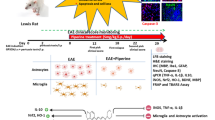
Abstract
Demyelination, inflammation, oxidative injury, and glial activation are the main pathological hallmarks of multiple sclerosis (MS). Vitamins, as essential micronutrients, seem to be crucial in the pathogenesis of MS, and particularly vitamins A and C were found to have a protective role in MS development or progression. In this study, the therapeutic potential of combined therapy of vitamins A and C on progression of experimental autoimmune encephalomyelitis (EAE) and myelin repair mechanisms was examined. EAE, an animal model of MS, was induced in female Lewis rats. The rats were treated with daily intraperitoneal injections of vitamins A and C and their combination. We found that co-supplementation of vitamins A and C mitigated neurological severity and EAE disease progression. Histological study confirmed a significant reduction in demyelination size, inflammation and immune cell infiltration as well as microglia and astrocyte activation following co-administration of vitamins A and C. Co-administration of vitamins A and C also decreased the levels of pro-inflammatory cytokines (TNF-α, IL1β) and iNOS and increased gene expressions of IL-10, Nrf-2, HO-1, and MBP. Combination therapy of vitamins A and C also increased the total antioxidant capacity and decreased levels of oxidative stress markers. Finally, we proved that co-administration of vitamins A and C has anti-apoptotic and neuroprotective impacts in EAE via decreasing caspase-3 and increasing BDNF and NeuN expressing cells. The present study suggests that combined therapy of vitamins A and C may be an effective strategy for development of alternative medicine in boosting myelin repair in demyelinating diseases.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS) which leads to neurological disability in young people [1, 2]. Experimental autoimmune encephalomyelitis (EAE) is a typical animal model for studying MS [3]. Inflammation, demyelination, oligodendrocytes death, gliosis (astrocytes and microglial activation), and axonal loss are the common pathological hallmarks of MS and EAE diseases [4, 5]. The reactivated astrocytes and microglia produce inflammatory mediators such as interleukin-1β (IL-1β), IL-17, IL-6, tumor necrosis factor alpha (TNF-α), and reactive oxygen species (ROS), in MS and EAE [6]. Inflammation and oxidative stress in the CNS have been shown to cause acute neuronal apoptosis [6]. Oxidative stress and inducible nitric oxide synthase (iNOS) are involved in the pathobiology of EAE and MS diseases [7]. The current therapeutic approach for MS patients is based on immunomodulatory drugs [8]; however, their therapeutic efficiency is limited by various side effects and there are no effective treatments to stop the disease progression [9]. There is an improving trend to use complementary and alternative medicine (CAM) in treating MS due to fewer adverse effects, more safety, and preventative impacts [10]. Vitamins, as essential micronutrients, seem to be critical in the pathogenesis of MS, and particularly vitamins A and C were found to have a protective role in MS development or progression [11]. It is also well-established that MS patients have decreased levels of vitamin C and vitamin A [12, 13].
Vitamin C is important for correct functioning of CNS and has been shown to be involved in biosynthesis of collagen and myelin [14]. Vitamin C scavenges ROS to protect cells from oxidative stress injury [15]. It also promotes oligodendrocyte progenitor cell (OPC) differentiation and myelin repair [16]. Vitamin A is essential for diverse physiological functions including development, growth, immune responses and vision [11]. It activates transcription of various genes involved in immune system [17]. Vitamin A acts as an anti-inflammatory agent, reduces synthesis of inflammatory cytokines such as IL-2, IL-6, IL-17 and IL-23 [11, 18, 19], while enhancing anti-inflammatory cytokines like IL-4 and IL-10 in MS and EAE [20]. Vitamin A also suppresses T-cell mediated autoimmunity and the progression of disease in EAE [18, 19].
Both vitamin C and vitamin A supplements have emerged as potential complementary treatments for MS patients. Several studies have assessed the therapeutic impacts of vitamin C [14, 16, 21] and vitamin A [20, 22] supplementations in MS patients and its animal models. Despite some promising results, the effects of combined therapy of vitamin C and vitamin A on neurological symptoms and myelin repair in MS remain to be established. Thus, the aim of this study was to examine the therapeutic efficacy of vitamin A, vitamin C and their combination on EAE symptoms and myelin repair mechanisms. We evaluated expression level of myelin protective factor, inflammatory and anti-inflammatory cytokines and oxidative stress markers by qPCR. Inflammation, immune cell infiltration and glia activation were also assessed. Moreover, to determine the mechanisms of combined therapy of vitamin C and vitamin A, the neuroprotective impacts of treatments were evaluated in EAE model.
Material and Methods
Animals
Adult female Lewis rats (180–200 g) were purchased from Daru-Pakhsh pharmaceutical Company (Tehran, Iran). The animals were housed under standard states with proper temperature (22 ± 2 °C), humidity (60%) and a 12 h light and 12 h dark cycle and allowed to free access to food and water. Guinea pig was obtained from Razi institute (Tehran, Iran) to prepare the Guinea pig spinal cord homogenate (GPSCH) for EAE induction. In this study, all experiments were performed according to the international guidelines for care and use of laboratory animals and approved by the ethical committee of Babol University of Medical Sciences (Ethic code number: IR.TUMS.VCR.REC.1396.3350).
Chemicals
Vitamin A (retinol palmitate, 46,959-U), vitamin C (L-Ascorbic acid analytical standard, 47,863), Complete Freund’s adjuvant (F5881) and Pertussis toxin (P7208) were acquired from Sigma-Aldrich (St. Louis, MO, USA). Anti-myelin basic protein (MBP) (MAB386, Millipore), anti-NeuN (MAB-377-Millipore), anti-Iba1 (019–19,741-Wako), anti-glial fibrillary acidic protein (GFAP) (Z0334-Dako), anti-CD45 (14–0451-82-Invitrogen) and anti-Caspase-3 (9661-Cell signaling) antibodies were used for immunohistochemistry. All data related to primary and secondary antibodies are presented in Table S1.
Experimental Groups and Interventions
Fifty female Lewis rats were randomly divided into six groups (n = 8) as follows: (1) control (untreated rats), (2) EAE (immunized rats), (3) EAE + Veh (immunized rats receiving daily intraperitoneal (i.p.) injections of 200 μl of corn oil and saline (as vitamin A and C solvents), (4) EAE + Vit A (immunized rats receiving daily i.p. injection of 200 μl of Vitamin A ( 7.5 mg/kg/day), (5) EAE + Vit C (immunized rats receiving daily i.p. injection of 200 μl of Vitamin C (200 mg/kg/day) and (6) EAE + Vit A + C (immunized rats receiving daily i.p injections of 200 μl of combination of vitamin A (7.5 mg/kg/day) and vitamin C (200 mg/kg/day). All groups received treatment from day 8 until the day 29 post immunization. All rats presented clinical symptoms at the time of initiating vitamin/vehicle treatment (Supplementary Table S1). Dose selection for vitamin A (12500 IU or 7.5 mg/rat/day) [19, 23] and vitamin C (200 mg/kg/day) [16] was based on prior studies. The vitamins were freshly prepared by dissolving of vitamin C in 0.9% saline and vitamin A in corn oil (final volume 200 μl). For combined therapy of vitamins A and C, each one was dissolved in 100 μl to reach the final volume 200 μl. Eight rats were included in each experimental group, and at the end of experiment, they were randomly divided to two sets (4 rats), one set was used for qPCR and biochemical assays and another used for histological and IHC studies.
EAE Induction
Guinea pig spinal cord homogenate (GPSCH) was prepared as previously described [24, 25]. Each gram of spinal cord was homogenized in 1 ml of distilled water. GPSCH was mixed with complete Freund’s adjuvant (CFA) (1:1, v/v), to acquire the immunization emulsion. CFA contained 5 mg/ml heat inactivated Mycobacterium tuberculosis (M.t., strain H37 RA, Difco, Germany). Immunization of rats was performed with subcutaneous injection of immunization emulsion (0.4 ml) at the base of their tail (0.2 ml each side) on day 0. Intraperitoneal injection of Pertussis toxin (250 ng/0.2 ml of distilled water) was performed on the day of immunization and 48 h later. The rats were daily checked for clinical signs of EAE and scored as shown previously score 0 (no symptoms); score 1 (complete tail paralysis); score 2 (mild paresis of hind limbs); score 3 (complete paralysis of one hind limb); score 4 (bilateral hind limb paralysis); score 5 (complete paralysis (tetraplegia), moribund state, or death) [24]. If the rats were at borderlines of two different scores, it was given an average score. Vitamins supplementation was started at day 8 post immunization until the end of the experiment (29 days post immunization).
