Abstract
Alzheimer’s disease (AD), currently the single leading cause of death still on the rise, almost always coexists alongside vascular cognitive impairment (VCI). In fact, the ischemic disease affects up to 90% of AD patients, with strokes and major infarctions representing over a third of vascular lesions. Studies also confirmed that amyloid plaques, typical of AD, are much more likely to cause dementia if strokes or cerebrovascular damage also exist, leading to the term “mixed pathology” cognitive impairment. Although its incidence is expected to grow, there are no satisfactory treatments. There is hence an urgent need for safe and effective therapies that preserve cognition, maintain function, and prevent the clinical deterioration that results from the progression of this irreversible, neurodegenerative disease. To our knowledge, this is the first study to investigate the effects of long-term treatment with C21, a novel angiotensin II type 2 receptor (AT2R) agonist, on the development of “mixed pathology” cognitive impairment. This was accomplished using a unique model that employs the fundamental elements of both AD and VCI. Treatment with C21/vehicle was started 1 h post-stroke and continued for 5 weeks in mice with concurrent AD pathology. Efficacy was established through a series of functional tests assessing various aspects of cognition, including spatial learning, short-term/working memory, long-term/reference memory, and cognitive flexibility, in addition to the molecular markers characteristic of AD. Our findings demonstrate that C21 treatment preserves cognitive function, maintains cerebral blood flow, and reduces Aβ accumulation and toxic tau phosphorylation in AD animals post-stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Until recently, functional outcomes represented the only true measure of therapeutic efficacy for neurodegenerative conditions like Alzheimer’s disease (AD) and associated brain pathologies [1]. This notion changed with the advent of aducanumab, a new monoclonal anti-amyloid antibody, which was granted accelerated FDA approval, based solely on the drug’s ability to reduce Aβ, a “surrogate endpoint” deemed a likely predictor of clinical benefit in the brain [2]. Under the current circumstances, given the urgent need and lack of available alternatives, this agent was the only option for AD. Unfortunately, this new medication failed to show significant cognitive benefits in clinical trials and its continued approval for AD may be contingent upon verification of clinical benefit in confirmatory trials [3]. Moreover, it is associated with a high incidence (≥10%) of adverse reactions, namely, headaches, brain edema, and microhemorrhages [3]. There is an intense need for safe and effective medications that would preserve cognition, and effectively manage AD, possibly by 2025. This is the primary goal that forms the foundation of the US national plan for AD [4].
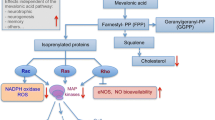
AD is a progressive, irreversible, neurodegenerative cerebral disorder that starts many years before symptoms appear and result in continuous cognitive decline, behavioral changes, psychiatric disturbances, dementia, and eventual death from complete brain deterioration [4]. The key histopathological hallmarks of AD are the presence of amyloid-β (Aβ) protein aggregates, which form senile plaques and neurofibrillary phospho-tau tangles. These processes trigger neuroinflammation, oxidative stress, and mitochondrial dysfunction, which continue to advance and ultimately lead to neurovascular degeneration and full-blown dementia [4].
AD is indeed the most common cause of dementia, accounting for an estimated 60–80% of cases. Nevertheless, it usually coexists alongside vascular cognitive impairment (VCI); the latter is defined by alterations in cognition resulting from a cerebrovascular pathology. This can range from a subclinical brain injury (seen only with neuroimaging) to a clinically manifested one, as an acute ischemic stroke. In fact, the largest proportion of dementia cases has this “mixed” pathology, with features of AD (amyloid plaques) in addition to the cerebrovascular characteristics of VCI [4].
It was previously thought that repeated ischemic events were necessary for causing vascular dementia, but recent data from a large, NIH-funded, epidemiologic trial showed that patients often experience a gradual yet incessant cognitive decline after a single-stroke lesion [5]. This continuous deterioration occurs even in the absence of any new stroke lesions and is further aggravated by amyloid pathology [6]. This was further supported by results from the Harvard Aging Brain study which showed concurrence of the 2 pathologies with a strong synergistic effect of vascular risks with Aβ for impending cognitive decline [7].
We developed a novel animal model, combining features of cerebrovascular disease (stroke) in an established strain of β-amyloid-expressing transgenic mice. These mice acquired the human APP and PSEN1 transgenes, with a total of five AD-linked mutations: the Swedish (K670N/M671L), Florida (I716V), and London (V717I) mutations in APP gene, and the M146L and L286V mutations in the PSEN1 gene and harbor the behavioral and histopathological signatures of AD [8]. These animals display age-associated cognitive impairments in addition to early Aβ accumulation, microglial activation, neuroinflammation, chronic astrogliosis, demyelination, and progressive neurodegeneration [8]. Aβ induces a toxic, pro-oxidative, neuroinflammatory environment, which is further aggravated by cerebrovascular insufficiency, hypoperfusion, and hypoxia, resulting from the acute ischemic event (stroke). The ischemic insult activates β-secretase enzyme to increase the production of Aβ, which suppresses endothelium-dependent responses, thereby further reducing cerebral blood flow. This limits trans-vascular transport of Aβ and reduces its clearance, leading to further buildup and toxicity.
Our group was the first to show that the selective AT2R agonist C21, when administered continuously, supports long-term cognitive function in young and aged hypertensive and normotensive animals post-stroke [9,10,11,12] Stimulation of AT2R has since developed into a novel therapeutic strategy for various CNS diseases by virtue of its vasodilatory, anti-inflammatory, and neuroprotective properties. The AT2R agonist C21 was selected for several reasons. Firstly, it is a stable non-peptide agonist with a much higher selectivity (up to 25,000-fold) for AT2R over AT1R [13]. It has favorable physicochemical and pharmacokinetic properties, including high water solubility and rapid absorption with median Tmax at 40 min in humans and 90 min in rats, in addition to an oral bioavailability of 20–30% and a half-life (t1/2) of 4 and 5.4 hours (h) in rats and humans, respectively [4, 9]. Moreover, C21 was found to be safe and well-tolerated in human subjects, in doses as high as 100 mg [14].
Although C21 has been shown by multiple groups to have cerebrovascular benefits, in a variety of disease states, this is the first study to investigate its effects in a new more intricate and translational model of “mixed” AD. The purpose of this investigation was to determine the effect of continuous treatment with C21, on long-term cognitive function and development of PSCI in AD mice. Given that AD is a progressive disease that worsens over time and is further accelerated by cerebrovascular events like stroke, we opted to treat animals long-term. This was intended to better simulate the clinical scenario, since like with other chronic clinical conditions, “mixed” AD may require lifelong treatment.
