Abstract
First-line therapy with interferon beta (IFN-β), involved in gene expression modulation in immune response, is widely used for multiple sclerosis. However, 30–50% of patients do not respond optimally. Variants in CBLB, CTSS, GRIA3, OAS1 and TNFRSF10A genes have been proposed to contribute to the variation in the individual response. The purpose of this study was to evaluate the influence of gene polymorphisms on the IFN-β response in relapsing–remitting multiple sclerosis (RRMS) patients. CBLB (rs12487066), GRIA3 (rs12557782), CTSS (rs1136774), OAS1 (rs10774671) and TNFRSF10A (rs20576) polymorphisms were analysed by Taqman in 137 RRMS patients. Response to IFN-β and change in the Expanded Disability Status Scale (EDSS) after 24 months were evaluated using multivariable logistic regression analysis. Carriers of at least one copy of the C allele of CTSS-rs1136774 had a better response to IFN-β (p = 0.0423; OR = 2.94; CI95% = 1.03, 8.40). Carriers of TT genotype of TNFRSF10A-rs20576 had a higher probability of maintaining their EDSS stable after 24 months of IFN-β treatment (p = 0.0251; OR = 5.71; CI95% = 1.39, 31.75). No influence of CBLB (rs12487066), OAS1 (rs10774671) and GRIA3 (rs12557782) gene polymorphisms in the variation of the individual response to IFN-β was shown. Our results suggest that the TNFRSF10A-rs20576 and CTSS-rs1136774 gene polymorphisms influence the response to IFN-β after 24 months, while the CBLB (rs12487066), OAS1 (rs10774671) or GRIA3 (rs12557782) gene polymorphisms had no effect on the variation of the individual response to IFN-β.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is one of the most common neurological diseases causing permanent disability among young adults [1, 2]. Epidemiological data indicate an estimated total prevalence of 83 affected per 100,000 people, an estimated annual average incidence rate of 4.3 cases per 100,000 inhabitants and a ratio female:male of ≈ 2.0 in the last three decades in the European continent, with the highest rates corresponding to Northern countries [3]. Although there are different types of MS, defined by the course of the disease [4], the relapsing–remitting multiple sclerosis (RRMS) pattern, manifested by 85% of patients, is characterized by the recurrence of weakening and recovery episodes [5, 6].
The mechanisms underlying the immunopathogenesis of MS have not yet been fully determined, despite the intense research [7, 8]. Some evidence points to autoimmune processes involving several cell subtypes and proinflammatory substances [8]. The process occurs through the production of cytokines by dendritic cells and lymphocytes; when the dendritic cells cross the blood–brain barrier reaching the central nervous system (CNS), induce polarization and activation of T helper cells (Th1 and Th17) [9, 10]. Both IFN gamma and interleukin 17 (IL-17), produced by Th1 and Th17 cells respectively, are active during the disease, promoting CNS inflammation and axonal damage [11,12,13].
Most MS patients are treated with first-line disease-modifying drugs, such as IFN-β, a natural polypeptide highly synthesized by fibroblasts with anti-inflammatory properties. IFN-β modulates the expression of certain genes, interfering with the antigen presentation process, inhibiting the activation and proliferation of T cells [14] and also reducing the expression of proinflammatory cytokines [15]. It has important effects on the molecules involved in the permeability of the blood–brain barrier, preventing the adhesion of T lymphocytes to the endothelium and their extravasation to the CNS [16, 17].
Two therapeutic options of recombinant IFN-β are used in the treatment of MS. IFN-β 1a is obtained from hamster ovary cells, with identical amino acid sequence to native IFN, which may be administered intramuscularly (IM) or subcutaneously (SC) [18]. The second form of recombinant IFN-β is the pegylated IFN 1a (PEG 1a), obtained by conjugation with polyethylene glycol molecules. The pegylation contributes to a reduction in antigenicity and immunogenicity, as well as increased exposure, half-life and serum concentrations of the therapeutic agent [19, 20]. IFN-β 1b, administered SC, is synthesized by Escherichia coli, has significant differences in certain amino acids compared to IFN-β of natural origin and is not glycosylated. These differences influence the specific biological activity, and therefore, a higher dose is required [18], which implies a greater probability of developing neutralizing antibodies (NAbs) [21].