Quantitative Real-Time PCR (q-PCR)
Total RNA was extracted from lumbar spinal cord of rats using RNA extraction kit (FABRK001, Yektatajhiz Azma, and Tehran-Iran) as described in the manufacturer’s instruction. RNA concentration was measured by nanodrop spectrophotometer (Thermo scientific, Wilmington, DE, USA). To verify the integrity of isolated RNA, 2 µg of RNA was run on agarose gel. cDNA was synthesized from 1 µg of total RNA with a reverse transcription reagent kit as explained by the manufacturer’s protocol (YT4500, Yektatajhiz Azma kit, Tehran, Iran). Rotor-Gene® Q Real Time PCR System device (Qiagen GmbH, Hilden, Germany) was used to perform q-PCR. Cyber®Green Master Mix (Qiagen, Germany, Cat No. IZ.30–5) was used in the quantitative real-time PCR (q-PCR) protocol. The mRNA expression is relative to the non-immunized control group. The selective forward and reverse primers for inducible nitric oxide synthase (iNOS), Heme oxygenase1(HO-1), nuclear factor erythroid 2 related factor 2(Nrf-2), brain derived neurotrophic factor (BDNF), myelin basic protein (MBP), tumor necrosis factorα (TNF-α), interleukin 1 beta (IL-1β) and interleukin 10 (IL-10), as target genes, and GAPDH gene, as reference gene, were designed (Fazapajooh, Tehran, Iran) the sequences of which are available in Table 1. The mixture of q-PCR reaction comprises 2 μl of cDNA, 10 μl SYBR™ green master mix and 1 μl of each primer (5 µM) in a total volume of 20 μl mixes of each reaction. Forty cycles (94 °C for 30 s, 60 °C for 45 s and 72 °C for 45 s) were applied in PCR reaction. An optimal annealing temperature for each primer was set and shown in Table 2. The cycle threshold (CT) of each gene was normalized to GAPDH (as the reference gene) to diminish the impact of changes in sample and to determine the relative expression of the target genes by the 2-delta-delta CT method [26]. We used 4 rats per experimental group for Quantitative real-time PCR determinations and qPCR was done with duplication in three independent qPCR experiments for each sample.
Biochemical Assays
Ferric Reducing Antioxidant Power (FRAP) Assay
The FRAP assay is a typical method for measuring the antioxidant power of a sample. This assay is performed based on the reduction of the ferric ion (Fe3 +) to the ferrous ion (Fe2 +) as the reaction as a FRAP value, which is tied to a color change by donor electrons in the sample and done as previously explained [27]. Briefly, we mixed 50 μl of sample from each experimental group with 1500 μl of FRAP reagent (10 volumes of 300 mM acetate buffer, pH 3.6 + 1 volume of 10 mM TPTZ in 40 mM HCl + 1 volume of 20 mM iron (III) chloride) and incubated 5 min at 37° C and after the incubation time, the absorbance was read at 593 nm. The FRAP value was calculated as follows and expressed in μmol/l.:
Thiobarbituric Acid Reactive Substances Assay (TBARS)
Malondialdehyde (MDA) is an end-product of lipid peroxidation that is used as an oxidative stress marker. The TBARS assay was carried out as reported previously [28]. TBARS assay involves the reaction of MDA to thiobarbituric acid (TBA) that results in generation of a pink colored dimeric compound. Briefly, we added 100 μl of sample to 20 ml of TBAR solution (15%( v/v) TCA, 0.375% (w/v) TBA and 0.25 M HCL) in acid washed tube and incubated the mix in a boiling water bath for 15 min. Then, we cooled the solution and centrifuged at 1000 g for 10 min. The absorbance of the supernatant was read at 535 nm. MDA value was calculated as previously described and expressed in μmol/l [28].
Histological Analysis
Luxol Fast Blue (LFB) Staining
Luxol fast blue (LFB) staining was performed to assess spinal cord demyelination in the rats [29]. After deep anesthesia, the rats were perfused with PBS (0.1 M), followed by paraformaldehyde 4% (pH 7.4). Then, lumbar part of spinal cord was dissected, post-fixed in PFA 4% overnight, and dehydrated via graded alcohol concentrations. After tissue processing, it was incubated in xylene and embedded in paraffin. Serial coronal sections of 6 μm from the lumbar spinal cord were collected using a microtome (Leica RM2135, Germany). The tissue sections were deparaffinized in xylene, rehydrated in a descending alcohol series, and then stained by 0.1% LFB (Atom Scientific, UK) at 60 °C for 3 h. Thereafter, the sections were treated with 0.1% cresyl violet (Sigma, Germany) for 10 min at room temperature and washed with distilled water. Again, the slides were dehydrated via a series of reducing concentrations of alcohols, cleaned in xylene and mounted by entellan (Merck Chemicals, Germany). A light Olympus Cx-23 microscope was used to observe the slides. Photos from spinal cord were captured with C-P6 OPTIKA camera. The size of demyelination was assessed as the ratio of demyelinated area/total area using Image J software (National Institutes of Health, USA) [29]. The images from coronal serial Sects. (6 μm) were evaluated from 12 various points of the lumbar spinal cord (100 μm interval). The data was averaged 12 sections from a single animal in each group (n = 3); thus, 36 sections were evaluated for each group. We also quantified the optical density (OD) of the spinal cord with the Image J software and corrected against background signal levels. Optical density was represented in arbitrary units. Threshold was set and the percentage of the total area was evaluated. We assessed the mean OD of 9 sections for each rat (n = 3), which was used as the observed value for group comparisons.
Hematoxylin and Eosin (H&E) Staining
To evaluate the inflammatory scores and cell infiltration in spinal cord sections, we used H&E staining. After deparaffinization with xylene, the sections were rehydrated with alcohol and stained with Hematoxilin for 5 min at room temperature. After clearing in xylene, sections were rinsed with PBS several times and counterstained with Eosine for 10 min at room temperature, and then washed and cover slipped. A light Olympus Cx-23 microscope was used to observe the slides and images captured by C-P6 OPTIKA camera for future analysis. The inflammation scores were evaluated in whole spinal cord section as previously explained [30]. The inflammation scores were determined as follows: 0: no inflammation; 1: inflammation only in the perivascular areas and meninges; 2: mild inflammation in less than one third part of the spinal cord Sect. 3: moderate inflammation in more than one third part of the spinal cord section; and 4: deep inflammation throughout the spinal cord section [30].
Immunohistochemistry
The immunostaining was performed as we previously described [26]. After perfusion of rats, the lumbar spinal cord was dissected, post-fixed 2 h in PFA (4%) and incubated in 20% sucrose solution overnight. Then, tissue was submersed in a frozen embedding medium (Thermo Scientific). We acquired 12-µm-thick coronal spinal cord sections using Cryostat (MICROM HM 525, Thermo Scientific) and collected the sections on superfrost plus slides (Thermo Scientific). The sections were then air-dried for 1 h prior to the IHC experiment and rehydrated in PBS three times for 5 min. After several washes with PBS, slides were incubated with blocking buffer (4% bovine serum albumin (BSA) in PBS and 0.3% Triton X-100) for 1 h at room temperature. Slides were then incubated with primary antibodies [anti-CD45 (1/100, MCD4500-Invitrogen), anti-Caspase-3 (1/50, 9661-Cell signaling), Iba1 (1:250, 019–19,741-Wako), GFAP (1:500, Dako, Z0334), and anti-NeuN (1/50, MAB-377-Millipore); antibodies were listed in Supplementary Table S2] overnight at 4 °C. After incubation and several washes with PBS, the sections were incubated with relevant secondary antibodies (Supplementary Table S2) 2 h at room temperature and then washed three times (5 min) with PBS. Finally, to observe the cell nuclei, the sections were incubated with Hoechst (33,342) 5 μg/ml for 30 min and after several washes with PBS, the slides were mounted by Fluor-mounting medium (SouthernBiotech, USA). Images were acquired with a C5 camera and BX-51 fluorescent microscope (Olympus, Japan). IHC data were quantified by Image J software [26]. The number of CD45+, GFAP+, Iba1+, Caspase-3+ and NeuN+ cells was counted and averaged from three sections in each slide and three slides from each animal (150 μm apart) (n = 3 animals/group). Results are represented as the mean number of cells ± SD per square millimeter.
Statistical Analysis
EAE mean clinical scores were evaluated by repeated measures two-way analysis of variance (ANOVA) and the significance was assessed with a Bonferroni post-test. The cumulative scores, peak clinical scores, histological and gene expression data were statistically analyzed by one-way ANOVA followed by Tukey test. Data analysis was carried out using the Graph-Pad prism-6 software (Graph-Pad software, CA, USA).P < 0.05 was considered statistically significant. The result is expressed as means ± SD of three independent experiments.
Results
Co-supplementation of Vitamins A and C Improves Clinical Symptoms and Myelin Repair in EAE Disease
EAE model is a suitable animal model for evaluating the therapeutic potential of different compounds and study pathophysiology of MS disease [24]. Therefore, to evaluate the impacts of combined therapy of vitamins A and C, EAE-induced rats received daily injections of vitamin A (7.5 mg/kg/day), vitamin C (200 mg/kg/day), combined therapy of vitamin A and C (same doses as mentioned above for both) and the vehicle (saline solution with corn oil) from the onset of clinical signs at day 8 post immunization (dpi) until 29 dpi. The neurological symptoms of EAE were then checked as mean clinical score, cumulative score and peak clinical score. EAE and EAE + Veh groups indicated typical EAE disease course, with clinical scores enhancing gradually from 8 to 15 dpi, and the peak neurological score up to level 5 was between 10 and 12 dpi (Fig. 1a–b). The acute phase was followed by a remitting phase during days 15 to 19 after immunization, and second phase of neurological symptoms was started between 20 and 23 dpi (Fig. 1a). In contrast, rats treated with vitamin A or C alone displayed a significant reduction of the mean clinical score, peak clinical score and cumulative score compared to EAE and EAE + Veh groups (Fig. 1a–c, n = 8, F (3, 28) = 84.11, p < 0.0001). Combined therapy of vitamins A and C markedly reduced daily mean clinical score compared with EAE and EAE + Veh groups over the course of disease (Fig. 1a, p < 0.0001, F (3, 28) = 84.11). Combined vitamins A + C treated animals indicated lower peak and cumulative scores compared with EAE, EAE + Veh, EAE + Vit A and EAE + VitC groups, respectively (Fig. 1b–c, n = 8, P < 0.0001, F (3, 28) = 111.9). Relapse of disease signs was also significantly reduced after co-administration of vitamins A + C compared with EAE, EAE + Veh, EAE + Vit A and EAE + Vit C groups, respectively (Fig. 1a, n = 8, p < 0.0001, F (3, 28) = 84.11).