Experimental Design
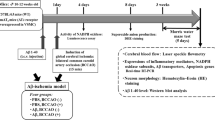
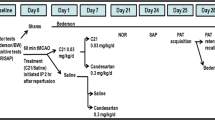
A total of 24 male and female mice—wild type (8 animals) and 5XFAD (16 animals)—obtained from Dr. McDonald at UTHSC, were housed in standard conditions (21–25 °C, 45–50% humidity) on a 12:12-h light–dark cycle, with free access to food and water. Once they reached 6 months of age, their baseline neurobehavioral performance was obtained before they were subjected to the Rose Bengal (RB) dye–based photothrombotic stroke (PTS) model of permanent focal ischemia [15].
The 5XFAD animals were randomly assigned to receive daily C21 or vehicle, initiated 1 h post-stroke (Figure 1). This was achieved by the simple randomization method [16]. All experiments were conducted in accordance with guidelines established by the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals [17, 18] with procedures and protocols approved by the Institutional Animal Care and Use Committee (IACUC) at UTHSC, Memphis TN, and functional outcomes assessed using validated, reproducible tests and reported in accordance with the most recent ARRIVE guidelines 2.0 (Animal Research: Reporting of in Vivo Experiments-Updated guidelines for reporting animal research) (see supplementary material for details).
Materials and Methods
(Please refer to the supplementary materials section for details)
Induction of Ischemic Photothrombotic Stroke in AD Mice
The animal was anesthetized, its head shaved, and an incision made along the right side of the scalp, at the eye level, to expose the scull. Rose Bengal (Photodynamic dye) in sterile saline was injected into the retroorbital sinus (10 μl/mouse) after which a high-intensity photobeam was applied at the location of the middle cerebral artery for 10 min with saline continually added to the area for rehydration. After surgery, the mice were housed in groups of 2–5 per cage, with free access to food and water, and continually monitored for signs of pain or distress. Pain medications and fluid supplementation were provided, as deemed necessary. Details of this procedure are provided in the supplementary material.
Treatment
The 5XFAD animals were assigned to receive daily C21 (VicorePharma, GӦteborg, Sweden) or vehicle, initiated 1 h post-stroke, with the first dose administered by intraperitoneal (IP) injection. Follow-up doses were incorporated into the drinking water and adjusted, according to animals’ average daily intake [9, 10] to provide them with 0.12 mg/kg/day oral dose of C21, an oral dose equivalent to 0.03 mg/kg/day IP (calculated based on bioavailability of 25%), selected based on our previous publications.
Assessment of Functional Outcome
Body Weight and Blood Glucose Concentration
Weight monitoring gives an independent and unambiguous measure of an animal’s overall health and well-being [11]. For our studies, animals were weighed at baseline, after stroke, and then periodically until the day of sacrifice. Blood glucose concentrations were measured from the tail tip using a glucometer (Contour Blood Glucose Monitoring System).
Memory, Cognitive, and Neurobehavioral Testing
AD is fundamentally a clinical diagnosis, with cognitive and neurobehavioral outcomes serving as the primary criteria (cornerstone for diagnosis) in clinical practice [1, 4].
Moreover, the large clinical studies currently focus primarily on behavioral and functional outcomes as the main measure of therapeutic success [4]. Our main focus, therefore, centers on preclinical functional measures, which were assessed using validated, reproducible, blinded tests established to simulate those employed in the clinic, performed at standard conditions during the animal’s natural active cycle, to ensure reliable results [9].
Animals underwent neurobehavioral testing at baseline (before the stroke) and again 30 days after stroke/treatment. The testing sequence was selected based on the nature and purpose of each individual test and in accordance with the recommendation of a neurobehavioral specialist, in a way designed to minimize task interference.
Sensorimotor Testing
To assess sensorimotor functions, animals underwent the Bederson test, for which they were assigned a score from 0 to 3. This score was based on the parameters: forelimb flexion; diminished resistance to lateral push; and contralateral circling. The animal was given one point for each parameter, with lower scores indicating better performance and a score of zero indicating a complete absence of deficit [10]. Animals’ swimming abilities were evaluated using the Morris water maze (MWM) (limb movement, swim speed, and ability to climb onto the platform).
The Morris Water Maze
The MWM was used to assess spatial learning, long-term/reference memory, and cognitive flexibility in addition to motor performance , as was described in detail in our previous publications [9, 10]. All tests were conducted in a large pool of opaque water, separated into quadrants, one of which (NW) contained a transparent escape platform, submerged below the water surface and obscured from view. Visual extra-maze cues were mounted to aid spatial navigation [9, 10].
The initial MWM learning/training phase consisted of 32 total trials provided as 4 trials/day, for 8 consecutive days [9, 10]. This phase was followed with a standard place task/ probe test, conducted 24 h (pre-op) and 30 days (post-op) after the last daily training session to assess spatial reference/long-term memory and consolidation [9]. Reversal training was conducted 24 h after the post-stroke probe test to assess “new” learning/updating and cognitive flexibility, which allows for updating of representations in response to changing environmental contingencies, post-stroke. For this, all parameters were kept the same, except that the platform was moved to a new location in a different (SE) quadrant. Mice were given a total of 4 sessions (4 trials each) over a period of 4 days, to learn the new location followed by a probe test 24 h after the last session. Performance was evaluated by measuring initial latency to the current target location as well as time spent in the current and previous target quadrants/zones [9, 10]
Elevated Plus Maze
The elevated plus maze (EPM) test, being a test of choice in pre-clinical studies, was used to assess overall levels of anxiety [19]. This test relies on a rodent’s affinity toward dark, enclosed spaces (closed arms) and its unconditioned fear of heights/open spaces leading to anxiety induced avoidance. It possesses several advantages including high degrees of validity and reproducibility in addition to absence of noxious stimuli (i.e., electric shock, food/water deprivation, loud noises, exposure to predator odor, etc.) that induce a conditioned response. The apparatus consists of 4 equidistant arms arranged to form a “plus” shape on an elevated, sturdy metal stand. Two of the alternate (oppositely positioned) arms are each separately enclosed by 15-cm-high walls and a top cover made of clear Plexiglas, externally lined with opaque white paper. The remaining 2 arms are completely open from all sides. Anxiety level was determined as a function of the time spent in the open arms relative to total time as well as number of open arm entries relative to total number of entries, with lower anxiety levels corresponding to an increase in open arm activity (duration and/or entries). Details of the procedure are provided in the supplementary material.
The Novel Object Recognition Test
The Novel Object Recognition (NOR) test was performed to evaluate non-spatial short-term working memory involving frontal-subcortical circuits. [20]. This test, based on the spontaneous tendency of animals to explore and interact with a novel object more than a familiar one, typically consists of 2 trials separated by a retention period and preceded by a habituation phase [11, 20,21,22].