The efficacy of IFN-β as first-line therapy has been demonstrated in different studies [22, 23]. However, approximately 30 to 50% of patients do not respond optimally to IFN treatment, showing no indication of response in some cases [24, 25]. The influence of genetic variants on MS susceptibility and/or in the response to IFN-β therapy has been investigated in several genome-wide association (GWA) and candidate gene association studies [26,27,28,29,30,31,32,33,34,35], revealing potential roles for different genes in the response to IFN-β [26,27,28,29,30, 36,37,38]. Although many single-nucleotide polymorphisms (SNPs) have been proposed, there is in general a lack of replication in other studies. Among them, we selected SNPs in GRIA3, TNFRSF10A, CTSS, OAS1 and CBLB genes, previously identified with potential roles in response to IFN-β [30], as response modifiers [28], increased disease activity [29] or mechanistically involved in IFN treatment [26]. In a GWAS evaluating 428,867 SNPs in 210 RRMS patients of Caucasian origin, the most significant SNP associated with response to IFN-β was rs12557782 in GRIA3 gene [30]. The GRIA3 gene (glutamate receptor ionotropic Ampa 3), located on the X chromosome, plays an important role in the synaptic transmission in the CNS [39, 40]. The rs12557782 (G allele) variant of this gene was identified as a possible biomarker of response to IFN-β therapy in 144 Spanish RRMS women (p = 0.002; OR = 2.7; CI95% = 1.5, 5.2) [30]. The TNFRSF10A (tumour necrosis factor receptor superfamily 10A) gene encodes a protein that induces cellular apoptosis [41] and is involved in autoimmune diseases mediated by T cells, such as MS [42]. The rs20576 polymorphism (CC genotype) was identified as a predictor of positive response to IFN-β (p = 8.88·10−4; OR: 0.30; CI95% = 0.14, 0.63) in the joint analysis of an original cohort of 509 and a validation cohort of 226 Spanish RRMS patients [38]. The C allele carriers of the rs1136774 polymorphism, located in the CTSS (cathepsin S) gene [43], encoding a protease involved in the degradation of antigens from antigen-presenting cells [44], showed a greater response to IFN-β in 230 RRMS patients from Belfast, UK (p = 0.02; OR = 0.38; CI95% = 0.18, 0.84) [28]. The OAS1 gene (oligoadenylate synthetase 1) is induced by IFN and encodes a protein involved in mechanisms of regulation of viral infection [45]. The presence of the AA genotype in the rs10774671 variant of this gene conferred susceptibility to MS in 401 RRMS patients treated with IFN-β and 394 healthy controls from Dublin, Ireland, while the GG genotype protected against disease activity (p = 0.04; hazard ratio = 1.47; CI95% = 1.01, 2.16) [29]. The CBLB gene (Casitas B-lineage lymphoma proto-oncogene B gene) is a key regulator of the activation thresholds of the peripheral immune system and T lymphocytes, involved in immunological tolerance and autoimmunity [26, 36, 37]. In 37 RRMS patients from Hamburg, Germany, T allele carriers of CBLB rs12487066 polymorphism showed a reduction in CBLB expression compared to CC homozygotes in the presence of IFN (p = 0.012), not inhibiting the proliferation of T lymphocytes [26].
Given the evidence of a potential role of genetics on the variability of the response to IFN-β in MS, the objective of this study was to investigate the influence of five polymorphisms (CBLB-rs12487066, GRIA3-rs12557782, CTSS-rs1136774, OAS1-rs10774671 and TNFRSF10A-rs20576) in the response to IFN-β in RRMS patients.
Material and Methods
An ambispective cohort study was carried out.
Study Population
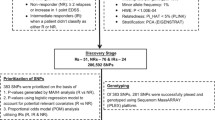
This study was carried out at the Virgen de las Nieves University Hospital (VNUH) in Granada, Spain, during the period from May 2016 to June 2020. One hundred thirty-seven RRMS patients over 18 years old and treated with IFN-β therapy were included and followed for 24 months. No patients with clinically or radiologically isolated syndrome were included. This study was approved by the VNUH Ethics and Research Committee and performed conform the declaration of Helsinki. All patients signed an informed consent.
Clinical and Sociodemographic Variables
The clinical and sociodemographic data were collected by reviewing and monitoring the medical records of the patients included in the study. The variables included were gender, family history of cancer, age at the time of the diagnosis of MS, duration of disease (years), Expanded Disability Status Scale (EDSS) [46], Multiple Sclerosis Severity Score (MSSS), Progression Index (EDSS/years of disease) [47] and treatment (IFN1a IM, 1a SC, 1a PEG, 1b SC) [18].
Two outcome variables were considered: response to IFN-β, as described by Rio et al. [48, 49], and change in the EDSS. For the variable response to IFN-β, patients were classified as non-responders if there was evidence of magnetic resonance disease activity and/or increase of at least one point on the EDSS scale and/or had one or more relapses that persisted for a minimum of two consecutive scheduled visits separated by a 6-month interval and/or the medication was discontinued in absence of toxicity [48, 49].
The variable change in the EDSS was defined as a variation or not in the EDSS after 24 months.
Genetic Variables
Analysis of Gene Polymorphisms
The following SNPs were determined, using real-time PCR with TaqMan probes (Supplementary Table 1), according to previously described protocols [50]: CBLB (rs12487066), GRIA3 (rs12557782), CTSS (rs1136774), OAS1 (rs10774671), TNFRSF10A (rs20576). DNA extraction from saliva samples was performed using the QIAamp Mini Kit (Qiagen Gmbh, Hilden Germany), according to the extraction protocols indicated by the manufacturer.
Statistical Analysis
Quantitative data were expressed as the mean and standard deviation for variables with a normal distribution or median and 25th and 75th percentiles for variables with a non-normal distribution. The Lilliefors test (Kolmogorov–Smirnov) was used to assess the normality of the variables.
To analyse the association between response and polymorphisms (genotypic, additive, allelic, dominant and recessive models), a bivariate test was performed using Pearson’s chi-square with Yates’ correction or the Fisher test for expected frequencies below 5%. The relative risk (RR) and its 95% confidence interval (CI95%) were also calculated. The genetic models were defined as follows: genotypic (mm vs. Mm vs. MM), dominant (mm and Mm vs. MM), recessive (mm vs. Mm and MM) and allelic (M vs. m), m being the minor allele and M being the major allele. Bivariate analysis for independent quantitative variables was carried out using the Wilcoxon test.
To verify the influence of SNPs on response to IFN-β, a multivariable logistic regression model was applied, taking a level for significance of p < 0.05. Statistical analyses were carried out using R 3.0.1 [51]. Hardy–Weinberg equilibrium was estimated using the free, open-source whole-genome association analysis toolset PLINK 1.9 [52].
The statistical analysis of rs12557782 polymorphism in the GRIA3 gene (X chromosome) was stratified by sex.
Results
Clinical Characteristics of Patients
We included a total of 137 patients of Caucasian origin diagnosed with RRMS who had been treated with IFN-β (1a, PEG 1a, 1b).