Combined therapy of vitamins A and C attenuates clinical score and severity of EAE. a Daily mean EAE clinical score of Lewis rats (8 rats per group) on EAE, EAE + Veh, EAE + vit A, EAE + vit C and EAE + vits A + C groups. Vitamins A and C were administered through daily intraperitoneally injection at day 8 after EAE induction until day 29. Data are presented as mean ± SD and repeated measure two way ANOVA with Tukey post hoc were done, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. b Comparison of the average of peak score shows that vitamin A, vitamin C and more effectively their combined therapy significantly reduced the peak clinical score compared to EAE and EAE + Veh groups (****P < 0.0001). The peak clinical score in EAE + vit A + C group was significantly less than that in EAE + vit A and EAE + vit C groups (++++p < 0.0001). c Comparison of the sum of EAE scores in different groups indicates that vitamin A, vitamin C and their combined therapy significantly decreased the cumulative scores compared to EAE and EAE + Veh groups (****P < 0.0001). The cumulative scores in EAE + vit A + C group was significantly less than that in EAE + vit A and EAE + vit C groups (++++p < 0.0001). Data is shown as mean ± SD and for mean clinical score: two-way repeated measures ANOVA and for peak and cumulative scores: one-way ANOVA followed by Tukey post hoc, **P < 0.01, ***P < 0.001,****P < 0.0001
To understand the underlying mechanisms of clinical recovery by vitamins A and C and their combined therapy, the lumbar spinal cord of rats was stained with luxol fast blue (LFB) and cresyl violet in different experimental groups (Fig. 2 a–f). Demyelination extension and optical density (OD) of myelin were quantified using Image J software and compared between groups. Severe demyelination was detected in EAE group, and demyelination extension was considerably increased in EAE and EAE + Veh groups compared with control (Fig. 2 a–c, h, n = 3, p < 0.0001, F (4, 10) = 29.52). Histological analysis of LFB stained sections indicated a significant reduction in demyelination extension in EAE + Vit A (P < 0.05), EAE + VitC (P < 0.05) and EAE + Vit A + C (P < 0.001) groups compared with EAE and EAE + Veh groups (Fig. 2 a–f, h, n = 3). Demyelination extension in combined therapy of vitamins A + C was markedly diminished compared to EAE + Vit A and EAE + Vit C groups (P < 0.05). The results were further confirmed by measuring optical density (OD) in LFB stained sections (Fig. 2 g). The OD in EAE and EAE + Veh (P < 0.0001), EAE + Vit A and EAE + Vit C (p < 0.001) groups was noticeably less than that in control group (Fig. 2 a–g, n = 3, p < 0.0001, F (4, 10) = 37.11). Treatment with vitamin A (P < 0.05), Vitamin C (P < 0.05) and combined therapy of vitamins A + C (p < 0.001) reduced demyelination severity, as shown by elevated levels of the OD in both groups compared with EAE and EAE + Veh (Fig. 2 a–g, n = 3). The level of OD in EAE + Vit A + C group was significantly more than that in EAE + Vit A and EAE + Vit C (P < 0.05) groups, confirming that combined therapy with vitamins A + C is more effective than vitamins A and C alone, enhance myelin protection.
Co-supplementation of vitamin A and C decreased the extent of demyelination in EAE rats. a–f Representative micrographs were prepared from the lumbar spinal cord sections of (a) control, (b) EAE, (c) EAE + Veh, (d) EAE + Vit A, (e) EAE + Vit C, and (f) EAE + Vit A + C groups stained with Luxol fast blue respectively. Boxes show magnified image of demyelinated areas, arrows show demyelinated area and dashed line show the magnified images regions. Scale bar for the first images in the row is 200 μm and for magnified images are 100 μm. g Optical density (OD) was calculated to measure the severity of demyelination with internal control in LFB-stained images. Quantitative analysis of OD shows vitamin A, vitamin C and more effectively their combined therapy decreased demyelination severity compared to EAE and EAE + Veh groups (+P < 0.05, ++++P < 0.0001, One way ANOVA, Tukey post-hoc). The enhancement of OD in EAE + Vit A + C group was more than EAE + Vit A and EAE + Vit C groups (#P < 0.05), One way ANOVA, post hoc Tukey was done. h Quantitative analysis of the extent of demyelinated area in different experimental groups in LFB stained images. Vitamin A, vitamin C and their combination significantly reduced the extent of demyelination compared to EAE and EAE + Veh groups (+P < 0.05, +++P < 0.001). The reduction of demyelination extension in EAE + Vit A + C group was more than EAE + Vit A and EAE + Vit C groups (#P < 0.05), One way ANOVA, Tukey post hoc was done. Data is expressed as mean ± SD. n = 3 for histological analysis
We also assessed the level of myelin basic protein (MBP) as a critical factor in the formation and stabilization of the myelin membrane. In our study, the MBP level in EAE and EAE + Veh was markedly lower than that in the control (Fig. 3 a, n = 4, p < 0.01, F (4, 15) = 21.91). The MBP expression level in EAE + Vit A (P < 0.01), EAE + Vit C (P < 0.0001) and EAE + Vit A + C (P < 0.0001) was markedly enhanced compared with EAE and EAE + Veh (Fig. 3 a, n = 4). Moreover, MBP level in EAE + Vit A + C group was considerably more than that in control, EAE + VitA and EAE + VitC groups (Fig. 3 a, P < 0.01, n = 4).
Co-supplementation of vitamin A and C treatment enhances MBP and BDNF gene expression in EAE animals. Lumbar spinal cord of rats was harvested and evaluated for gene expression analysis. The level of a MBP (***P < 0.001) and b BDNF (*P < 0.05) was significantly decreased in EAE and EAE + Veh groups compared with control groups. The data confirmed combined therapy of vitamin A + C significantly increased a MBP (++++P < 0.0001) and b BDNF (++++P < 0.0001) levels compared with EAE and EAE + Veh groups. Moreover, enhancement of MBP (##P < 0.01) and BDNF (#P < 0.05) in EAE + Vit A + C group was significantly more than EAE + Vit A or EAE + Vit C group. One way ANOVA, post hoc Tukey was used. Data is stated as mean ± SD
Brain-derived neurotrophic factor (BDNF) has a key role in re-myelination process [31]. Our data revealed a significant decrease in BDNF level in EAE and EAE + Veh compared with control group (Fig. 3b, P < 0.05). While, the BDNF level was noticeably enhanced in the EAE + Vit A (P < 0.05), EAE + Vit C (P < 0.01) and EAE + Vit A + C (P < 0.0001) groups compared with the EAE and EAE + Veh (Fig. 3b, n = 4, F (4, 15) = 12.29). Co-administration of the vitamin A + C significantly enhanced BDNF expression compared with EAE + VitA and EAE + VitC (P < 0.05) groups and even the control group (P < 0.001). These data suggest that combined therapy of vitamins A and C effectively protect myelin damage in EAE model via increasing MBP level (Fig. 3a) and BDNF (Fig. 3b) as a crucial protein in myelin repair.
Co-administration of Vitamin A and Vitamin C Decreased Cell Infiltration in the Spinal Cords and Modulated the Level of Pro-inflammatory and Anti-inflammatory Cytokines in EAE Model
To study the impact of vitamin A and vitamin C and their combined therapy on inflammatory processes and cell infiltration in EAE model, histopathological and molecular analyses were performed. To assess the infiltration of immune cells into the CNS, lumbar spinal cord sections were stained with Hematoxylin and Eosin (H&E) in different experimental groups. Then, the inflammation scores were assessed as described above [30]. In EAE and EAE + Veh groups, the infiltration of inflammatory cells into the spinal cord at widespread area was markedly enhanced, and inflammatory scores were markedly higher than those in control group (Fig. 4 a–c, g, n = 3, p < 0.0001, F (4, 15) = 41.47). Treatment with vitamin A (P < 0.0001) and vitamin C (P < 0.05) markedly decreased inflammatory scores and infiltrated cells compared with EAE and EAE + Veh groups, while inflammatory scores in both groups were still higher than those in control group (Fig. 4 a–e, g, n = 3, P < 0.01, F (4, 15) = 41.47, P < 0.0001). Co-administration of vitamins A and C more effectively reduced inflammatory scores and infiltrated cells compared to EAE and EAE + Veh (P < 0.0001) and EAE + Vit C (P < 0.0001) groups. To further approve our data, immune staining against CD45 antibody was done in lumbar spinal cord sections. CD45 is known as a leukocyte common antigen [32]. We quantified the CD45 positive cells in the lumbar spinal cord sections. The number of CD45 positive cells in EAE and EAE + Veh (P < 0.0001), EAE + Vit A (P < 0.01), EAE + Vit C (P < 0.0001) and EAE + Vit A + C (P < 0.05) groups was markedly more than that in the control group. However, in vitamin A, vitamin C and vitamin A + C treated groups, the number of CD45 positive cells was markedly decreased compared with EAE and EAE + Veh groups (Fig. 4 a–f, h, n = 3, p < 0.0001, F (4, 15) = 88.39). Moreover, the number of CD45 + cell in EAE + Vit A (P < 0.01) and EAE + Vit A + C (P < 0.01) was significantly diminished compared with EAE + Vit C group. Thus, combined therapy of vitamins A and C negatively regulates the progression of EAE via a decrease in inflammation and demyelination in the treated animals.
