
Cho H-M, Lee H-A, Kim HY, Lee D-Y, Kim IK (2013) Recruitment of specificity protein 1 by CpG hypomethylation upregulates Na+-K+-2Cl− cotransporter 1 in hypertensive rats. J Hypertens 31(7):1406–1413. https://doi.org/10.1097/HJH.0b013e3283610fed
Kindlundh-Högberg AMS, Svenningsson P, Schiöth HB (2006) Quantitative mapping shows that serotonin rather than dopamine receptor mRNA expressions are affected after repeated intermittent administration of MDMA in rat brain. Neuropharmacology 51(4):838–847. https://doi.org/10.1016/jneuropharm200605026
Lau JC, Kroes RA, Moskal JR, Linsenmeier RA (2013) Diabetes changes expression of genes related to glutamate neurotransmission and transport in the Long-Evans rat retina. Mol vision 19:1538–1553
Pershina EV, Mikheeva IB, Kamaltdinova ER, Arkhipov VI (2018) Expression of mGlu receptor genes in the hippocampus after intoxication with trimethyltin. J Mol Neurosci 67(2):258–264. https://doi.org/10.1007/S12031-018-1233-9
Hamilton SM, Green JR, Veeraragavan S et al (2014) Fmr1 and Nlgn3 knockout rats: novel tools for investigating autism spectrum disorders. Behav Neurosci 128(2):103–109. https://doi.org/10.1037/A0035988
Acknowledgements
We are grateful to Ainhoa Sánchez Gil for its initial help with the F0 mating/breeding protocol
Funding
This study was supported by the Grant PID2020-113812RB-C32, MCIN/AEI/ https://doi.org/10.13039/501100011033.
Author information
Authors and Affiliations
Contributions
P-F C made the original conceptualization of the experimental design, completed the animals’ maintenance, behavioral tasks, initial statistical analyses, figures design, and the first version of the manuscript. MM M made most of the gene expression analyzed. M–N M helped with the original conceptualization of the study design, behavioral experiments, and sacrifices. G-E L, C M, and G E completed the genotyping protocol (including optimization of the PCR settings) and helped with the gene expression analyses. CM T and S–S F had the original conceptualization of this set of experiments, completed continuous supervision of the work, get the economical and material resources, and review this manuscript until its last version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The present study is part of the project ES040130002260 and was conducted following the Spanish Royal Decree 53/2013, the European Community Directive (2010/63/EU) for animal research and complies with the ARRIVE guidelines for animal research. The Animal Research Committee of the University of Almeria approved the experiments described here.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Perez-Fernandez, C., Matamala Montoya, M., Morales-Navas, M. et al. Influence of Gestational Chlorpyrifos Exposure on ASD-like Behaviors in an fmr1-KO Rat Model. Mol Neurobiol 59, 5835–5855 (2022). https://doi.org/10.1007/s12035-022-02933-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02933-0