The clinical characteristics and sociodemographic data are detailed in Table 1. The median age of diagnosis was 31 years, with a mean duration of the disease of 2 years. The proportion of women was 65.7% (90/137). Sixty-nine patients maintained their EDSS stable after 24 months of therapy (84.2%) and the remaining 15.9% increased their EDSS score in a median of 0.5 points (Table 1). Good response to IFN-β therapy was shown by 77.3% of patients (Table 1). There were no differences in the distribution of responders and non-responders to IFN-β or change in the EDSS among different IFN-β therapeutic options (Table 2).
Baseline EDSS was the only clinical variable associated with response to IFN-β (p = 0.008) and change in the EDSS (p = 0.036) (Table 2).
Influence of SNPs and the Response to IFN-β
The bivariate analysis of genetic polymorphisms and the response to IFN is detailed in Table 3. Bivariate analysis showed that patients with CC genotype in the rs1136774 polymorphism of the CTSS (recessive model) gene had greater response to IFN-β (Table 3: p = 0.047; RR = 1.3; CI95% = 1.0, 1.7); women with the GG genotype of the rs12557782 polymorphism in the GRIA3 gene (recessive model) showed greater response to IFN-β (Table 3: p = 0.033; RR = 1.4; CI95% = 1.0, 2.1). The CBLB-rs12487066 and TNFRSF10A-rs20576 gene polymorphisms showed a trend to association with change in the EDSS after 24 months of IFN treatment, but non-significant (p < 0.1; Table 3). The OAS1-rs10774671 gene polymorphism did not predict the variation of the individual response to IFN-β or change in the EDSS.
Multivariable logistic regression analysis showed that patients with lower baseline EDSS (p = 0.0694; OR = 0.06; CI95% = 0.34, 1.04) and carriers of at least one copy of the C allele of CTSS-rs1136774 had a better response to IFN-β (p = 0.0423; OR = 2.94; CI95% = 1.03, 8.40). Patients with lower baseline EDSS (p = 0.0060; OR = 0.36; CI95% = 0.016, 0.71) and carriers of TT genotype of TNFRSF10A-rs20576 had a higher probability of maintaining their EDSS stable after 24 months of treatment with IFN-β (p = 0.0251; OR = 5.71; CI95% = 1.39, 31.75) (Table 4). The GRIA3-rs12557782 association with response in women and trend shown by CBLB-rs12487066 on change in the EDSS in the bivariate analysis were not confirmed as independent associations after multivariable analyses.
Discussion
IFN-β is one of the most used first-line treatment therapies for MS. Although its mechanism of action is not yet fully known, several studies indicate that it plays a role in modifying the expression of genes involved in the immune response [18, 53,54,55,56], by inhibiting the synthesis of inflammatory cytokines (IL-12, IL-17, IL-23), and favouring the production of anti-inflammatory cytokines (IL-4, IL-10) and Th2 type cells [57]. The response to IFN-β has also been suggested to be determined by certain genetic variants in the GRIA3, CBLB, CTSS, OAS1 and TNFRSF10A genes, among others [26,27,28,29,30, 36,37,38]. Our results point at the TT genotype for rs20576 in TNFRSF10A as a possible indicator of better response to IFN-β since it was associated with stability in the EDSS score after 24 months (p = 0.0251; OR = 5.71; CI95% = 1.39, 31.75). However, this was not reflected in an association with response to IFN-β when defined as the compound EDSS/relapse/imaging variable. The beneficial effect of the TT genotype found in our study is in contrast with the findings of a previous study including 509 MS patients of Spanish origin treated with IFN-β and an additional validation cohort of 226 patients, which showed an association of the CC genotype with a positive response to IFN-β, defined in a similar way to ours and also after 24 months (OR: 0.30; CI95% = 0.14–0.63; p = 8.88·10−4) [38]. The clinical characteristics of the patients were also similar. Another study with 73 Iranian MS patients did not found a significant association of rs20576 with IFN-β response (p = 0.87) [58]. No other studies have explored the influence of rs20576 on IFN-β response in MS patients. In follicular lymphoma, no association with response to first-line rituximab was found either in 125 patients [59]. Its role as a susceptibility marker for systemic lupus erythematosus [60], hepatocellular carcinoma [61], Alzheimer’s disease [62] or lymphomas [63] has also been investigated, although unsuccessfully.
Our patients also showed a better response to IFN-β for the C allele of the rs1136774 CTSS in the multivariable analysis (Table 4: p = 0.042; OR: 2.94; CI95%: 1.03, 8.40), in line with the results of a study with 230 RRMS patients of European origin (UK) in which C allele carriers had a better response to IFN-β after 2 years of follow-up (p = 0.02; OR: 0.38; CI95%: 0.18, 0.84) [28]. The CTSS protein (lysosomal cysteine proteinase) has been suggested to participate in the presentation of microglia antigens through MHC II-associated invariant chain degradation [64] and the degradation of myelin basic protein in vitro [65]. Serum cathepsin S and cystatin C levels influenced disease activity in 73 RRMS patients, specifically in those responding to IFN-β, through the reduction of the levels of serum cathepsin S [66].