The habituation phase (15 min/day) was conducted on 2 separate days, before the start of the test, to allow animals to acclimate to their arena. On the designated test day, each animal was first introduced into the box containing 2 identical sample objects (acquisition/sample trial) and allowed to explore these for 10 min before being taken back to its home cage for a 1-h retention period [11, 19]. The retention period is then followed by the 2nd preference/test trial (5 min), where a new/novel object replaced one of the familiar/sample objects. The time spent exploring each object, during the preference/test trial, was computed by the software and used to calculate the recognition index (RI), which is an established indicator of working memory. Details of the procedure are provided in the supplementary material.
Cerebral Blood Flow
A high-resolution laser speckle contrast imaging system (PSI System, Perimed Inc.) was used to determine relative variations in cerebral blood flow (perfusion) in the 2 hemispheres and confirm that stroke surgery was successful. The high-resolution 2D images obtained from measuring cerebral blood flow were automatically analyzed by the software and mean values recorded [23]. Cerebral blood flow was measured again at the end of the study, on the day of sacrifice to evaluate the effect of treatment.
Animal Sacrifice and Tissue Collection
Animals were deeply anesthetized and transcardially perfused with 200–300 ml of ice-cold PBS. Animals were then decapitated, and their brains collected. Sections A and B, from the brain matrix, were snap frozen in liquid nitrogen and stored at −80 °C until further processing for quantitative determinations. The remaining brain tissue was immersed in 10% formalin (Thermo Fischer Scientific, Waltham, MA, USA) and fixed overnight before being transferred to a 30% sucrose solution and stored at 4 °C for future sectioning [11].
Protein Expression
Quantitative determination of Aβ1-42 concentrations, in ipsilateral brain homogenates, was carried out using a sandwich amyloid-β ELISA kit (Wako, USA) [9].
Protein expression of a standard panel of inflammatory mediators (IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, KC/GRO, IL-10, IL-12p70, TNF-α) was achieved using a species-specific MSD V-PLEX, multi-array pro-inflammatory electro-chemiluminescence detection kit (Catalog # K15048D1). This was used for the simultaneous measurement of multiple inflammatory biomarkers according to the manufacturer’s protocol.
Western Blotting
Brain tissue samples were homogenized, and an aliquot containing 20 µg total protein was loaded into each well for gel electrophoresis. This was followed by transfer/blotting of protein bands onto PVDF membranes, which were blocked and probed with primary antibodies specific for the desired proteins [24]. Please refer to the supplementary materials section for details.
Brain Sectioning/Histology
Tissue sections were prepared, processed, and stained following a standard technique, as previously described [11]. Please refer to the supplementary materials section for details.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 8.0 software, and statistical significance was assessed using an alpha level of 0.05, unless otherwise noted. Cognitive functional outcomes as well as molecular parameters and behavioral data measures among the experimental groups, at specific time points, were evaluated by a one-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple-comparison test to examine post hoc pair-wise differences. Descriptive statistics for behavioral data measures in animals, within group, and at various indicated measurement times were determined and expressed as mean ± SEM. Two-way repeated measure ANOVA mixed models, with Greenhouse–Geisser correction, were used to examine differences between the 3 groups over time for parameters such as body weights and MWM learning/training sessions [24].
Results
5XFAD Animals Showed Moderate Impairments in Spatial Learning and Short-term Working Memory
WT non-AD animals acquired the paradigm rather quickly and displayed a typical learning curve, characterized by a gradual reduction in the mean escape latency over the 8-day training period. However, 5XFAD animals showed impaired learning and short-term working memory, as indicated by relatively higher mean escape latencies compared to WT non-AD/control animals (Figure 2a).
Effect of strain (WT vs. 5XFAD) on spatial learning and memory. Evaluated by animals’ a learning curve which represents the number of sessions it took for animals to acquire the paradigm and use the surrounding cues for spatial navigation of the maze to locate the hidden platform, b swim speed, c initial latency to the target zone, d time spent in the target quadrant during the 24-h probe test, and e heat maps illustrating relative time spent in the various locations during the probe test. The animal was introduced into the maze from various positions and relies on its working memory to reach the target (n = 8 animals/group). Symbols and error bars indicate mean ± SEM. A paired t-test was used to examine differences in outcomes, between the 2 groups over time, with statistical significance for post hoc pair-wise comparisons, denoted by *P < 0.05, **P < 0.01
5XFAD Animals Showed Clear Impairments in Reference Memory
The probe test was conducted 24 h after the final training sessions. As expected, 5XFAD animals failed to remember the platform location and hence showed significantly longer latencies to reach the target (platform) zone compared to WT animals despite similar swim speeds (Figure 2b, c). These animals also spent significantly less time (cumulative duration) in the target quadrant, compared to WT animals (Figure 2d), indicating deficits in reference memory. This was further illustrated with heat maps depicting the relative duration spent in the various quadrants (Figure 2e).
5XFAD Animals Showed a Loss of Normal Stress Response
Wild-type non-AD animals displayed a typical stress response, reflective of a normal rodent’s fear of heights/open spaces and its preference for dark, more restricted areas. These animals appeared to be in “flight mode”: they moved around much more quickly/at a higher speed (Figure 3a), than 5XFAD animals. The 5XFAD animals on the other hand showed a loss of the normal rodent’s affinity to dark, confined spaces, its inherent fear of heights/open spaces, and its avoidance of potentially threatening areas. These animals spent much longer in the open arms than that of normal controls (Figure 3b). This duration represented a significant proportion of their total time in the maze (Figure 3c). 5XFAD animals also showed a significantly greater number of open arm entries relative to total number of entries (Figure 3d). This was further illustrated with heat maps depicting time spent in different arms (Figure 3e).
Effect of strain (WT vs. 5XFAD) on normal stress response. An ordinary rodent’s fear of heights/open spaces and its preference for dark, more restricted spaces is an inborn safety feature demonstrated by normal mice. To assess this response, each animal was placed in the center of an elevated plus maze (EPM), for 5 min. 5XFAD animals ambulated at a a much slower speed, a leisurely pace contrary to the standard stress response. These animals also spent much longer b more total time, which was also c a significantly larger portion of the total allotted period in the “dangerous” open arms and less in the closed arms, with d a considerably greater number of open arm entries, relative to total entries, compared to normal WT controls. e Heat maps illustrating relative time spent in the various arms. Symbols and error bars indicate mean ± SEM. A Student’s t-test was used to examine differences in outcomes, between the 2 groups over time, with statistical significance for post hoc pair-wise comparisons, denoted by *P < 0.05, **P < 0.01
All Animals Displayed Transient Motor Deficits Followed by Complete Recovery Post-stroke
All animals showed mild sensorimotor deficits, including impaired forelimb flexion, poor gait, loss of balance, and diminished resistance to lateral push. These were apparent right after photothrombotic stroke and did not differ between the groups, at any point throughout the study (Figure 4a). Moreover, all animals showed similar and rapid sensorimotor recovery, as evident by similarly perfect Bederson scores and comparable swimming abilities 30 days post-stroke (Figure 4b–d).
