Abstract
Cholesterol is an indispensable component of the cell membrane and plays vital roles in critical physiological processes. Brain cholesterol accounts for a large portion of total cholesterol in the human body, and its content must be tightly regulated to ensure normal brain function. Disorders of cholesterol metabolism in the brain are linked to neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and other atypical cognitive deficits that arise at old age. However, the specific role of cholesterol metabolism disorder in the pathogenesis of neurodegenerative diseases has not been fully elucidated. Statins that are a class of lipid-lowering drugs have been reported to have a positive effect on neurodegenerative diseases. Herein, we reviewed the physiological and pathological conditions of cholesterol metabolism and discussed the possible mechanisms of cholesterol metabolism and statin therapy in neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Cholesterol is one of the important components of the cell membrane and plays an important role in maintaining the structural integrity of the plasma membrane and transmembrane signal transmission. Cholesterol concentration in the human brain is about 23 mg/g of brain tissue [1]. Brain cholesterol is mainly involved in the formation of plasma membrane and axon myelin sheath. Also, it is present in large quantities in the synaptic membrane and helps in the transmission of electroneurographic signals [2]. Cholesterol-rich myelin sheath serves as an insulator to increase nerve conduction speed [2]. Impeccable regulatory mechanisms in the brain exist to keep stable the cholesterol level in the brain to maintain its normal functions. Impaired cholesterol homeostasis can cause neurodegenerative diseases, of which Alzheimer’s disease (AD) and Parkinson’s disease (PD) are representative diseases. Also, statins that are a class of lipid-lowering drugs have been reported to have a positive effect on neurodegenerative diseases. However, the specific mechanism of statin treatment of AD and PD is still unclear. Herein, we reviewed the physiological and pathological conditions of cholesterol metabolism and discussed the possible mechanisms of cholesterol metabolism and statin therapy in neurodegenerative diseases.
Brain Cholesterol Metabolism
Cholesterol Synthesis
Because peripheral cholesterol cannot enter the central nervous system through the blood–brain barrier (BBB), most intracerebral cholesterol (over 95%) is supplied by de novo syntheses mainly in the glia and slightly in the neurons [1]. In the endoplasmic reticulum (ER), acetyl-CoA is first catalyzed to 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) by HMG-CoA-synthetase, an irreversible step in cholesterol synthesis. Subsequently, HMG-CoA, under the action of HMG-CoA-reductase, is converted to mevalonate, 3-isopentenyl pyrophosphate, farnesyl pyrophosphate, squalene, and lanosterol [3]. After lanosterol synthesis, two pathways have been suggested to participate in cholesterol synthesis in the brain [4]. In the Bloch pathway of astrocytes, desmosterol (DE) is ultimately converted to cholesterol by 24-dehydrocholesterol reductase (DHCR24). The Kandutsch–Russel pathway in neurons mainly includes the precursors lathosterol (LT) and 7-dehydrocholesterol (7D) [4]. Finally, under the action of 7-dehydrocholesterol reductase (DHCR7), 7D is converted to cholesterol.
During perinatal and adolescent years, cholesterol is synthesized in large quantities to form the myelin that surrounds the axons. The rate of synthesis is fastest when the myelination process is at its peak [5]. After myelination, the efficiency of cholesterol synthesis reduces by about 90% and mainly happens in oligodendrocytes and astrocytes, especially astrocytes [6]. The newly synthesized cholesterol is rapidly transferred from the ER to the plasma membrane (PM). The synthesis process relies on ATP but is independent of the Golgi complex [7,8,9]. The complete synthesis process mainly happens in the endoplasmic reticulum (ER). Cholesterol levels in the ER vary more than those in the plasma membrane. Indeed, the environment of ER affects the total cholesterol levels of the cell. Sterol regulatory element-binding protein (SREBP-2), an inactive transcription factor immobilized on the ER membrane, plays a vital role in the regulation of cholesterol synthesis. It binds to the cholesterol detector SCAP (SREBP cleavage-activating protein). When cholesterol levels in the ER are low, SCAP guides SREBP-2 into the Golgi compartment [10]. Within the organelle, SCAP releases the N-terminal domain of SREBP-2, which is translocated to the nucleus and binds to sterol regulatory elements (SRE) in promoter regions of more than 30 target genes encoding cholesterol biosynthesis, thereby raising the cholesterol level [11, 12].
Cholesterol Transport
Cholesterol is insoluble in water and mainly exists in the form of lipoprotein, including the following types: high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), and very-low-density lipoprotein cholesterol (VLDL). In the brain, cholesterol mainly combines with apolipoprotein E (APOE) which is principally synthesized by astrocyte to form lipoprotein and then secreted through ATP-binding cassette (ABC) transporters on the cytomembrane. With dimerization and depolymerization of ABC transporters, the binding substrate is transferred to the other side of the membrane by a conformational change of ABC transporters. There are mainly three kinds of ABC transporters (ABCA1, ABCG1, and ABCG4) in the central nervous system, and ABCA1 is a key molecule in cholesterol homeostasis [13]. Lipoproteins are secreted into extracellular fluids with the help of ABCA1 transporter on the cell membrane of astrocyte and then transported to neurons. Neurons ingest lipoprotein mainly through low-density lipoprotein family receptors (LDLR).
These receptors include LRP, LRP1B, megalin/LRP2, LRP4, LRP5/6, LRP8/APOER2, and LRP11/SORL1 [14, 15]. Among them, LRP and LRP1 are the primary receptors for apolipoprotein particles in the brain. The latter is expressed in neurons, whereas the former is expressed in glial cells [16]. The APOE–lipid complex binds to the receptors to form vesicles followed by endocytosis. In the cells, APOE is separated from the lipid components and recycled back to the plasma membrane. The vesicles deliver the lipid particles to the late endosomes/lysosomes [17]. Subsequently, cholesterol leaves the late endosome/lysosome and travels to the plasma membrane or ER via the NPC1- and NPC2-mediated pathways [18].