We could not find any association of response to IFN-β or change in EDSS with gene polymorphisms in GRIA3, CBLB or OAS1. RRMS women carrying the G allele in rs12557782-GRIA3 responded better to IFN-β (p = 0.002; OR = 2.7; CI95% = 1.5, 5.2) in a GWAS including 106 RRMS patients of Caucasian origin (Spanish) that evaluated 428,867 SNPs [30]. No association was found in men [30]. Despite the size, follow-up period of 24 months and female:male ratio (2:1) in our study being very similar and GG women showing greater response to IFN-β in our bivariate analysis, this association did not prove to be independent after multivariable analysis (Table 3: p = 0.033). No association with response to IFN-β was shown in 73 MS patients of Iranian origin, neither in females or males (p = 0.15 and 0.4, respectively), although we cannot rule out a lack of statistical power, given the limited size of the study [58]. The GRIA3 gene encodes an AMPA-type glutamate receptor, which participates in most excitatory synaptic transmissions of the CNS, potentially explaining a relationship between the response to IFN-β and genes encoding channels activated by neurotransmitters [27]. The rs12487066 polymorphism of the CBLB gene was associated with MS risk in a GWAS in which 334,923 SNPs were evaluated in 931 family trios of European origin (UK = 928 cases and 1475 controls) and American (US = 1394 cases and 1512 controls) [67]. The replication study showed an increased risk of MS in T allele carriers (p = 0.035; OR: 1.08; CI95% = 1.03, 1.16) [67]. Another study with 342 patients of Saudi origin (99 MS with no antecedents of MS, 22 with a family member with MS, 89 controls related to MS and 132 independent controls) showed rs12487066 associated with MS when compared to the independent control group [68]. This SNP has been suggested to regulate the expression of the CBLB gene so that in carriers of the T allele, CBLB is inhibited by transcription factors, and lymphocytic proliferation does not cease [26]. In our study, CBLB-rs12487066 had no influence on response to IFN-β in RRMS patients, but we cannot compare it to other populations since this is the first study evaluating its possible predictive value for response in MS. The role of OAS1-rs10774671 polymorphism in disease activity in MS patients treated with IFN-β was investigated in 198 RRMS Irish patients and 394 controls [29]. The relapse time after treatment with IFN-β was significantly shorter in patients with AA genotype (hazard ratio: 1.47; CI95% = 1.01–2.16; p = 0.04) [29]. RRMS patients were divided into two groups according to disease activity (low and high); those with low activity were defined as those who had a maximum relapse after 24 months of treatment with IFN-β and did not have a sustained increase in disability. High activity was for those who had two or more relapses in the 24-month follow-up, with or without a sustained increase in disability (EDSS) [29]. This approach is similar to the response criteria applied in our study; however, OAS1-rs10774671 did not show an influence on response to IFN-β in our RRMS patients. There are no more studies of rs10774671 related to response to IFN.
Other SNPs have been proposed as indicators of response to IFN-β in Caucasian patients in various studies, some of them as response predictors (FHIT, GAPVD1, ZNF697 [35], GPC5, COL25A1, HAPLN1 [27], IRF5 [31], CD46 [32], PELI3, GABRR3 [33]), or other signs of response, as lower relapse rates (IFNAR1) [34]. Some of these results were not adjusted by multiple comparisons [27] or have not been replicated in other studies [28, 34].
The main limitation of our study was the sample size. In our study, 160 patients treated with IFN-β were included as an initial cohort, 23 were excluded due to unavailability of their clinical history for follow-up, leaving an even smaller sample size. However, our population has a great homogeneity in terms of the definition and application of the response criteria since these are patients treated in the same hospital, evaluated, monitored and treated according to the same protocols by the same team of physicians.
It has been shown that patients treated with IFN-β could develop neutralizing antibodies that can negatively affect the therapeutic response. In Korea, a study was conducted with 150 patients from nine different centres to evaluate the development of NAbs in MS patients treated with IFN-β-1a and IFN-β-1b. NAbs were found in 35% of patients treated with IFN-β-1b, 15% with IFN-β-1a SC and 0% with IFN-β-1a IM. Persistent NAb positivity was associated with disease activity in MS patients treated with IFN-β (p = 0.004) [69]. The frequency of MS patients developing NAbs against IFN-β was also significantly higher with IFN-β-1b therapy compared with IFN-β-1a therapy [70]. In our study, with only 14 patients (Table 1, 10.2%) treated with IFN-β 1b SC, the influence of NAbs development could not be well-powered investigated. Consequently, a further limitation of our study would be that patients were not screened for NAbs.
It is therefore necessary that future studies include larger cohorts that allow greater statistical power to elucidate associations, not only to warrantee sufficient statistical power for the investigation of a greater number of SNPs, but also to include different therapeutic forms of IFN-β or several follow-up periods, which would allow to explore the effect of the different genetic variants on the short or long-term response, as well as the need for a change in therapy and the moment during the follow-up when this occurs, which will allow a better therapeutic control of these patients.
Conclusions
Our results suggest that treatment of RRMS patients with IFN-β for 24 months helps maintaining EDSS score in TT carriers of the TNFRSF10A-rs20576 gene polymorphism, whereas carriers of the C allele of CTSS-rs1136774 show a better response. A lower baseline EDSS is associated with a better response to IFN-β and EDSS stability, while CBLB (rs12487066), OAS1 (rs10774671) or GRIA3 (rs12557782) gene polymorphisms did not influence the variation of the individual response to IFN-β after 24 months.
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information files. The datasets generated during and/or analysed during the current study cannot be publicly available due to that option was not included in the informed consent for participants.