Effect of acute ischemic insult and treatment on the (1) motor function, (2) body weights, and (3) blood glucose levels of 5XFAD animals. Sensorimotor function was determined by a Bederson test of gait and motor coordination and b–d swim speeds on both initial and reversal tests of the Morris water maze as measures of swimming ability (a parameter of motor function) for the different treatment groups. All animals showed initial sensorimotor deficits, apparent right after stroke followed by complete motor recovery, evident by perfect Bederson scores and comparable swimming abilities 30 days post-stroke. Animals’ e body weights and f blood glucose concentrations were obtained periodically throughout the study. Although C21 treatment had a positive effect on 5XFAD animals’ body weight, it did not influence blood glucose concentrations (n = 6–7 animals/group). Symbols and error bars indicate mean ± SEM. Repeated measures ANOVA mixed models were used to examine differences in body weights, between the 3 groups over time. For the blood glucose, a one-way ANOVA was used to examine differences between the groups, with a Tukey-Kramer multiple comparisons test used to examine post hoc pair-wise differences. A one-way ANOVA was used to examine differences between the groups with a Tukey-Kramer multiple comparisons test used to examine post hoc pair-wise differences
AT2R Agonist C21 Reduced Weight Loss and Enhanced Overall Recovery Post-stroke
All groups showed moderate reductions in body weight at 24 h post-stroke. Such reductions were temporary in wild-type non-AD control animals, which achieved complete recovery of their baseline pre-stroke body weight by the end of the study period (Figure 4e). However, the 5XFAD animals failed to reach their baseline weight; their percentage of baseline body weight was significantly lower than that of non-AD controls, with C21-treated animals showing no statistically significant differences from those of non-AD WT controls at the end of the study.
5XFAD Animals Showed a Sharp Rise and Fall in Blood Glucose Levels Post-stroke
Both WT and 5XFAD animals showed elevations in BG levels post-stroke. The rise seen in WT animals was relatively more moderate and followed by a gradual reduction and stabilization that was not seen in 5XFAD animals (Figure 4f). The rise and fall seen in 5XFAD animals were drastic and not affected by treatment.
AT2R Agonist C21 Preserved the Recognition and Non-spatial Short-term Working Memory in 5XFAD Mice Post-stroke
Vehicle-treated 5XFAD animals had significantly lower recognition indices (RI) on the novel object recognition (NOR) test, indicating defects in non-spatial working memory, compared to WT non-AD and C21-treated 5XFAD animals, under similar conditions (Figure 5).
Effect of long-term C21 treatment on the non-spatial working memory of 5XFAD animals post-stroke. a The recognition index (RI)—time spent exploring the novel object relative to the total time of exploration revealed deficits in non-spatial working memory of 5XFAD animals which had significantly lower recognition indices (RI) compared to both WT non-AD and C21-treated 5XFAD animals, under similar conditions. b Heat maps illustrating relative time spent in the various locations during the retention test. The novel object was located within the white-rimmed area. TF and TN are the times spent interacting with the familiar and novel object respectively (n ≈ 6–7 animals/group), Symbols and error bars indicate mean ± SEM. A one-way ANOVA was used to examine differences between the groups with a Tukey-Kramer multiple-comparison test used to examine post hoc pair-wise differences. Statistical significance is denoted by **P < 0.01
AT2R Agonist C21 Preserved Long-term/Reference Memory Retrieval in 5XFAD Mice Post-stroke
The positive effect of C21, on long-term/reference memory retrieval in 5XFAD animals post-stroke, was demonstrated using the MWM probe test. The C21-treated animals had a lower latency to target zone and a relatively higher number of target platform crossings (Figure 6a, b). They also displayed more focused swim patterns, with a greater time spent searching for the platform in the correct quadrant, compared to vehicle-treated 5XFAD animals (Figure 6c, d). The vehicle-treated 5XFAD animals had a significantly lower number of target platform crossings compared to stroked WT animals, whereas the performance of C21-treated animals was not much different from that of WT.
Effect of C21 treatment on long-term/reference memory retrieval in 5XFAD mice post-stroke. Long-term reference memory was evaluated at 30 days post-stroke by a repeat Morris water maze probe test. Performance was evaluated by measuring a initial latency to the original (NW) target zone, b number of target crossings, and c time spent in the target quadrant. The C21-treated 5XFAD animals tended to performed better than those treated with vehicle. They spent more time searching for the platform in the correct quadrant and showed a relatively higher number of target crossings, compared to those treated with vehicle; their performance did not differ much from WT animals. d Heat maps illustrating relative time spent in the various locations during the post-op probe test. The target (platform) zone is outlined in white (n ≈ 6–7 animals/group). Symbols/bars and error bars indicate mean ± SEM. A one-way ANOVA was used to examine differences between the groups with a Tukey-Kramer multiple-comparison test used to examine post hoc pair-wise differences. Statistical significance is denoted by **P < 0.01
AT2R Agonist C21 Was Associated with Improved Cognitive Flexibility and Reduced Preservative Behavior in 5XFAD Mice Post-stroke
The non-AD animals learned the new platform location within the first few sessions; their latency to the new target remained notably lower than that of AD animals post-stroke, and it stayed more or less constant throughout the 4-day training period (Figure 7a). This lower latency to the new target zone was also reflected in the probe test (Figure 7b). During the probe test, the WT and C21-treated 5XFAD animals spent significantly more time searching for the platform in the new (SE) target quadrant than the vehicle-treated 5XFAD animals (Figure 7c). The 5XFAD animals, on the other hand, spent much longer in the previous (NW) target quadrant and less time in the correct quadrant than did the WT animals (Figure 7d). This “preservative” behavior, typical of defective updating and impaired cognitive flexibility, was not seen in the animals treated continually with C21.