Combined therapies of vitamin A and C reduces immune cells infiltration in EAE. a–e Representative micrographs were prepared from the lumbar spinal cord sections of control (a), EAE (b), EAE + Veh)c), EAE + Vit A (d), EAE + Vit C (e), and EAE + Vit A + C (f) groups stained with H&E and CD45 (red) respectively. Boxes show magnified image of selected areas. Scale bars for images of H&E in the first column are 200 μm, and second and third (CD45) column are 50 μm. g Quantitative analysis of the inflammatory score in both white and gray matter area in spinal cord. In each group 20 sections were scored by a pathologist who was blinded to the experimental groups. Higher inflammatory scores were observed in EAE and vehicle groups compared with control (**P < 0.01,****P < 0.0001) and vitamins treated animals (+P < 0.05,++++P < 0.0001). h Quantitative analysis of the number of CD45+ (red) cells in the inflammatory regions of the lumbar spinal cord sections. Vitamin A, C and A + C treatment significantly reduced the number of CD45+ cells in the lumbar spinal cord of EAE rat (++++P < 0.0001). Combination therapy of vitamin A + C more effectively reduced CD45 + cell compared to the treatment with either vitamin alone (##P < 0.01). ***P < 0.001, ****P < 0.0001 for comparison between control, EAE and EAE + Veh groups. n = 4 for histological analysis, One way ANOVA, post hoc Tukey was used. Data is shown as mean ± SD
Furthermore, we quantified the levels of inflammatory cytokines of TNF-α and IL-1β as well as anti-inflammatory cytokine of IL-10 by qPCR. Our results showed that in EAE and EAE + Veh (P < 0.0001), EAE + Vit A (P < 0.01, P < 0.001), EAE + Vit C (P < 0.0001) and EAE + Vit A + C (P < 0.05, P < 0.01) groups, TNF-α and IL-1β levels were significantly augmented compared with control group, respectively (Fig. 5 a–b, n = 4, FTNF-α (4, 15) = 31.44, FIL-1β (4, 15) = 56.02). Daily treatment with vitamin A (P < 0.0001) and vitamin C (P < 0.01, P < 0.001) decreased TNF-α and IL-1β levels compared with EAE and EAE + Veh groups, respectively (Fig. 5 a–b, n = 4). However, co-administration of vitamins A and C effectively reduced TNF-α and IL-1β levels compared with EAE and EAE + Veh (P < 0.0001) and EAE + Vit C (P < 0.05, P < 0.01), respectively. In EAE + Vit A and EAE + Vit A + C groups, IL-10 level was higher than that in control group (Fig. 5 c, p < 0.0001, n = 4, F (4, 15) = 52.12) and IL-10 level in EAE + Vit A (p < 0.0001), EAE + Vit C (p < 0.01) and EAE + Vit A + C (p < 0.0001) groups was higher than that in EAE and EAE + Veh groups. Moreover, the enhancement of IL10 expression in EAE + Vit A + C (p < 0.001) and EAE + Vit A (p < 0.01) was more than EAE + Vit C group. Therefore, co-supplementation of vitamins A and C has an immunomodulatory impact in EAE model via eliminating inflammatory cytokines and increasing anti-inflammatory cytokines.
Co-supplementation of vitamin A and C suppressed the expression level of the inflammatory cytokines and enhanced IL-10 in EAE. Lumbar spinal cord of rats was harvested and evaluated for the expression of a TNF-α, b IL-1β and c IL-10 by real time-PCR. a TNF-α and b IL-1β expression level considerably increased in EAE and EAE + Veh groups compared to control (****P < 0.0001). The level of TNF-α and IL-1β gene expression in EAE + VitA, EAE + VitC and EAE + Vit A + C groups significantly diminished compared with EAE and EAE + Veh groups (++++P < 0.0001, ++P < 0.01, +P < 0.05). Also in EAE + vitamin A + C groups the expression levels of TNF-α (##P < 0.01) and IL-1β (#P < 0.05) was considerably decreased compared with EAE + Vit C group. However, the level of TNF-α (*P < 0.05,**P < 0.01, ****P < 0.0001) and IL-1β (**P < 0.01, ***P < 0.001,****P < 0.0001) gene expression in Vitamins A, vitamin C and their combination A + C treated animals was still more than that control. c The expression level of IL-10 as an anti-inflammatory cytokine in vitamin A, C and A + C treated groups was significantly higher than that in EAE, EAE + Veh groups (++P < 0.01,++++p < 0.0001) and control groups(****P < 0.0001). The level of IL-10 in EAE + Vit A + C (###P < 0.001) was significantly more than that in EAE + Vit C and in EAE + Vit A (###P < 0.001) was less than that in EAE + Vit C. n = 4 for gene expression analysis, One way ANOVA, post hoc Tukey was used. Data is shown as mean ± SD
Co-supplementation of Vitamins A and C Decreased Astrocytes and Microglial Activation in EAE Model
Microglia and astrocytes activation promotes pro-inflammatory cytokines production and inflammatory infiltration into the CNS of EAE animals [33]. Consequently, we investigated the effect of vitamin A and C and their combined therapy on microglia and astrocytes activation in EAE animals. We conducted immunostaining against glial fibrillary acidic protein (GFAP; an astrocyte marker) and ionized-calcium binding adaptor protein (Iba-1; a microglia marker) in lumbar spinal cord sections (Fig. 6 a–f). Our data revealed a significant increase in the number GFAP positive cells in EAE and EAE + Veh (P < 0.0001) and EAE + Vit C (P < 0.01) groups compared with controls in both white matter (WM) and gray matter (GM) (Fig. 6 a–f, g). In vitamin A (P < 0.001), vitamin C (P < 0.05) and vitamin A + C (P < 0.001) treated groups the number GFAP positive cells was markedly reduced compared with EAE and EAE + Veh groups in WM and GM (Fig. 6 a–h, n = 3, F (4, 10) = 25.12). Moreover, Co-administration of the vitamin A + C significantly reduced the number of GFAP + cell in GM compared with EAE + Vit C group (P < 0.05). The quantification of Iba-1 positive cell number revealed a significant increase in the number of Iba-1 + cells in EAE and EAE + Veh (P < 0.0001) and EAE + Vit C (P < 0.01) groups compared with control group. Following vitamins treatment, the number of Iba-1 positive cells was markedly reduced in EAE + Vit A (P < 0.001), EAE + Vit C (P < 0.05) and EAE + Vit A + C (P < 0.001) groups compared with EAE group in WM and in GM (P < 0.0001) (Fig. 6 a–f, I,j, n = 3, F (4, 10) = 23.78). These data confirmed that treatment with vitamins A and C and their combined therapy inhibit microglia/astrocytes reactivation during EAE disease.