References
Sadovnick AD, Ebers GC (1993) Epidemiology of multiple sclerosis: a critical overview. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. Can J Neurol Sci 20(1):17–29
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG (2000) Multiple sclerosis. N Engl J Med 343(13):938–952. https://doi.org/10.1056/nejm200009283431307
Pugliatti M, Rosati G, Carton H, Riise T, Drulovic J, Vecsei L, Milanov I (2006) The epidemiology of multiple sclerosis in Europe. Eur J Neurol 13(7):700–722. https://doi.org/10.1111/j.1468-1331.2006.01342.x
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302. https://doi.org/10.1002/ana.22366
Loma I, Heyman R (2011) Multiple sclerosis: pathogenesis and treatment. Curr Neuropharmacol 9(3):409–416. https://doi.org/10.2174/157015911796557911
Weiner HL (2008) A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J Neurol 255(Suppl 1):3–11. https://doi.org/10.1007/s00415-008-1002-8
Lazibat I, RubinićMajdak M, Županić S (2018) Multiple sclerosis: new aspects of immunopathogenesis. Acta Clin Croat 57(2):352–361. https://doi.org/10.20471/acc.2018.57.02.17
Grigoriadis N, van Pesch V (2015) A basic overview of multiple sclerosis immunopathology. Eur J Neurol 22(Suppl 2):3–13. https://doi.org/10.1111/ene.12798
Ifergan I, Kebir H, Bernard M, Wosik K, Dodelet-Devillers A, Cayrol R, Arbour N, Prat A (2008) The blood-brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain : a journal of neurology 131(Pt 3):785–799. https://doi.org/10.1093/brain/awm295
Nuyts AH, Lee WP, Bashir-Dar R, Berneman ZN, Cools N (2013) Dendritic cells in multiple sclerosis: key players in the immunopathogenesis, key players for new cellular immunotherapies?. Mult Scler 9(8):995–1002. https://doi.org/10.1177/1352458512473189
Montes M, Zhang X, Berthelot L, Laplaud DA, Brouard S, Jin J, Rogan S, Armao D, Jewells V, Soulillou JP, Markovic-Plese S (2009) Oligoclonal myelin-reactive T-cell infiltrates derived from multiple sclerosis lesions are enriched in Th17 cells. Clin Immunol 130(2):133–144. https://doi.org/10.1016/j.clim.2008.08.030
Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A (2007) Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13(10):1173–1175. https://doi.org/10.1038/nm1651
Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schroder R, Deckert M, Schmidt S, Ravid R, Rajewsky K (2000) Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med 192(3):393–404. https://doi.org/10.1084/jem.192.3.393
Jiang H, Milo R, Swoveland P, Johnson KP, Panitch H, Dhib-Jalbut S (1995) Interferon beta-1b reduces interferon gamma-induced antigen-presenting capacity of human glial and B cells. J Neuroimmunol 61(1):17–25
Guo B, Chang EY, Cheng G (2008) The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Investig 118(5):1680–1690. https://doi.org/10.1172/jci33342
Karabudak R, Kurne A, Guc D, Sengelen M, Canpinar H, Kansu E (2004) Effect of interferon beta-1a on serum matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of matrix metalloproteinase (TIMP-1) in relapsing remitting multiple sclerosis patients. One year follow-up results. J Neurol 251(3):279–283. https://doi.org/10.1007/s00415-004-0285-7
Graber J, Zhan M, Ford D, Kursch F, Francis G, Bever C, Panitch H, Calabresi PA, Dhib-Jalbut S (2005) Interferon-beta-1a induces increases in vascular cell adhesion molecule: implications for its mode of action in multiple sclerosis. J Neuroimmunol 161(1–2):169–176. https://doi.org/10.1016/j.jneuroim.2004.11.017
Goodin DS (2005) Treatment of multiple sclerosis with human beta interferon. Int MS J 12(3):96–108
Baker DP, Pepinsky RB, Brickelmaier M, Gronke RS, Hu X, Olivier K, Lerner M, Miller L, Crossman M, Nestorov I, Subramanyam M, Hitchman S, Glick G, Richman S, Liu S, Zhu Y, Panzara MA, Davar G (2010) PEGylated interferon beta-1a: meeting an unmet medical need in the treatment of relapsing multiple sclerosis. J Interferon Cytokine Res 30(10):777–785. https://doi.org/10.1089/jir.2010.0092
Fishburn CS (2008) The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J Pharm Sci 97(10):4167–4183. https://doi.org/10.1002/jps.21278
Govindappa K, Sathish J, Park K, Kirkham J, Pirmohamed M (2015) Development of interferon beta-neutralising antibodies in multiple sclerosis–a systematic review and meta-analysis. Eur J Clin Pharmacol 71(11):1287–1298. https://doi.org/10.1007/s00228-015-1921-0
Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, Fischer JS, Goodkin DE, Granger CV, Simon JH, Alam JJ, Bartoszak DM, Bourdette DN, Braiman J, Brownscheidle CM, Coats ME, Cohan SL, Dougherty DS, Kinkel RP, Mass MK, Munschauer FE 3rd, Priore RL, Pullicino PM, Scherokman BJ, Whitham RH et al (1996) Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Annals of neurology 39(3):285–294. https://doi.org/10.1002/ana.410390304
(2001) Interferon beta-lb is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. 1993 [classical article]IFNB Multiple Sclerosis Study Group. Neurology 57(12 Suppl 5):S3–9