Effect of long-term C21 treatment on new learning/updating and cognitive flexibility in 5XFAD mice post-stroke. Cognitive flexibility and new learning/updating were assessed by reversal training/test. Performance measures included a latency to new (SE) target zone during the 4-day training period, b latency to the new target zone, c duration of time spent in the new target quadrant which indicated effective consolidation and retention of the new memory, d duration in the previous target (NW) quadrant which indicates preservative behavior. e Heat maps illustrating relative time spent in the various locations during the reversal test. The target (platform) zone is outlined in white and the previous target in red (n ≈ 6–7 animals/group). Symbols/bars and error bars indicate mean ± SEM. A one-way ANOVA was used to examine differences between the groups with a Tukey-Kramer multiple-comparison test used to examine post hoc pair-wise differences. Statistical significance is denoted by *P < 0.05
AT2R Agonist C21 Improved Cerebral Blood Flow in the Ipsilateral Hemisphere of 5XFAD Mice Post-stroke
In the current study, cerebral blood flow measurements were obtained directly after stroke (before treatment) and again at the end of the study period (after long-term treatment) on the day of sacrifice, with values presented relative to the contralateral (non-stroked) hemisphere (Figure 8). Initially, all animals had considerable reductions in ipsilateral blood flow post-stroke (≈50%). At the end of the treatment period, each of the WT non-AD and the C21-treated 5XFAD animals recovered up to 90% of their non-stroked CBF while the vehicle-treated 5XFAD animals did not show substantial improvement. Their mean value was about 70%, significantly lower than that of the other 2 groups.
Effect of long-term C21 treatment on cerebral blood flow in 5XFAD mice post-stroke. a Cerebral blood flow measurements, obtained using the high-resolution laser speckle contrast imaging system, illustrated a relative preservation in cerebral blood flow with long-term C21 treatment. b Heat maps illustrating CBF directly after stroke and right before sacrifice (n = 7 animals/group). Symbols and error bars indicate mean ± SEM. A one-way ANOVA was used to examine differences between the groups with a Tukey-Kramer multiple-comparison test used to examine post hoc pair-wise differences. Statistical significance is denoted by *P < 0.05
AT2R Agonist C21 Reduced Ipsilateral Aβ1-42 Accumulation in 5XFAD Mice Post-stroke
5XFAD animals treated continually with C21, post-stroke, had markedly lower concentrations of amyloid beta (Aβ1-42) in their ipsilateral hemisphere at the end of the 4-week period than those treated with vehicle (Figure 9a). They also tended to show less severe astrogliosis, evident by lower expression of GFAP (astroglial marker) compared to vehicle-treated 5XFAD animals under similar conditions (Figure 9b, c).
Effect of long-term C21 treatment on ipsilateral Aβ1-42 concentrations, Tau Ser202/Thr205 phosphorylation, and astrogliosis in 5XFAD animals post-stroke. Quantitative determinations showed that animals treated daily with C21, starting at 1 h after stroke, had markedly lower ipsilateral concentrations of a Aβ1-42 as determined by ELISA and b GFAP expression as determined by western blot, compared to vehicle-treated 5XFAD animals, the latter being an astroglial marker that is upregulated with increased inflammation. This was confirmed with immunofluorescent staining as illustrated by c representative 20× immunofluorescent images showing Aβ/green and GFAP/red with blue/DAPI nuclear staining, scale bar represents 20 μm. These animals also showed lower d phospho Tau Ser202/Thr205 relative to total tau (n = 7 animals/group). Symbols and error bars indicate mean ± SEM. A one-way ANOVA was used to examine differences between the groups with a Tukey-Kramer multiple-comparison test used to examine post hoc pair-wise differences. Statistical significance is denoted by *P < 0.05, **P < 0.01
AT2R Agonist C21 Reduced Ipsilateral Tau Ser202/Thr205 Phosphorylation and Astrogliosis in 5XFAD Mice Post-stroke
Another novel finding is that 5XFAD animals treated long-term with C21 showed reduced phosphorylation of Tau at Ser202/Thr205 residues compared to vehicle-treated 5XFAD animals (Figure 9d).
AT2R Agonist C21 Reduced Overall Cerebral Inflammation in 5XFAD Mice Post-stroke
As was expected, the brains of 5XFAD animals showed higher overall levels of inflammation in the ipsilateral hemisphere, compared to that of non-AD WT controls (Figure 10). Although this pattern of occurrence was significant in the brains of vehicle-treated animals, this was not the case with C21-treated animals. In fact, no significant differences were observed between 5XFAD animals treated long-term with C21 and WT non-AD controls for any of the inflammatory mediators.
Effect of long-term C21 treatment on overall cerebral inflammation in 5XFAD mice post-stroke. Comprehensive multiplex analyses showed an overall increase in the level of expression of a standard panel of proinflammatory cytokines in 5XFAD animals compared to that of WT control animals post-stroke. This was significant for a IL-1β and b KC/GRO with a similar albeit less significant difference for c IL-12p70, d IL-4, e IL-6, and f TNF-α. Animals treated daily with C21, starting at 1 h after stroke, showed lower levels of cytokine expression compared to those treated with vehicle (n = 7 animals/group). Symbols and error bars indicate mean ± SEM. A one-way ANOVA was used to examine differences between the groups with a Tukey-Kramer multiple-comparison test used to examine post hoc pair-wise differences. Statistical significance is denoted by *P < 0.05, **P < 0.01
Discussion
To our knowledge, this is the first study to investigate the effects of long-term treatment with the AT2R agonist C21 in AD animals post-stroke. This was accomplished specifically in relation to cognitive function and the molecular biomarkers characteristic of “mixed” pathology AD, which is in fact the most common form of AD [25, 26]. This is therefore an essential study since dementia occurs in up to 1/3 of elderly patients with stroke, a large subset of whom already suffer from AD [26] . This thorough investigation included a comprehensive sequence of neurobehavioral tests assessing the various aspects of learning, memory, and executive function in 5XFAD animals post-stroke. This unique model incorporated a vascular component (focal ischemic stroke) with typical AD pathology (transgenic β-amyloid-expressing mice). The acute vascular insult/focal ischemic stroke disrupts local blood flow resulting in hypoperfusion and hypoxia, a state of vascular insufficiency that results in further accumulation of Aβ. The toxic buildup of Aβ sustains neuroinflammation, toxic tau phosphorylation, chronic astrogliosis, and neurodegeneration, as part of a devastating feed-forward loop [4, 9].
The photothrombotic stroke model was selected for its high reproducibility and accuracy and the consistency of location and size of the infarct, which also allows for visual confirmation of successful MCAO. It is also associated with low mortality and high long-term survival rates, making it a preferred model in 5XFAD animals, which are more susceptible to complications, given their overall health condition. Furthermore, the Stroke Therapy Academic Industry Roundtable (STAIR) recommends that a permanent MCAO model be studied first before proceeding to transient models. Since we were the first to study the effect of long-term oral C21 treatment in 5XFAD mice, we opted for this model [9].