Combined therapies of vitamin A and C efficiently inhibited microglia and astrocyte activation. Immunostaining against GFAP (red) as an astrocyte marker, Iba1 (green) as a microglia and DAPI (blue) as a nucleus marker was performed on a lumbar spinal cord sections from different experimental groups including: (a) control, (b) EAE, EAE + Veh)c), EAE + Vit A (d), EAE + Vit C (e) and EAE + Vit A + C (f). Boxes show magnified image of selected areas from white (WM) and gray (GM) mater. Scale bar for images from the first and third columns are 200 μm, and for second and fourth columns are 50 μm. g Quantification of GFAP+ cell number in white matter (WM) showed that the number of GFAP+ cells in EAE, EAE + Veh and EAE + Vit C groups was significantly more than that in control (****P < 0.0001, **P < 0.01). Treatment with Vitamin A (+++p < 0.001), vitamin C (+p < 0.05) and vitamins A + C (+++p < 0.001) significantly decreased the number of GFAP+ cells compared to EAE group (One way ANOVA, post hoc Tukey, n = 3). h Quantification of GFAP+ cell number in gray matter (GM) showed that the number of GFAP+ cells in EAE, EAE + Veh and EAE + Vit C groups was significantly increased compared with control (****P < 0.0001, **P < 0.01). The number of GFAP+ cells in Vitamin A (+++p < 0.001), vitamin C (+p < 0.05) and vitamins A + C (+++p < 0.001) treated groups significantly decreased compared with EAE and EAE + Veh groups. The number of GFAP+ cells in EAE + Vit A + C was significantly less than that in EAE + Vit C (#P < 0.05, One way ANOVA, post hoc Tukey, n = 3). i Quantitative analysis of the number of Iba1+ cells (microglia) in WM showed the number of Iba1+ cells was considerably increased in EAE, EAE + Veh and EAE + Vit C compared to control group, respectively (****P < 0.0001, **P < 0.01). Treatment with Vitamin A (+++p < 0.001), vitamin C (+p < 0.05) and vitamins A + C (+++p < 0.001) significantly reduced the number of Iba1+ cells compared to EAE group. j Quantification of Iba1+ cell number in GM showed that the number of GFAP+ cells in EAE, EAE + Veh and EAE + Vit C groups was significantly increased compared with control (****P < 0.0001). The number of Iba1+ cells in Vitamin A, vitamin C and vitamins A + C (++++p < 0.0001) treated groups significantly decreased compared with EAE and EAE + Veh groups. Data is displayed as mean ± SD. One way ANOVA, post hoc Tukey was used, n = 3
Combined Therapy of Vitamins A and Vitamin C Reduced Oxidative Stress and Improved Antioxidant Capacity in the EAE Model
To understand the mechanisms underlying therapeutic impacts of vitamin A, vitamin C and their co-administration in EAE model, the levels of iNOS, Nrf2 and HO-1 in all experimental groups was assessed by q-PCR. The iNOS is known as one of the major oxidative-related enzymes involved in cytotoxicity, inflammation and oxidative stress damage [34]. The iNOS level in EAE and EAE + Veh (p < 0.0001), EAE + Vit A (p < 0.001) and EAE + Vit C (p < 0.05) groups was significantly higher than that in control group (Fig. 7 a). The level of iNOS in EAE + Vit A (p < 0.01), EAE + Vit C (p < 0.0001) and EAE + Vit A + C (p < 0.0001) groups was markedly reduced compared with EAE and EAE + Veh groups. Moreover, the reduction in iNOS expression in EAE + Vit A + C (p < 0.01) group was greater than that in EAE + Vit A (Fig. 7 a, n = 4, F (4, 15) = 30.8). Nrf2 is an important transcription factor which is involved in cellular antioxidant defenses against oxidative stress [35]. We found that there was no notable difference in Nrf2 level between EAE, EAE + Veh and control groups. However, the expression level of Nrf2 in EAE + Vit C (p < 0.01) and EAE + Vit A + C (p < 0.001) groups was markedly higher than that in EAE and EAE + Veh groups. Moreover, the expression of Nrf2 in EAE + Vit A + C group was more than that in EAE + Vit A (p < 0.01). In all treated groups, the Nrf2 level was higher than that in control group (Fig. 7 b, n = 4, F (4, 15) = 21.89,). HO-1, the inducible isoform of heme oxygenase, is a cyto-protective enzyme that has anti-inflammatory and anti-oxidant impacts [36]. The evaluation of HO-1 gene expression in lumbar spinal sections showed a significant increase in the HO-1 level in EAE + Vit C (p < 0.05) and EAE + Vit A + C (p < 0.001) groups compared with EAE and EAE + Veh groups. Moreover, the expression level of HO-1 in EAE + Vit A + C group was higher than that in EAE + Vit A group (p < 0.05). The level of HO-1 in all treated groups was also higher than that in the control group (Fig. 7 c, p < 0.001, P < 0.0001, n = 4, F (4, 15) = 27.72).
Co-supplementation of vitamin A and C modulated the level of oxidant and antioxidant mediators in EAE rats. Lumbar part of spinal cord was harvested and evaluated for the gene expression analysis of iNOS (a) Nrf-2 (b) and HO-1 (c). a The results indicated that the expression level of iNOS in EAE (****P < 0.0001), EAE + Veh (****P < 0.0001), EAE + Vit A (***P < 0.001) and EAE + Vit C (*P < 0.05) groups was significantly more than control group. Treatment with Vitamin A (++p < 0.01), vitamin C (++++p < 0.0001) and vitamins A + C (++++p < 0.0001) significantly decreased the level of iNOS compared to EAE and EAE + Veh groups. The level of iNOS in EAE + Vit A + C group was significantly less than that in EAE + Vit A group (##p < 0.01, One way ANOVA, post hoc Tukey, n = 4). b The level of Nrf-2 in Vitamin A, vitamin C and vitamins A + C treated groups was significantly higher than those in EAE, EAE + Veh (+++p < 0.001,++++P < 0.0001) and control groups (**P < 0.01, ****P < 0.0001, One way ANOVA, post hoc Tukey, n = 4). c The level of HO-1 was significantly increased in Vitamin A, vitamin C and vitamins A + C treated groups compared to EAE, EAE + Veh (+p < 0.05,+++P < 0.001) and control (***P < 0.001, ****P < 0.0001) groups. Moreover, the level of HO-1 in EAE + Vit A + C group was significantly higher than that in EAE + Vit A group (#p < 0.05, One way ANOVA, post hoc Tukey, n = 4). d The level of MDA (as an oxidative stress marker) was measured by TBARS assay. The level of MDA in EAE and EAE + Veh groups was considerably higher than that in control (**P < 0.01, *P < 0.05). However, treatment with vitamin C and vitamins A + C significantly decreased the MDA level compared to EAE and EAE + Veh groups (++P < 0.01,+++p < 0.001, One way ANOVA, post hoc Tukey, n = 4). e we measured the total antioxidant capacity of the lumbar spinal cord with FRAP assay. The level of FRAP in vitamins A + C treated groups was significantly higher than those in EAE and EAE + Veh groups (++p < 0.01) and control (***P < 0.001) and EAE + Vit A (#P < 0.05) groups and in vitamin C group was more than that in control (**P < 0.01). Data is presented as mean ± SD. One way ANOVA, post hoc Tukey was used, n = 4
To further approve the protective impact of vitamin A and vitamin C and their combination on oxidative damage, the malondialdehyde (MDA) (TBARS assay) and total antioxidant capacity (FRAP assay) were measured in the lumbar spinal cord tissue. MDA is the end-product of lipid peroxidation and is considered as an oxidative stress marker. We found that MDA level in EAE and EAE + Veh groups was higher than that in control group (P < 0.001). The MDA level in EAE + Vit C and EAE + Vit A + C groups was markedly reduced compared with EAE and EAE + Veh groups (p < 0.0001). Combined therapy of vitamins A + C more effectively reduced MDA level compared with EAE + Vit A group (P < 0.001) (Fig. 7 d, n = 4, F (4, 15) = 20.16). The ferric reducing ability of plasma (FRAP) is a novel procedure for evaluating antioxidant activity [27]. Our data indicated that EAE + Vit A + C group had profound reducing power capacity compared with EAE and EAE + Veh (P < 0.01), EAE + Vit A (p < 0.05) and control groups (p < 0.001) (Fig. 7 e, n = 4, F (4, 15) = 11.06). Thus, combination therapy with vitamin A and C could reduce oxidative stress and enhance total antioxidant capacity of the CNS in EAE model.
Co-administration of Vitamin A and Vitamin C Decreased Caspase-3 Expression and Neuronal Loss in the EAE Model
To assay the neuroprotective impact of vitamin A, vitamin C and their co-administration, immunostaining against neuronal nuclear protein (NeuN; a marker of mature neurons) and caspase-3 (a marker of apoptosis) was performed in the lumbar spinal sections (Fig. 8 a–f). We quantified the number of NeuN + and caspase3 + cells in the lumbar spinal sections. We found that the number of NeuN + cells in EAE and EAE + Veh (p < 0.001), EAE + Vit A (p < 0.01) and EAE + Vit C (p < 0.01) groups was markedly lower than that in control group. Co-administration of vitamins A + C significantly enhanced the number of NeuN + cells compared with EAE and EAE + Veh (p < 0.001) and EAE + Vit A (p < 0.05) groups (Fig. 8 a–g, n = 3, P < 0.05, F (4, 10) = 21.6). Caspase-3 is a major mediator of apoptosis in neuronal cells [37]. We demonstrated that the number of caspase-3 positive cells in EAE and EAE + Veh (p < 0.0001), EAE + Vit A (p < 0.01) and EAE + Vit C (p < 0.001) groups was significantly higher than that in control group. Treatment with vitamin A, vitamin C and their co-administration reduced the number of capase3 + cells in EAE + Vit A (p < 0.05) and EAE + Vit C (p < 0.05) and EAE + Vit A + C (p < 0.0001) groups compared to EAE and EAE + Veh groups. Combined therapy of vitamin A + C more effectively reduced caspase3 + cell number compared to EAE + Vit A group (p < 0.05) (Fig. 8 a–f, h, n = 3, F (4, 10) = 31.81). Thus, combination therapy with vitamin A and C reduced apoptosis through inhibiting the caspase-3 and increased the survival of neuronal cells in EAE animals.