Rio J, Nos C, Tintore M, Borras C, Galan I, Comabella M, Montalban X (2002) Assessment of different treatment failure criteria in a cohort of relapsing-remitting multiple sclerosis patients treated with interferon beta: implications for clinical trials. Ann Neurol 52(4):400–406. https://doi.org/10.1002/ana.10290
Gauthier SA, Glanz BI, Mandel M, Tsagkaropoulos A, Neema M, Stankiewicz J, Arora A, Duan Y, Liptak Z, Egorova S, Buckle GJ, Bakshi R, Guttmann CR, Khoury SJ, Weiner HL (2009) Incidence and factors associated with treatment failure in the CLIMB multiple sclerosis cohort study. J Neurol Sci 284(1–2):116–119. https://doi.org/10.1016/j.jns.2009.04.020
Sturner KH, Borgmeyer U, Schulze C, Pless O, Martin R (2014) A multiple sclerosis-associated variant of CBLB links genetic risk with type I IFN function. J Immunol (Baltimore, Md: 1950) 193(9):4439–4447. https://doi.org/10.4049/jimmunol.1303077
Byun E, Caillier SJ, Montalban X, Villoslada P, Fernandez O, Brassat D, Comabella M, Wang J, Barcellos LF, Baranzini SE, Oksenberg JR (2008) Genome-wide pharmacogenomic analysis of the response to interferon beta therapy in multiple sclerosis. Arch Neurol 65(3):337–344. https://doi.org/10.1001/archneurol.2008.47
Cunningham S, Graham C, Hutchinson M, Droogan A, O’Rourke K, Patterson C, McDonnell G, Hawkins S, Vandenbroeck K (2005) Pharmacogenomics of responsiveness to interferon IFN-beta treatment in multiple sclerosis: a genetic screen of 100 type I interferon-inducible genes. Clin Pharmacol Ther 78(6):635–646. https://doi.org/10.1016/j.clpt.2005.08.018
O’Brien M, Lonergan R, Costelloe L, O’Rourke K, Fletcher JM, Kinsella K, Sweeney C, Antonelli G, Mills KH, O’Farrelly C, Hutchinson M, Tubridy N (2010) OAS1: a multiple sclerosis susceptibility gene that influences disease severity. Neurology 75(5):411–418. https://doi.org/10.1212/WNL.0b013e3181ebdd2b
Comabella M, Craig DW, Morcillo-Suarez C, Rio J, Navarro A, Fernandez M, Martin R, Montalban X (2009) Genome-wide scan of 500,000 single-nucleotide polymorphisms among responders and nonresponders to interferon beta therapy in multiple sclerosis. Arch Neurol 66(8):972–978. https://doi.org/10.1001/archneurol.2009.150
Vosslamber S, van der Voort LF, van den Elskamp IJ, Heijmans R, Aubin C, Uitdehaag BM, Crusius JB, van der PouwKraan TC, Comabella M, Montalban X, Hafler DA, De Jager PL, Killestein J, Polman CH, Verweij CL (2011) Interferon regulatory factor 5 gene variants and pharmacological and clinical outcome of Interferonbeta therapy in multiple sclerosis. Genes Immun 12(6):466–472. https://doi.org/10.1038/gene.2011.18
Alvarez-Lafuente R, Blanco-Kelly F, Garcia-Montojo M, Martinez A, De Las HV, Dominguez-Mozo MI, Bartolome M, Garcia-Martinez A, De la Concha EG, Urcelay E, Arroyo R (2011) CD46 in a Spanish cohort of multiple sclerosis patients: genetics, mRNA expression and response to interferon-beta treatment. Mult Scler (Houndmills, Basingstoke, England) 17(5):513–520. https://doi.org/10.1177/1352458510393263
Bustamante MF, Morcillo-Suarez C, Malhotra S, Rio J, Leyva L, Fernandez O, Zettl UK, Killestein J, Brassat D, Garcia-Merino JA, Sanchez AJ, Urcelay E, Alvarez-Lafuente R, Villar LM, Alvarez-Cermeno JC, Farre X, Lechner-Scott J, Vandenbroeck K, Rodriguez-Antiguedad A, Drulovic JS, MartinelliBoneschi F, Chan A, Oksenberg J, Navarro A, Montalban X, Comabella M (2015) Pharmacogenomic study in patients with multiple sclerosis: responders and nonresponders to IFN-beta. Neurol Neuroimmunol Neuroinflamm 2(5):e154. https://doi.org/10.1212/nxi.0000000000000154
Sriram U, Barcellos LF, Villoslada P, Rio J, Baranzini SE, Caillier S, Stillman A, Hauser SL, Montalban X, Oksenberg JR (2003) Pharmacogenomic analysis of interferon receptor polymorphisms in multiple sclerosis. Genes Immun 4(2):147–152. https://doi.org/10.1038/sj.gene.6363946
Mahurkar S, Moldovan M, Suppiah V, Sorosina M, Clarelli F, Liberatore G, Malhotra S, Montalban X, Antigüedad A, Krupa M, Jokubaitis VG, McKay FC, Gatt PN, Fabis-Pedrini MJ, Martinelli V, Comi G, Lechner-Scott J, Kermode AG, Slee M, Taylor BV, Vandenbroeck K, Comabella M, Boneschi FM, King C (2017) Response to interferon-beta treatment in multiple sclerosis patients: a genome-wide association study. Pharmacogenomics J 17(4):312–318. https://doi.org/10.1038/tpj.2016.20
Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A, Le J, Ohashi PS, Sarosi I, Nishina H, Lipkowitz S, Penninger JM (2000) Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature 403(6766):211–216. https://doi.org/10.1038/35003228
Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H (2000) Cbl-b regulates the CD28 dependence of T-cell activation. Nature 403(6766):216–220. https://doi.org/10.1038/35003235
Lopez-Gomez C, Pino-Angeles A, Orpez-Zafra T, Pinto-Medel MJ, Oliver-Martos B, Ortega-Pinazo J, Arnaiz C, Guijarro-Castro C, Varade J, Alvarez-Lafuente R, Urcelay E, Sanchez-Jimenez F, Fernandez O, Leyva L (2013) Candidate gene study of TRAIL and TRAIL receptors: association with response to interferon beta therapy in multiple sclerosis patients. PLoS ONE 8(4):e62540. https://doi.org/10.1371/journal.pone.0062540