Compound 21 (C21), was selected as our AT2R agonist of choice, given its promising history and favorable physicochemical and pharmacokinetic properties, in addition to its established safety and tolerability in human subjects [4]. This agent has consistently shown positive results in multiple studies of cognition [4]. It improves cerebrovascular autoregulation and blood flow, reduces oxidative stress and inflammation, enhances resistance to ischemia, and inhibits neurodegeneration post-stroke [4, 27,28,29,30]. Therapy with C21 was initiated 1 h post-stroke, a slightly “deferred therapeutic strategy” intended to improve the translational value of our study, as it accurately simulated the inevitable time delay between onset of symptoms and start of treatment in actual stroke patients, who often show up late to the emergency department. Treatment was continued for the full duration of the study, until the day of sacrifice. This is essential, especially for therapies intended to target an ongoing process as with VCI/mixed pathology AD, where neuronal degeneration progresses for days after occurrence of the initial ischemic event [11].
5XFAD animals showed a loss of normal stress response as well as impairments in spatial learning and short-term working memory. Fear is an instinctive animal emotion, naturally triggered in response to unfamiliar environments or potentially dangerous situations, as an essential measure of safety and well-being. This primitive protective mechanism was defective in 5XFAD animals, which wandered about the EPM at a leisurely pace and spent significantly longer in the “dangerous” open arms, compared to healthy WT controls. The 5XFAD animals also showed impairments in learning and short-term working memory, as indicated by relatively higher mean escape latencies on MWM training sessions, compared to WT non-AD/controls. These differences were apparent, even before the onset of stroke, but were modest possibly due to variations in levels of anxiety between the species, with WT non-AD animals showing more anxious behavior than 5XFAD animals. Nevertheless, 5XFAD animals showed obvious impairments in reference memory compared to WT control animals.
Following the photothrombotic stroke, all animals showed mild sensorimotor deficits. These initial deficits did not vary much between the groups and were relatively transient. All 3 groups showed rapid and spontaneous motor recovery. Within 72 h, animals displayed dexterous limb movement, comparable swim speeds, and excellent gait and coordination. The spontaneous motor recovery seen in our study is in agreement with that of others using similar models [9]. Unlike the case with motor function, only those WT/ non-AD control animals achieved complete recovery of their baseline pre-stroke body weight by the end of the study period. However, 5XFAD animals failed to reach their baseline pre-stroke body weight, which was also considerably less than that of WT controls. Their percentage of baseline body weight at the end of the study was significantly lower than that of non-AD controls, with the exception of C21-treated animals which were no different from controls. C21 on the other hand did not affect the BG levels of 5XFAD animals which continued to be relatively lower than that of WT controls. This is because although all animals had some elevations in BG levels, and 5XFAD animals had a much more drastic “spike” compared to that of WT controls. This sharp rise and drastic fall in 5XFAD animals may have partly contributed to some of the negative consequences seen in those animals post-stroke.
The 5XFAD animals, which were maintained on C21 long-term, also showed a preservation of their non-spatial, short-term working memory post-stroke. This was demonstrated by a much higher recognition index (RI), reflecting the animal’s greater affinity for the novel object during the NOR preference trial. In fact, they displayed RI values that were not much different from those of WT non-AD animals. The NOR test has several distinctive advantages over others. It is highly sensitive and specific. It reliably evaluates non-spatial working memory related to the frontal sub-cortical circuits, as it does not involve reference memory components (e.g., explicit rule learning) making it selective for non-spatial, short-term working memory [22, 24, 31,32,33,34,35].
It does not include confounders such as positive reinforcements (e.g., food rewards, which could influence the motivational aspects of the performed task) or punishment/negative reinforcements with strong aversive stimuli (e.g., electric shocks likely to produce stress and may influence performance) that make it more comparable to the memory tests used in humans. Furthermore, this test is relatively quick and easy to implement, making it widely used for assessing cognitive impairment in pre-clinical research [11].
Those 5XFAD animals treated with C21 also demonstrated better hippocampal-dependent long-term/reference memory, evident by long-term retention (more time spent in target quadrant) and rapid retrieval (low latencies) not seen with their vehicle-treated counterparts post-stroke. These C21-treated animals also tended to show better performance on the reversal test of updating, consolidation, and cognitive flexibility, compared to vehicle-treated animals. Nevertheless, the difference in latency between the groups, while apparent, did not reach statistical significance.
This may have been because, although the non-AD animals quickly learned the approximate location of the platform, it initially took them a long time to locate the exact spot, an issue that they did not have before the stroke. They did however spend significantly longer in the new target quadrant which is indicative of effective updating, as opposed to vehicle-treated 5XAD animals which tended to display typical preservative behavior. This preservative behavior is attributed to difficulty with the updating of information in response to changing environmental contingencies and is possibly a result of impaired consolidation or to problems with the retrieval of brand new memories post-stroke, or to difficulty inhibiting the adaptive, previously learned response when it became no longer appropriate. This behavior is characteristic of the poor cognitive flexibility often associated with mixed dementia in patients. Fortunately, the 5XFAD animals treated continually with C21 did not exhibit this behavior. Although some of these animals proceeded to swim to the previous location, they appeared to remember half-way and change their course of action. These animals committed significantly fewer preservative errors, defined as visiting of the previous platform location, used during the initial reference memory training. The average time these C21-treated animals spent in the new target quadrant was also significantly longer than that of vehicle-treated 5XFAD animals. In fact, the time these animals spent in the correct quadrant was not very different from that of non-AD animals. This indicates that C21-treated 5XFAD animals maintained their ability to learn new information post-stroke, as did the non-AD animals. They were also able to effectively update information that had been previously learned before the stroke.
The relatively preserved neurological function seen in C21-treated 5XFAD mice post-stroke appears to be a result of multiple molecular mechanisms. Firstly, the cerebral blood flow of 5XFAD animals is known to be compromised even in the absence of stroke. This is essentially a result of the high amyloid (Aβ) burden, which suppresses endothelium-dependent relaxation and disrupts vessel patency, all of which further aggravate the injurious effects of ischemic stroke and hypoperfusion. We discovered that C21 improved CBF in our 5XFAD animals post-stroke. Although C21 is known to increase cerebral perfusion in models of vascular cognitive impairment and ischemic stroke, this effect has never been studied in 5XFAD animals post-stroke [9, 12, 27, 36,37,38].
During the acute phase (first few hours) post-stroke, an increase in cerebral blood flow is essential, to allow perfusion through collateral vessels and optimize salvage of penumbral tissue. This is even more critical when we add the clogging effect of toxic amyloid to the equation, as is the case with AD, as it will hamper blood flow further and accelerate the development of cognitive impairment.