Combination therapies with vitamin A and C inhibited apoptosis in EAE model. Representative micrographs were prepared from the lumbar spinal cord sections of all experimental groups (a) control, (b) EAE, EAE + Veh)c), EAE + Vit A (d), EAE + Vit C (e) and EAE + Vit A + C (f) stained with NeuN as mature neuron marker (green) and caspase-3 as marker of apoptosis (red) and DAPI (blue) as a nucleus marker. Boxes show magnified image of selected areas. Scale bar for images from the first column is 200 μm and for second and third column is 50 μm. g Quantitative analysis of the number of NeuN+ cells (neuron) in the lumbar spinal cord sections. The number of NeuN+ cells was considerably decreased in EAE (***P < 0.001), EAE + Veh (***P < 0.001), EAE + Vit A (**P < 0.01), EAE + Vit C (**P < 0.01) compared with control group. However, the number of NeuN+ cells in EAE + Vit A + C group was significantly higher than that in EAE and EAE + Veh (+++P < 0.001), EAE + Vit A and EAE + Vit C (#P < 0.05) groups. h Quantification of the number of caspase-3+ cells in the lumbar spinal cord sections indicated that it significantly increased in EAE and EAE + Veh (****P < 0.0001), EAE + Vit A (**P < 0.01) and EAE + Vit C (***P < 0.001) compared with control group. Vitamin A (+p < 0.05), vitamin C (+p < 0.05) and combination of vitamins A + C (++++p < 0.0001) effectively reduced caspase-3 expression level compared with EAE and EAE + Veh groups. The number of caspase-3+ cells in EAE + Vit A + C groups was significantly less than that EAE + Vit A (##P < 0.01) and EAE + Vit C (#P < 0.05) groups. Data is shown as mean ± SD. One way ANOVA, post hoc Tukey was used, n = 3
Discussion
The present study showed for the first time that the co-administration of vitamin A and vitamin C provides synergistic benefits in EAE model. We found that combined therapy of vitamins A and C decreased clinical symptoms of EAE and spinal cord demyelination. Subsequently, we observed a decrease in infiltration of immune cells, activation of microglia and astrocytes and inflammatory mediators (TNF-α, IL-1β and iNOS). We also observed an increase in anti-inflammatory cytokine of IL-10 in the spinal cord of EAE rats. The level of MDA as an oxidative stress marker was decreased, while the total antioxidant capacity and Nrf2 and HO-1 levels as antioxidant mediators were increased. Moreover, co-administration of vitamins A and C enhanced the expression of BDNF, MBP and NeuN + cells, while reduced the level of caspase-3 in EAE model. Thus, co-supplementation with vitamins A and C has a neuroprotective and therapeutic potential in EAE through modulating inflammation and oxidative stress.
In this study, we used intrapritoeanal (i.p.) injection for vitamin supplementation in rats based on several researches that used i.p. injection for vitamin treatment [16, 38]. The administration of drugs to animals through daily i.p. injections results in similar therapeutic responses compared to oral gavage [39]. Previous reports indicated that vitamin A compounds delayed the onset and development of EAE in different animal models [19, 20]. It was also shown that vitamin A alone or as complementary medicine has a therapeutic impact on MS patients [11, 22]. Vitamin C improves impaired memory in local model of demyelination in the hippocampal rat [21]. Moreover, vitamin C was found to be effective to improve EAE symptoms and the protective properties of vitamin C in EAE may be related to its ability to neutralize oxidative stress [40]. In line with these researches, we found that co-administration of vitamins A and C more effectively attenuates EAE disease severity compared with vitamin C or vitamin A alone, as proved by histological analyses of EAE lesions. Consequently, we checked cell infiltration, glia activation, the trends of pro-inflammatory and anti-inflammatory cytokines, oxidative stress markers, apoptosis, neuronal survival and myelin repair to explore the underlying mechanisms of positive impacts of vitamin A, vitamin C and their combination in EAE model.
In the early phase of MS disease and EAE, immune cells activation and infiltration into the CNS result in disease onset and progression [41]. Thus, inhibition of the early activated immune system is essential in the treatment of MS disease and EAE. In this study, we found that co-administration of vitamins A and C significantly decreased inflammatory scores and the number of infiltrated cells (CD45 + cells) in the spinal cord of EAE rats. CD45 (as a common antigen) is expressed on all leucocytes, and which plays a crucial role in their function [32]. The immune cells activation in the early phase of MS disease results in the production of TNF-α, IL-1β and IFN-γ, which are crucial for MS and EAE diseases relapse [41]. However, anti-inflammatory cytokines such as IL-4, IL-10 and TGF-β, seem to be involved in disease remission [41]. The immunomodulatory and anti-inflammatory impacts of vitamin A in several inflammatory neurological diseases such as EAE have been reported [19, 20]. Vitamin C takes a role in immune defense by supporting diverse cellular actions of both the innate and adaptive immune systems [42]. Vitamin C also increases T-reg cells differentiation and modulates secretion of TNF-α, IL-1β, IL-6, IFN-γ, IL-10, and type I interferons [43]. Our results indicated that combination therapy with vitamin A and vitamin C efficiently decreased the level of pro-inflammatory cytokines (TNF-α and IL-1β) and increased IL-10 level in the EAE rats. In this respect, retinoic acid (RA) was found to increase the levels of IL-4, IL-13 and IL-5 and reduce TNF-α, IFN-γ, IL-12 and IL-2 levels [44]. Synthetic retinoid (AM80) protected animals from EAE induction due to reduced IL-17 and IFN-ץ production [19, 45]. It was also demonstrated that anti-inflammatory activity of vitamin A in MS patients was associated with an increase in the ratio of IL-10/TNF-α [20]. Some studies demonstrated that IL-10 as a multifunctional cytokine can suppress the activity of Th-1 cells, NF-κB and inflammatory macrophages [46, 47]. Moreover, IL-10 is essential for recovery of EAE and the lack of IL-10 has been shown to increase severity of EAE [48]. An in vitro study showed that incubation of vitamin C with peripheral blood lymphocytes reduced lipopolysaccharide (LPS)-induced production of the pro-inflammatory cytokines TNF-α and IFN-γ, and enhanced anti-inflammatory IL-10 production [49]. Although vitamin C is an effective antioxidant, it is possible that its action as a cofactor for several biosynthetic and gene regulatory enzymes contribute to its immune-modulating impacts [43]. Therefore, co-administration of vitamins A and C could modulate pro-inflammatory and anti-inflammatory cytokines and cause an anti-inflammatory impact in EAE model.
Astrocytes and microglia are implicated in the pathology of MS and EAE and their reactivation is critical for development and progression of the diseases. Ablation of microglia inhibits EAE development [50]. Activated astrocytes and microglia release cytokines and other inflammatory factors, which in turn induce inflammation, demyelination, axonal loss and gliosis [50]. Hence, the modulation of microglial and astrocytes activation may be a promising therapeutic approach for MS [51]. Consistent with these reports, we found that the expression of GFAP and Iba1 extremely increased in EAE group, while treatment with vitamin A and vitamin C alone and in combination significantly suppressed the activity of microglial and astrocyte in EAE. In this respect, several studies reported that inhibition of microglial activation attenuated clinical symptoms, inflammation, and demyelination in relapsing–remitting EAE model [52, 53]. It was also reported that vitamin A ameliorates anti-inflammatory impacts of astrocytes, inhibits immune responses and improves myelin repair process in MS [22, 54]. The protective impact of vitamin C on the ethanol-induced toxicity in human glial cells was also reported [55]. It has been found that vitamin C treatment markedly inhibits the activation of microglia and astrocytes and decreases Bax/Bcl-2 ratio, and ethanol-induced caspase-3 and caspase-9 activation in rat brain [56]. Our results are in line with aforementioned studies and propose that co-administration of vitamins A and C has a therapeutic potential in EAE model via inhibiting astrocyte and microglial activation.
Oxidative stress is supposed to play a crucial role in the pathology of EAE and MS [6]. Malondialdehyde (MDA) is a main mediator of oxidative stress. The iNOS is involved in inflammation, oxidative injury and neuronal death in numerous neurological diseases [7]. Several studies demonstrated an increase in the levels of iNOS and MDA in demyelinating diseases [24, 57]. We found that co-supplementation of vitamins A and C efficiently reduced the levels of MDA and iNOS and increased Nrf2 and HO-1 levels in EAE model. Nrf2 as a transcription factor regulates various antioxidant genes including HO-1, a downstream target of the Nrf2-ARE (antioxidant responsive element) pathway [35]. It was also found that Nrf2 suppresses NF-kB activation [58]. HO-1 decreases the oxidative damage and controls apoptosis and inflammation [36]. The pervious study has reported that HO-1 overexpression improved the clinical disability of EAE [59], and induction of EAE in HO-1 knockout mice caused a more severe EAE [60]. Several lines of evidence revealed that vitamin C inhibits oxidative stress through the Nrf2/HO-1 pathway in different pathological conditions [61, 62]. Together, we propose that Nrf2-HO-1 pathway is a potential target of vitamin A and vitamin C for their protective impact in EAE model.
Axonal damage and neuronal loss are the most important factors driving of disability in MS and EAE. Neural apoptosis is a key pathological finding in MS and EAE [63]. Caspase-3 is involved in apoptosis [37] and its activation induces neuronal apoptosis during EAE disease [63]. Here, we quantified the number of caspase-3 + and NeuN + cells in the rat spinal cord in all groups. NeuN is a marker of mature neuron and its quantification can be considered as an indicator of neural health [64]. Consistent with previous studies we found a considerable enhancement in the number of caspase-3 + cells and a decrease in NeuN + cells in EAE rats. Our data proved that combined therapy of vitamin A and vitamin C effectively decreased caspase-3 level and enhanced neuronal health in EAE model. Vitamin C inhibits apoptosis through modulating caspase-3 in oxidative stress and pathological conditions [65]. Vitamin C has a neuroprotective effect and can alleviate ethanol-induced oxidative stress, neuro-inflammation and apoptotic neuronal loss [56]. The protective effect of vitamin A on reproductive system was associated with its anti-apoptotic properties through inhibition of caspase-3 and Fas expression level [66]. Thus, we can suggest that co-administration of vitamin A and C has neuroprotective effect in EAE model.