Sommer B, Keinanen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg PH (1990) Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science (New York, NY) 249(4976):1580–1585. https://doi.org/10.1126/science.1699275
Vaden JH, Tian T, Golf S, McLean JW, Wilson JA, Wilson SM (2019) Chronic over-expression of ubiquitin impairs learning, reduces synaptic plasticity, and enhances GRIA receptor turnover in mice. J Neurochem 148(3):386–399. https://doi.org/10.1111/jnc.14630
Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, Yuan J, Gurney A, Goddard AD, Godowski P, Ashkenazi A (1997) A novel receptor for Apo2L/TRAIL contains a truncated death domain. Current biology : CB 7(12):1003–1006. https://doi.org/10.1016/s0960-9822(06)00422-2
Dorr J, Bechmann I, Waiczies S, Aktas O, Walczak H, Krammer PH, Nitsch R, Zipp F (2002) Lack of tumor necrosis factor-related apoptosis-inducing ligand but presence of its receptors in the human brain. J Neurosci 22(4):Rc209
Shi GP, Webb AC, Foster KE, Knoll JH, Lemere CA, Munger JS, Chapman HA (1994) Human cathepsin S: chromosomal localization, gene structure, and tissue distribution. J Biol Chem 269(15):11530–11536
Steimle A, Kalbacher H, Maurer A, Beifuss B, Bender A, Schafer A, Muller R, Autenrieth IB, Frick JS (2016) A novel approach for reliable detection of cathepsin S activities in mouse antigen presenting cells. J Immunol Methods 432:87–94. https://doi.org/10.1016/j.jim.2016.02.015
Hovnanian A, Rebouillat D, Mattei MG, Levy ER, Marie I, Monaco AP, Hovanessian AG (1998) The human 2’,5’-oligoadenylate synthetase locus is composed of three distinct genes clustered on chromosome 12q24.2 encoding the 100-, 69-, and 40-kDa forms. Genomics 52(3):267–277. https://doi.org/10.1006/geno.1998.5443
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11):1444–1452. https://doi.org/10.1212/wnl.33.11.1444
Roxburgh RH, Seaman SR, Masterman T, Hensiek AE, Sawcer SJ, Vukusic S, Achiti I, Confavreux C, Coustans M, le Page E, Edan G, McDonnell GV, Hawkins S, Trojano M, Liguori M, Cocco E, Marrosu MG, Tesser F, Leone MA, Weber A, Zipp F, Miterski B, Epplen JT, Oturai A, Sorensen PS, Celius EG, Lara NT, Montalban X, Villoslada P, Silva AM, Marta M, Leite I, Dubois B, Rubio J, Butzkueven H, Kilpatrick T, Mycko MP, Selmaj KW, Rio ME, Sa M, Salemi G, Savettieri G, Hillert J, Compston DA (2005) Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology 64(7):1144–1151. https://doi.org/10.1212/01.wnl.0000156155.19270.f8
Rio J, Nos C, Tintore M, Tellez N, Galan I, Pelayo R, Comabella M, Montalban X (2006) Defining the response to interferon-beta in relapsing-remitting multiple sclerosis patients. Ann Neurol 59(2):344–352. https://doi.org/10.1002/ana.20740
Rio J, Castillo J, Rovira A, Tintore M, Sastre-Garriga J, Horga A, Nos C, Comabella M, Aymerich X, Montalban X (2009) Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler (Houndmills, Basingstoke, England) 15(7):848–853. https://doi.org/10.1177/1352458509104591
Jimenez-Varo E, Canadas-Garre M, Henriques CI, Pinheiro AM, Gutierrez-Pimentel MJ, Calleja-Hernandez MA (2014) Pharmacogenetics role in the safety of acenocoumarol therapy. Thromb Haemost 112(3):522–536. https://doi.org/10.1160/th13-11-0941
Team R (2006) A language and environment for statistical computing. Computing 1https://doi.org/10.1890/0012-9658(2002)083[3097:CFHIWS]2.0.CO;2
Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4:7. https://doi.org/10.1186/s13742-015-0047-8
Noronha A, Toscas A, Jensen MA (1990) Interferon beta augments suppressor cell function in multiple sclerosis. Ann Neurol 27(2):207–210. https://doi.org/10.1002/ana.410270219
Byrnes AA, McArthur JC, Karp CL (2002) Interferon-beta therapy for multiple sclerosis induces reciprocal changes in interleukin-12 and interleukin-10 production. Ann Neurol 51(2):165–174. https://doi.org/10.1002/ana.10084
Rudick RA, Ransohoff RM, Peppler R, VanderBrugMedendorp S, Lehmann P, Alam J (1996) Interferon beta induces interleukin-10 expression: relevance to multiple sclerosisR A Rudick 1, R M Ransohoff, R Peppler, S VanderBrug Medendorp, P Lehmann, J Alam. Ann Neurol 40(4):618–27. https://doi.org/10.1002/ana.410400412
Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S (2009) IFN-beta inhibits human Th17 cell differentiation. J Immunol (Baltimore, Md) 183(8):5418–5427. https://doi.org/10.4049/jimmunol.0803227
McGraw CA, Lublin FD (2013) Interferon beta and glatiramer acetate therapy. Neurotherapeutics 10(1):2–18. https://doi.org/10.1007/s13311-012-0163-4
Jazireian P, Sasani ST (2020) TRAILR1 (rs20576) and GRIA3 (rs12557782) are not associated with interferon-β response in multiple sclerosis patients. Mol Biol Rep 47(12):9659–9665. https://doi.org/10.1007/s11033-020-06026-w
Gutiérrez-Cívicos R, Hurtado AM, Torres-Moreno D, Sanchez-Blanco JJ, Español I, Consuegra-Sánchez L, Perez-Ceballos E, Gutiérrez-Meca MD, Jerez A, Conesa-Zamora P (2017) Rituximab response in follicular lymphoma is associated with the rs20575 polymorphism in TRAILR1 extrinsic apoptosis trigger. Pharmacogenet Genomics 27(2):70–77. https://doi.org/10.1097/fpc.0000000000000262