In addition to improving CBF, AT2R agonist C21 also resulted in a significant reduction in cerebral Aβ1-42 accumulation, which may be the result of improved cerebrovascular Aβ clearance, diminished Aβ synthesis, or a combination of the two mechanisms. This is because the reduction in CBF, induced by stroke, causes local tissue hypoxia, which facilitates Aβ production by activating β-secretase enzyme, necessary for Aβ synthesis. Aβ itself is also inherently vasoconstrictive; it suppresses endothelium-dependent responses, further reduces CBF, and worsens cerebral hypoperfusion, limiting transvascular transport. In other words, Aβ reduces its own clearance leading to further buildup and toxicity. C21 counteracts this process by stimulating CBF and increasing local tissue perfusion and hence Aβ elimination. We also discovered that C21 treatment reduces phosphorylation of Tau at Ser202/Thr205 residues, which is not surprising given that Ser202/Thr205 tau phosphorylation is mainly a result of Aβ accumulation. In fact, a causal relationship has been established, linking Aβ accumulation with tau hyperphosphorylation, aggregation, and associated cytoskeletal derangements [4]. Moreover, phosphorylation of Ser202/Thr205 residues is known to promote tau misfolding and formation of neurofibrillary tangles, which accumulate in neuronal cell bodies and synapses, causing neurotransmitter deficits and eventually neuronal death, all of which result in neurocognitive impairments and dementia.
5XFAD animals treated with C21 post-stroke also tended to show less severe astrogliosis, marked by lower GFAP, and lower overall levels of inflammation. This is essential, considering that elevated GFAP [39] and neuroinflammation are early and consistent features of AD [40]. In fact, Aβ plaques not only contribute to astrogliosis [39] and neuroinflammation but also have specifically been shown to increase IL-1β and IL-6 production in AD, the latter of which has very recently been linked to the precipitous cognitive decline and metabolic dysfunction seen in AD [40]. We found the specific pattern of inflammatory cytokine expression in our 5XFAD stroke model to be similar to that noted for other models of AD. Animals treated with C21 showed a favorable inflammatory profile compared to vehicle-treated animals. Note that these differences reached statistical significance for the major players, with a similar albeit less significant trend for IL-12p70, IL-4, and TNF-α. Such differences may become apparent in later studies with larger sample sizes or longer treatment durations.
Summary
We show for the first time that the AT2R agonist C21, which is also recognized for its antioxidant and anti-inflammatory effects [4] when given at a low dose for a prolonged period of time, effectively sustains cognitive function in 5XFAD animals subjected to stroke. It appears to accomplish this by preserving CBF and targeting the main pathological features of AD, namely, Aβ accumulation (responsible for senile plaque formation) and tau phosphorylation (the precursor for harmful neurofibrillary tangles). It also minimizes astrogliosis, inflammation, and progressive neurodegeneration. Although the results of this investigation were in accordance with our earlier findings supporting the beneficial effects of C21 on cognition [4], this had never been reported in animals with mixed-pathology AD (5XFAD animals with cerebrovascular deficiency/ischemic stroke). Moreover, this is the very first study to investigate the therapeutic effect of AT2R stimulation on the central histopathological hallmarks of AD, for which we report reductions in both Aβ accumulation and toxic tau hyperphosphorylation with C21 in AD animals after focal ischemic stroke.
Conclusion
The present study demonstrated that the AT2R agonist, C21, when started, 1 h post-stroke and continued for an extended period, effectively delayed the development of PSCI in 5XFAD animals. In fact, C21 not only provided a functional benefit (preserved cognition); it also managed the underlying pathology (reduced cerebral Aβ accumulation and toxic tau phosphorylation) in 5XFAD mice post-stroke. Therefore, therapy with C21, initiated in the early-phase post-ischemic insult, and continued long-term, may be an effective approach for slowing cognitive decline in subjects with mixed pathology AD or in such patients who have recently suffered a stroke.
Data Availability
The data generated and analyzed in this study will be made available from the corresponding author upon reasonable request.
References
McKhann GM, Knopmanc DS, Chertkowd H, Hymanf BT, Jack CR Jr, Kawash CH, Klunkk WE, Koroshetzl WJ et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3):263–9
Ciani O, Buyse M, Drummond M, Ras G, et al (2017) Time to review the role of surrogate end points in health policy : state of the art and the way forward. Value Health [Internet]. 20(3):487–95. Available from: https://doi.org/10.1016/j.jval.2016.10.011
AduhelmTM(aducanumab-avwa) (2021) [package insert]. Biogen Inc, Cambridge, MA Biogen and Eisai Pharmaceutical Companies
Ahmed HA, Ishrat T (2020) The brain AT2R — a potential target for therapy in Alzheimer’s disease and vascular cognitive impairment : a comprehensive review of clinical and experimental therapeutics. Mol Neurobiol 57:3458–3484
Levine DA, Galeck AT, Langa KM, Unverzagt FW, Kabeto MU et al (2015) Trajectory of cognitive decline after incident stroke. JAMA 314(1):41–51
Wiesmann MKA and Claassen J (2013) Vascular aspects of cognitive impairment and dementia. J Cereb Blood Flow Metab [Internet]. 1–11. Available from: https://doi.org/10.1038/jcbfm.2013.159
Rabin JS, Schultz AP, Hedden T, Viswanathan A, Marshall GA, Kilpatrick E et al (2019) Interactive associations of vascular risk and β-amyloid burden with cognitive decline in clinically normal elderly individuals findings from the Harvard Aging Brain Study. JAMA Neurol 75(9):1124–31
Alzforum (2019) Rersearch Models 5xFAD (B6SJL). Alzforum networking for a cure. 1–12
Ahmed HA, Ishrat T, Pillai B, Bunting KM, Vazdarjanova A, Waller JL, Ergul A, Fagan S (2019) Angiotensin receptor ( AT2R ) agonist C21 prevents cognitive decline after permanent stroke in aged animals — a randomized double- blind pre-clinical study. Behav Brain Res 359:560–9
Ahmed HA, Ishrat T, Pillai B, Bunting KM, Patel A, Vazdarjanova A et al (2018) Role of angiotensin system modulation on progression of cognitive impairment and brain MRI changes in aged hypertensive animals – a randomized double-blind pre-clinical study. Behav Brain Res 346:29–40
Ahmed HA, Ishrat T, Pillai B, Fouda AY, Sayed MA, Eldahshan W, Waller JL, Ergul A, Fagan S (2018) RAS modulation prevents progressive cognitive impairment after experimental stroke: a randomized, blinded preclinical trial. J Neuroinflammation 15(1):229–45