Brain derived neurotrophic factor (BDNF) has an essential role in the CNS development and myelination [31]. Several lines of evidence demonstrated that BDNF is an essential factor to increase re-myelination in several animal models of central demyelination [31, 67]. BDNF enhanced Bcl-2 and inhibited the caspase-3, thus having an anti-apoptotic impact on neurons [68]. The expression level of MBP can also be regulated by BDNF [67]. BDNF enhances OPC proliferation, migration and differentiation in the demyelination condition [69]. Moreover, BDNF treatment alleviated the pathological severity of EAE clinical scores [68]. In agreement with these reports, we found that MBP and BDNF levels were markedly reduced in EAE group. Treatment with vitamin A, vitamin C and their combination increased the levels of MBP and BDNF in EAE model. Recently it was proved that vitamin C enhances OPC differentiation and facilitates the myelination in OPC-neuron co-culture [16]. Vitamin C also promotes in vivo re-myelination and provides remarkable therapeutic impact in animal model of demyelination. Our findings suggest that combined therapy of vitamins A and C protect myelin damage via increasing BDNF and MBP levels in the CNS and causes anti-inflammatory, antioxidant and neuroprotective impacts in the EAE model. Altogether, we suggest that vitamin A mostly acts through its immunomodulatory and anti-inflammatory effects to inhibit EAE disease progression. Vitamin C commonly reduces the clinical course of EAE through its antioxidant and neuroprotective properties. Thus, combination of vitamin A and C as EAE treatment effectively boosts anti-inflammatory, antioxidant and neuroprotective system in the body and improves EAE symptoms.
Conclusion
Our study revealed new insights into the efficiency of combined therapy of vitamins A and C on neuroinflammation and re-myelination in experimental models of MS. Thus, this strategy may be effective for further improvements in MS treatment and development of alternative medicine to boost myelin repair in demyelinating diseases. The main aim of combined therapy is that the combination suppresses the disease and improves clinical consequences better than the treatment with either drug alone. Since vitamin A and vitamin C are widely used as dietary supplements, and have been approved by FDA for clinical uses, we suggest a routine co-supplementation of these two vitamins (A + C) in MS patients. Further research is required to evaluate the potential benefits of earlier, more frequent, and longer administration of combination therapy of vitamins A and C in MS patients.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rezapour A, Almasian Kia A, Goodarzi S, Hasoumi M, NouraeiMotlagh S, Vahedi S (2017) The impact of disease characteristics on multiple sclerosis patients’ quality of life. Epidemiol Health 39:e2017008
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372:1502–1517
Burrows DJ, McGown A, Jain SA, De Felice M, Ramesh TM, Sharrack B, Majid A (2019) Animal models of multiple sclerosis: from rodents to zebrafish. Mult Scler 25:306–324
Constantinescu CS, Farooqi N, O’Brien K, Gran B (2011) Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 164:1079–1106
Lassmann H, Bradl M (2017) Multiple sclerosis: experimental models and reality. Acta Neuropathol 133:223–244
Karpus WJ (2020) Cytokines and chemokines in the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol 204:316–326
Yuste JE, Tarragon E, Campuzano CM, Ros-Bernal F (2015) Implications of glial nitric oxide in neurodegenerative diseases. Front Cell Neurosci 9:322
Goldschmidt C, McGinley MP (2021) Advances in the treatment of multiple sclerosis. Neurol Clin 39:21–33
Foti Cuzzola V, Palella E, Celi D, Barresi M, Giacoppo S, Bramanti P, Marino S (2012) Pharmacogenomic update on multiple sclerosis: a focus on actual and new therapeutic strategies. Pharmacogenomics J 12:453–461
Namjooyan F, Ghanavati R, Majdinasab N, Jokari S, Janbozorgi M (2014) Uses of complementary and alternative medicine in multiple sclerosis. J Tradit Complement Med 4:145–152
Khosravi-Largani M, Pourvali-Talatappeh P, Rousta AM, Karimi-Kivi M, Noroozi E, Mahjoob A, Asaadi Y, Shahmohammadi A, Sadeghi S, Shakeri S et al (2018) A review on potential roles of vitamins in incidence, progression, and improvement of multiple sclerosis. eNeurologicalSci 10:37–44
Besler HT, Comoglu S, Okcu Z (2002) Serum levels of antioxidant vitamins and lipid peroxidation in multiple sclerosis. Nutr Neurosci 5:215–220
Loken-Amsrud KI, Myhr KM, Bakke SJ, Beiske AG, Bjerve KS, Bjornara BT, Hovdal H, Lilleas F, Midgard R, Pedersen T et al (2013) Retinol levels are associated with magnetic resonance imaging outcomes in multiple sclerosis. Mult Scler 19:451–457
Kocot J, Luchowska-Kocot D, Kielczykowska M, Musik I, Kurzepa J: Does Vitamin C influence neurodegenerative diseases and psychiatric disorders? Nutrients 2017, 9.
Du J, Cullen JJ, Buettner GR (2012) Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta 1826:443–457
Guo YE, Suo N, Cui X, Yuan Q, Xie X (2018) Vitamin C promotes oligodendrocytes generation and remyelination. Glia 66:1302–1316
Hall JA, Grainger JR, Spencer SP, Belkaid Y (2011) The role of retinoic acid in tolerance and immunity. Immunity 35:13–22
Huang JK, Jarjour AA, Nait Oumesmar B, Kerninon C, Williams A, Krezel W, Kagechika H, Bauer J, Zhao C, Baron-Van Evercooren A et al (2011) Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci 14:45–53
Klemann C, Raveney BJ, Klemann AK, Ozawa T, von Horsten S, Shudo K, Oki S, Yamamura T (2009) Synthetic retinoid AM80 inhibits Th17 cells and ameliorates experimental autoimmune encephalomyelitis. Am J Pathol 174:2234–2245
Eriksen AB, Berge T, Gustavsen MW, Leikfoss IS, Bos SD, Spurkland A, Harbo HF, Blomhoff HK (2015) Retinoic acid enhances the levels of IL-10 in TLR-stimulated B cells from patients with relapsing-remitting multiple sclerosis. J Neuroimmunol 278:11–18
Babri S, Mehrvash F, Mohaddes G, Hatami H, Mirzaie F (2015) Effect of intrahippocampal administration of vitamin C and progesterone on learning in a model of multiple sclerosis in rats. Adv Pharm Bull 5:83–87
Jafarirad S, Siassi F, Harirchian MH, Sahraian MA, Eshraghian MR, Shokri F, Amani R, Bitarafan S, Mozafari S, Saboor-Yaraghi A (2012) The effect of vitamin A supplementation on stimulated T-cell proliferation with myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. J Neurosci Rural Pract 3:294–298
Kang SG, Wang C, Matsumoto S, Kim CH (2009) High and low vitamin A therapies induce distinct FoxP3+ T-cell subsets and effectively control intestinal inflammation. Gastroenterology 137(1391–1402):e1391-1396
Mohajeri M, Sadeghizadeh M, Najafi F, Javan M (2015) Polymerized nano-curcumin attenuates neurological symptoms in EAE model of multiple sclerosis through down regulation of inflammatory and oxidative processes and enhancing neuroprotection and myelin repair. Neuropharmacology 99:156–167
Beeton C, Garcia A, Chandy KG: Induction and clinical scoring of chronic-relapsing experimental autoimmune encephalomyelitis. JoVE (Journal of Visualized Experiments) 2007:e224.
Ebrahim-Tabar F, Nazari A, Pouramir M, Ashrafpour M, Pourabdolhossein F (2020) Arbutin improves functional recovery and attenuates glial activation in lysolecethin-induced demyelination model in rat optic chiasm. Mol Neurobiol 57:3228–3242
Benzie I, Devaki M: The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: concepts, procedures, limitations and applications. Measurement of antioxidant activity & capacity: recent trends and applications Wiley, New York 2018:77–106.
Ghani MA, Barril C, Bedgood DR Jr, Prenzler PD (2017) Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem 230:195–207
Mousavi Majd A, Ebrahim Tabar F, Afghani A, Ashrafpour S, Dehghan S, Gol M, Ashrafpour M, Pourabdolhossein F (2018) Inhibition of GABA A receptor improved spatial memory impairment in the local model of demyelination in rat hippocampus. Behav Brain Res 336:111–121
Seno A, Maruhashi T, Kaifu T, Yabe R, Fujikado N, Ma G, Ikarashi T, Kakuta S, Iwakura Y (2015) Exacerbation of experimental autoimmune encephalomyelitis in mice deficient for DCIR, an inhibitory C-type lectin receptor. Exp Anim 64:109–119
Linker R, Gold R, Luhder F: Function of neurotrophic factors beyond the nervous system: inflammation and autoimmune demyelination. Critical Reviews™ in Immunology 2009, 29.