Sandoughi M, Salimi S, Shahraki-Ghadimi H, Saravani M (2020) The Impact of TRAIL (C1595T and G1525A) and DR4 (rs20576) Gene polymorphisms on systemic lupus erythematosus. Biochem Genet 58(4):649–659. https://doi.org/10.1007/s10528-020-09966-x
Alsalawy NF, Darwish RK, Kamal MM, ElTaweel AE, Shousha HI (2018) Evaluation of trail receptor 1 (DR4) polymorphisms C626G and A683C as risk factors of hepatocellular carcinoma. J Med Virol 90(3):490–496. https://doi.org/10.1002/jmv.24964
Edgünlü TG, Ozge A, Yalın O, Kul S, Erdal ME (2013) A Study of the Impact of Death Receptor 4 (DR4) Gene polymorphisms in Alzheimer’s disease. Balkan Med J 30(3):268–272. https://doi.org/10.5152/balkanmedj.2013.7455
Heredia-Galvez B, Ruiz-Cosano J, Torres-Moreno D, Español I, Morales-Lara MJ, Pérez-Ceballos E, González-Conejero R, Gutiérrez-Cívicos R, Vicente V, Pérez-Guillermo M, Conesa-Zamora P (2014) Association of polymorphisms in TRAIL1 and TRAILR1 genes with susceptibility to lymphomas. Ann Hematol 93(2):243–247. https://doi.org/10.1007/s00277-013-1864-4
Nakanishi H (2003) Microglial functions and proteases. Mol Neurobiol 27(2):163–176. https://doi.org/10.1385/mn:27:2:163
Beck H, Schwarz G, Schröter CJ, Deeg M, Baier D, Stevanovic S, Weber E, Driessen C, Kalbacher H (2001) Cathepsin S and an asparagine-specific endoprotease dominate the proteolytic processing of human myelin basic protein in vitro. Eur J Immunol 31(12):3726–3736. https://doi.org/10.1002/1521-4141(200112)31:12%3c3726::aid-immu3726%3e3.0.co;2-o
Haves-Zburof D, Paperna T, Gour-Lavie A, Mandel I, Glass-Marmor L, Miller A (2011) Cathepsins and their endogenous inhibitors cystatins: expression and modulation in multiple sclerosis. J Cell Mol Med 15(11):2421–2429. https://doi.org/10.1111/j.1582-4934.2010.01229.x
Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ, Pericak-Vance MA, Gregory SG, Rioux JD, McCauley JL, Haines JL, Barcellos LF, Cree B, Oksenberg JR, Hauser SL (2007) Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 357(9):851–862. https://doi.org/10.1056/NEJMoa073493
Al Jumah M, Al Balwi M, Hussein M, Kojan S, Al Khathaami A, Al Fawaz M, Al Muzaini B, Jawhary A, Al Abdulkareem I (2012) Association of SNPs rs6498169 and rs10984447 with multiple sclerosis in Saudi patients: a model of the usefulness of familial aggregates in identifying genetic linkage in a multifactorial disease. Mult Scler (Houndmills, Basingstoke, England) 18(10):1395–1400. https://doi.org/10.1177/1352458512440832
Hyun JW, Kim G, Kim Y, Kong B, Joung A, Park NY, Jang H, Shin HJ, Kim SH, Ahn SW, Shin HY, Huh SY, Kim W, Park MS, Kim BJ, Kim BJ, Oh J, Kim HJ (2018) Neutralizing antibodies against interferon-beta in Korean patients with multiple sclerosis. J Clin Neurol (Seoul, Korea) 14(2):186–190. https://doi.org/10.3988/jcn.2018.14.2.186
Rudick RA, Simonian NA, Alam JA, Campion M, Scaramucci JO, Jones W, Coats ME, Goodkin DE, Weinstock-Guttman B, Herndon RM, Mass MK, Richert JR, Salazar AM, Munschauer FE 3rd, Cookfair DL, Simon JH, Jacobs LD (1998) Incidence and significance of neutralizing antibodies to interferon beta-1a in multiple sclerosis. Multiple Sclerosis Collaborative Research Group (MSCRG). Neurology 50(5):1266–1272. https://doi.org/10.1212/wnl.50.5.1266
Acknowledgements
The results of this investigation are part of the doctoral thesis presented by María Isabel Carrasco Campos at the University of Granada. We appreciate the collaboration of the Asociación Granadina de Esclerosis Múltiple for liaising with additional participants for the inclusion in the cohort, as well as the Biobanco de Andalucía for the management of the corresponding samples.
Author information
Authors and Affiliations
Contributions
All authors are accountable for all aspects of the work, contributed to data interpretation, revised the manuscript critically and gave final approval. Additional contributions are as follows:
M.I.C.C.: Drafting the manuscript, conception and design of the work, data acquisition and analysis
C.P.R.: Data analysis
E.M.C.: Data acquisition
A.S.P.: Drafting the manuscript
C.A.G.: Data acquisition
M.A.C.H.: Funding acquisition
A.J.M.: Funding acquisition
M.C.G.: Drafting the manuscript, conception and design of the work, data acquisition and analysis
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the VNUH Ethics and Research Committee and performed conform the declaration of Helsinki. All patients signed an informed consent.
Consent to Participate
Written informed consent was obtained from all patients.
Consent to Publish
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carrasco-Campos, M.I., Pérez-Ramírez, C., Macías-Cortés, E. et al. Pharmacogenetic Predictors of Response to Interferon Beta Therapy in Multiple Sclerosis. Mol Neurobiol 58, 4716–4726 (2021). https://doi.org/10.1007/s12035-021-02454-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02454-2