Alhusban A, Fouda AY, Pillai B, Ishrat T, Soliman S, Fagan S (2015) Compound 21is pro-angiogenic in the brain and results in sustained recovery after ischemic stroke. J Hypertens 33(1):170–80
McCarthy CA, Vinh A, Miller AA, Hallberg A, Alterman M, Callaway JK et al (2014) Direct angiotensin AT2 receptor stimulation using a novel AT2 receptor agonist, compound 21, evokes neuroprotection in conscious hypertensive rats. PLoS ONE 9(4):1–10
Steckelings UM, Paulis L, Namsolleck P, Unger T (2012) AT2 receptor agonists: hypertension and beyond. Curr Opin 21(2):142–6
Watts LR, Zheng W, Garling RJ, Frohlich VC et al (2015) Rose Bengal photothrombosis by confocal optical imaging in vivo: a model of single vessel stroke. J Vis Exp 100(June):e52794 (1–10)
Suresh KP (2011) An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci 4(1):1–11
Kilkenny C, Browne W, Cuhill IC, Emerson M et al (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLOS Biol 8(6):6–10
Percie N, Hurst VA, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC et al (2020) Europe PMC Funders Group The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Exp Physiol 105(9):1459–66
Walf AA, Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2(2):322–8
Ma JX, Hou J-y, Yan W-w, Sun H-j, Huang Y, Jin S-w, Wang L et al (2012) Protective effect of carnosine on subcortical ischemic vascular dementia in mice. CNS Neurosci Ther 18:745–53
Wietrzych M, Meziane H, Sutter A, Ghyselinck N, Chapman PF, Chambon P et al (2005) Working memory deficits in retinoid X receptor gama-deficient mice. Learn Mem 12:318–26
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13:93–110
Khan MB, Hoda N, Vaibhav K, Giri S, Wang P, Waller JL et al (2015) Remote ischemic postconditioning: harnessing endogenous protection in a murine model of vascular cognitive impairment. Transl Stroke Res 6:69–77
Ahmed, Heba A, Ismael S, Mirzahosseini, Golnoush, Ishrat T (2021) Verapamil prevents development of cognitive impairment in an aged mouse model of sporadic Alzheimer’s disease. Mol Neurobiol. (Mar 11.):pp 14 (Online ahead of print). https://doi.org/10.1007/s12035-021-02350-9
Gorelick P, Nyenhuis D (2013) Understanding and treating vascular cognitive impairment. Continuum 19:425–37
Selnes O, Vinters H (2006) Vascular cognitive impairment. Nat Clin Pract Neurol 2(10):538–47
Fuchtemeier M, Brinckmann MP, Foddis M, Kunz A, Po C, Curato C et al (2015) Vascular change and opposing effects of the angiotensin type 2 receptor in a mouse model of vascular cognitive impairment. J Cereb Blood Flow Metab 35:476–84
Jing F, Mogi M, Sakata A, Iwanami J, Tsukuda K, Ohshima K et al (2012) Direct stimulation of angiotensin II type 2 receptor enhances spatial memory. J Cereb Blood Flow Metab 32:248–55
Iwanami J, Mogi M, Tsukuda K, Jing F, Ohshima K, Wang X et al (2014) Possible synergistic effect of direct angiotensin II type 2 receptor stimulation by compound 21 with memantine on prevention of cognitive decline in type 2 diabetic mice. Eur J Pharmacol 724:9–15
Iwanami J, Mogi M, Tsukuda K, Wang XL, Nakaoka H, Kan-No H et al (2015) Direct angiotensin II type 2 receptor stimulation by compound 21 prevents vascular dementia. J Am Soc Hypertens 9(4):250–6
Oliveira AMM, Hawk JD, Abel T, Havekes R (2010) Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Cold Spring Harbor Lab 215:155–60
Moscardo E, Salvetti B, Becchi S, Bertini G, Fabene PF (2012) The Novel Object Recognition test in rodents: which are the essential methodological aspects? Proc Meas Behav 2012:476–8
Kouwenberg A, Martin GM, Skinner DM, Thorpe CM, Walsh CJ (2012) Spontaneous object recognition in animals: a test of episodic memory. Adv Object Recog Syst 2012:25–40
Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P et al (2013) Object recognition test in mice. Nat Protoc 8(12):2531–7
Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ (2014) Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet 5(APR):1–23
Mateos L, Perez-alvarez MJ, Wandosell F (2016) Angiotensin II type-2 receptor stimulation induces neuronal VEGF synthesis after cerebral ischemia. Biochimica et Biophysica Acta J 1862:1297–308
Shan B-S, Mogi M, Iwanami J, Bai H-Y, Kan-no H, Higaki A, Min L-J et al (2018) Attenuation of stroke damage by angiotensin II type 2 receptor stimulation via peroxisome proliferator-activated receptor-gamma activation. Hypertens Res [Internet]. 41:839–48. Available from: https://doi.org/10.1038/s41440-018-0082-9
Mcfall A, Nicklin SA and Work LM (2020) The counter regulatory axis of the renin angiotensin system in the brain and ischaemic stroke : insight from preclinical stroke studies and therapeutic potential. Cell Signal [Internet]. 76(October):109809. Available from: https://doi.org/10.1016/j.cellsig.2020.109809
Cicognola C, Janelidze S, Hertze J, Zetterberg H, Blennow K (2021) Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimers Res Ther 13(68):1–9
Silva NML, Gonçalves RA, Pascoal TA L-FR et al (2021) Pro-inflammatory interleukin-6 signaling links cognitive impairments and peripheral metabolic alterations in Alzheimer’s disease. Transl Psychiatry [Internet]. 11:Article 251. Available from: https://doi.org/10.1038/s41398-021-01349-z
Acknowledgements
We acknowledge to Dr. Michael P. McDonald at UTHSC for providing a breeding pair for 5XFAD mice.
Funding
This work was supported by startup funds from the UTHSC Department of Anatomy and Neurobiology (TI) and by grants R01-NS097800 (TI), 1R01-AG058467-03 (FL), and 1R01 NS120327-01 (FL). This study was also supported by the National Institute of Health [R01-NS097800 (TI)].
Author information
Authors and Affiliations
Contributions
HA conducted the research, analyzed the data, prepared the figures, and drafted the manuscript. SI performed the stroke surgeries and cerebral blood flow determinations. MS performed the western blots. PD sectioned the brains and prepared the slides for immunostaining. MM and FFL also helped in experimental design and reviewed the manuscript. TI designed and oversaw the whole project including experimental design, randomization, data analysis, and managing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Compliance with Ethical Standards
All procedures performed in studies involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) at UTHSC in full accordance with the ethical guidelines of the National Institutes of Health for the care and use of laboratory animals.
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahmed, H.A., Ismael, S., Salman, M. et al. Direct AT2R Stimulation Slows Post-stroke Cognitive Decline in the 5XFAD Alzheimer’s Disease Mice. Mol Neurobiol 59, 4124–4140 (2022). https://doi.org/10.1007/s12035-022-02839-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02839-x