Altin JG, Sloan EK (1997) The role of CD45 and CD45-associated molecules in T cell activation. Immunol Cell Biol 75:430–445
Borggrewe M, Grit C, Vainchtein ID, Brouwer N, Wesseling EM, Laman JD, Eggen BJL, Kooistra SM, Boddeke E (2021) Regionally diverse astrocyte subtypes and their heterogeneous response to EAE. Glia 69:1140–1154
Choudhury MG, Saha N (2016) Induction of inducible nitric oxide synthase by lipopolysaccharide and the influences of cell volume changes, stress hormones and oxidative stress on nitric oxide efflux from the perfused liver of air-breathing catfish. Heteropneustes fossilis. PLoS One 11:e0150469
Kim J, Cha YN, Surh YJ (2010) A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res 690:12–23
Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S (2010) Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol 80:1895–1903
D’amelio M, Cavallucci V, Cecconi F (2010) Neuronal caspase-3 signaling: not only cell death. Cell Death Differ 17:1104–1114
Ajith TA, Abhishek G, Roshny D, Sudheesh NP (2009) Co-supplementation of single and multi doses of vitamins C and E ameliorates cisplatin-induced acute renal failure in mice. Exp Toxicol Pathol 61:565–571
Bagheri Y, Barati A, Nouraei S, Jalili Namini N, Bakhshi M, Fathi E, Montazersaheb S (2021) Comparative study of gavage and intraperitoneal administration of gamma-oryzanol in alleviation/attenuation in a rat animal model of renal ischemia/reperfusion-induced injury. Iran J Basic Med Sci 24:175–183
Spitsin SV, Scott GS, Mikheeva T, Zborek A, Kean RB, Brimer CM, Koprowski H, Hooper DC (2002) Comparison of uric acid and ascorbic acid in protection against EAE. Free Radic Biol Med 33:1363–1371
McFarland HF, Martin R (2007) Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 8:913–919
Estrada JA, Contreras I: Nutritional modulation of immune and central nervous system homeostasis: the role of diet in development of neuroinflammation and neurological disease. Nutrients 2019, 11.
Carr AC, Maggini S: Vitamin C and immune function. Nutrients 2017, 9.
Dawson HD, Collins G, Pyle R, Key M, Weeraratna A, Deep-Dixit V, Nadal CN, Taub DD (2006) Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression by human T lymphocytes. BMC Immunol 7:27
Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK (2008) Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol 181:2277–2284
Saraiva M, O’Garra A (2010) The regulation of IL-10 production by immune cells. Nat Rev Immunol 10:170–181
Couper KN, Blount DG, Riley EM (2008) IL-10: the master regulator of immunity to infection. J Immunol 180:5771–5777
Kallaur AP, Oliveira SR, Delicatode Almeida ER, Kaminami Morimoto H, Lopes J, de Carvalho Jennings Pereira WL, Marques Andrade R, Muliterno-Pelegrino L, Donizete Borelli S, Kaimen-Maciel DR (2013) Cytokine profile in relapsing-remitting multiple sclerosis patients and the association between progression and activity of the disease. Molecular Medicine Reports 7:1010–1020
Molina N, Morandi AC, Bolin AP, Otton R (2014) Comparative effect of fucoxanthin and vitamin C on oxidative and functional parameters of human lymphocytes. Int Immunopharmacol 22:41–50
Singh S, Metz I, Amor S, van der Valk P, Stadelmann C, Brück W (2013) Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathol 125:595–608
Traiffort E, Kassoussi A, Zahaf A, Laouarem Y (2020) Astrocytes and microglia as major players of myelin production in normal and pathological conditions. Front Cell Neurosci 14:79
Chen X, Ma X, Jiang Y, Pi R, Liu Y, Ma L (2011) The prospects of minocycline in multiple sclerosis. J Neuroimmunol 235:1–8
Starossom SC, Mascanfroni ID, Imitola J, Cao L, Raddassi K, Hernandez SF, Bassil R, Croci DO, Cerliani JP, Delacour D et al (2012) Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity 37:249–263
Villamor E, Fawzi WW (2005) Effects of vitamin a supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev 18:446–464
Sanchez-Moreno C, Paniagua M, Madrid A, Martin A (2003) Protective effect of vitamin C against the ethanol mediated toxic effects on human brain glial cells. J Nutr Biochem 14:606–613
Ahmad A, Shah SA, Badshah H, Kim MJ, Ali T, Yoon GH, Kim TH, Abid NB, Rehman SU, Khan S, Kim MO (2016) Neuroprotection by Vitamin C against ethanol-induced neuroinflammation associated neurodegeneration in the developing rat brain. CNS Neurol Disord Drug Targets 15:360–370
Roshanbakhsh H, Salmani ME, Dehghan S, Nazari A, Javan M, Pourabdolhossein F (2020) Piperine ameliorated memory impairment and myelin damage in lysolecethin induced hippocampal demyelination. Life Sciences 253:117671
Sivandzade F, Prasad S, Bhalerao A, Cucullo L (2019) NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biology 21:101059
Liu Y, Zhu B, Luo L, Li P, Paty DW, Cynader MS (2001) Heme oxygenase-1 plays an important protective role in experimental autoimmune encephalomyelitis. NeuroReport 12:1841–1845
Chora ÂA, Fontoura P, Cunha A, Pais TF, Cardoso S, Ho PP, Lee LY, Sobel RA, Steinman L, Soares MP (2007) Heme oxygenase–1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Investig 117:438–447
Mostafavi-Pour Z, Ramezani F, Keshavarzi F, Samadi N (2017) The role of quercetin and vitamin C in Nrf2-dependent oxidative stress production in breast cancer cells. Oncol Lett 13:1965–1973
Xu LL, Zhao B, Sun SL, Yu SF, Wang YM, Ji R, Yang ZT, Ma L, Yao Y, Chen Y et al (2020) High-dose vitamin C alleviates pancreatic injury via the NRF2/NQO1/HO-1 pathway in a rat model of severe acute pancreatitis. Ann Transl Med 8:852
Meyer R, Weissert R, Diem R, Storch MK, de Graaf KL, Kramer B, Bähr M (2001) Acute neuronal apoptosis in a rat model of multiple sclerosis. J Neurosci 21:6214–6220
Yousef A, Robinson JL, Irwin DJ, Byrne MD, Kwong LK, Lee EB, Xu Y, Xie SX, Rennert L, Suh E (2017) Neuron loss and degeneration in the progression of TDP-43 in frontotemporal lobar degeneration. Acta Neuropathol Commun 5:1–15
Vissers MC, Lee WG, Hampton MB (2001) Regulation of apoptosis by vitamin C. Specific protection of the apoptotic machinery against exposure to chlorinated oxidants. J Biol Chem 276:46835–46840
Chen F, Jiang Z, Jiang S, Li L, Lin X, Gou Z, Fan Q (2016) Dietary vitamin A supplementation improved reproductive performance by regulating ovarian expression of hormone receptors, caspase-3 and Fas in broiler breeders. Poult Sci 95:30–40
Fletcher JL, Murray SS, Xiao J (2018) Brain-derived neurotrophic factor in central nervous system myelination: a new mechanism to promote myelin plasticity and repair. Int J Mol Sci 19:4131
Makar TK, Trisler D, Sura KT, Sultana S, Patel N, Bever CT (2008) Brain derived neurotrophic factor treatment reduces inflammation and apoptosis in experimental allergic encephalomyelitis. J Neurol Sci 270:70–76
Van’t Veer A, Du Y, Fischer TZ, Boetig DR, Wood MR, Dreyfus CF (2009) Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J Neurosci Res 87:69–78
Acknowledgements
The authors would like to gratefully appreciate the kind support of Dr. Mohammad Javan from physiology department of Tarbiat Modares University Tehran-Iran during the study. Also we appreciate Babol University of Medical Sciences for the support and facilities used during the project.
Funding
This work was supported by a grant (No. 35425–139-02–96), from Electrophysiology Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
Mozhdeh Navidhamidi: conceptualization; investigation; data curation; funding acquisition; project administration; and writing—review & editing of the manuscript. Atena Nazari: methodology; investigation review & editing of the manuscript. Samaneh Deghan: investigation; formal analysis; review & editing of the manuscript. Anahita Ebrahimpour: investigation; review & editing of the manuscript. Reza Nasrnezhad: investigation; formal analysis Fereshteh Pourabdolhossein: Conceptualization; Investigation; Methodology; Data curation; funding acquisition; formal analysis; Software; Supervision; Validation; Writing—original draft; Writing—review & editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental procedures of this study were performed according to the international guidelines for care and use of laboratory animals and approved (Ethic code number: IR.TUMS.VCR.REC.1396.3350) by the ethical committee of Tehran University of Medical Sciences. This article does not contain any studies involving human participants performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Co-supplementation of vitamins A and C alleviated neurological symptoms of EAE disease
• Co-administration of vitamins A and C reduced gliosis, immune cell infiltration and demyelination in EAE
• Combination therapy of vitamins A and C increased the antioxidant capacity and decreased the oxidative stress
• Co-supplementation of vitamins A and C decreased TNF-α and IL-1β levels and increased IL-10
• Co-administration of vitamins A and C has anti-apoptotic and neuroprotective impacts in EAE model
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Navidhamidi, M., Nazari, A., Dehghan, S. et al. Therapeutic Potential of Combined Therapy of Vitamin A and Vitamin C in the Experimental Autoimmune Encephalomyelitis (EAE) in Lewis Rats. Mol Neurobiol 59, 2328–2347 (2022). https://doi.org/10.1007/s12035-022-02755-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02755-0