Cholesterol Turnover
Net cholesterol excretion occurs when cholesterol synthesis exceeds cellular requirements. Supererogatory cholesterol goes through a variety of pathways to maintain normal levels. Three pathways have been identified for cholesterol conversion. Firstly, about 1% of total cholesterol is esterified by acyl-coenzyme A: acyltransferase 1 (ACAT1/SOAT1) in the ER and then stored as cholesterol ester (CE), also known as lipid droplet [19, 20]. In the second pathway, the excessive cholesterol is released via members of the ABC transporters, especially ABCA1. Cholesterol forms a complex with APOA1 lipoprotein and is released into the cerebrospinal fluid (CSF). These lipoproteins are then removed from the brain through LRP1, or scavenger receptor class B1, expressed by endothelial cells in brain capillaries [21]. In the third pathway, cholesterol is hydroxylated to 24-hydroxycholesterol (24-OHC), which enables it to pass through the lipophilic membranes, such as the BBB, much faster than cholesterol [22, 23]. This process is catalyzed by the brain-specific enzyme CYP46A1, responsible for at least 40% of brain cholesterol conversion. CYP46A1 is a brain-specific enzyme that exists in specific regions of neurons and brain areas [24, 25]. It is highly expressed in the hippocampal and cortical pyramidal neurons, Purkinje cells in the cerebellum, and interneurons in the hippocampus and cerebellum, suggesting that these cells may be sensitive to cholesterol levels in the neurons.
Cholesterol Metabolism and Neurodegenerative Diseases
Alzheimer’s Disease
AD is the leading cause of dementia among the elderly. According to the World Alzheimer Report 2019, there are about 50 million dementia patients worldwide. Pathological hallmarks of AD include senile plaques made of aggregated amyloid-β (Aβ) and neurofibrillary tangles which are the twisted fibers of tau [26]. The condition is associated with gradual loss of memory and cognitive skills and, eventually, the ability to perform simple tasks. The occurrence of AD is influenced by both genetic and environmental risk factors. However, the precise mechanisms underlying its onset and progression have not been revealed. Since the failure of many clinical trials that target Aβ, current studies are beginning to explore other potential molecular mechanisms for AD. Altered cholesterol metabolism is considered a critical factor in the pathogenesis of AD (summarized in Table 1 and Fig. 1) [27].
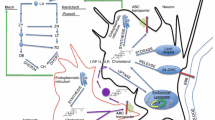
Cholesterol metabolism in Alzheimer’s disease. (1) Cholesterol is synthesized in the endoplasmic reticulum (ER) of astrocytes, and then binds APOE to form APOE-cholesterol (APOE-CH) particles. APOE-CH is secreted into extracellular fluids with the help of ABC transporters. The cholesterol is subsequently endocytosed by neurons with the LDLR/LRP2. (2) APOE-CH particles are separated inside neurons, LDLR/LRP2 return to the cell membrane, and cholesterol is metabolized in three ways. (a) One percent cholesterol is converted into lipid droplets. (b) Most of the cholesterol is catalyzed by CYP46A1 enzyme to 24-OHC. Subsequently, 24-OHC passes through the blood–brain barrier and enters the plasma, while plasma 27-OHC flows into the brain. (c) A small amount of cholesterol flows out of the cell and forms particles with ApoA1 under the mediation of the ABC transporter. 27-OHC promotes the formation of Aβ and 24-OHC inhibits Aβ production. (3) Aβ can form a complex with APOE-CH particles, which are eliminated by endocytosis (d), secreted to the peripheral system (e), or degraded by proteases (f). The APOE4 genotype shows the worst ability to mediate Aβ clearance. Abbreviations: ER, endoplasmic reticulum; BBB, brain–blood barrier; APOE-CH, APOE-cholesterol; ABC transporters, ATP-binding cassette (ABC) transporters
Hyperlipidemia in Midlife and High-Fat Diet Are Risk Factors for AD
The relationship between plasma cholesterol levels and AD is controversial (summarized in Table 1). Some studies have shown that individuals with high levels of plasma cholesterol are more prone to develop AD [27,28,29]. Also, evidence indicates that individuals with total cholesterol equal to or greater than 251 mg/dl have a doubled risk of developing AD [27]. In one study, patients with AD exhibited higher LDL levels and lower HDL levels compared with the control group. Also, it was demonstrated that increased LDL levels and decreased HDL levels had a significant positive correlation with Aβ42. This correlation was not associated with the APOE genotype [29, 32]. A prospective study including 2514 participants found that higher serum concentrations of LDL-C, TC, non-HDL-C, and LDL-C/HDL-C ratio in midlife were positively associated with accelerated global cognitive decline [44]. Intriguingly, some epidemiological studies have shown that hyperlipidemia is related to decreased risks for developing AD among the elderly [34, 35]. A recent meta-analysis found that people who suffer from hypercholesterolemia in midlife and early stages of aging had a higher risk of developing AD. However, this was not associated with the risk of cognitive impairment in late life [36]. Notably, hypocholesterolemia in late life has also been related to increased risks of cognitive impairment [27, 34]. Dementia patients often have a more conspicuous cholesterol decline in late life. One possible explanation that hypocholesterolemia in late life aggravates the cognitive impairment is that the elderly, especially in dementia patients, have low cholesterol synthesis ability, and the role of cholesterol in synaptic function is impaired, which ultimately leads to increased cognitive impairment. In another study, 266 out of 2844 people with middle-aged cholesterol levels above 240 mg/dl were diagnosed with dementia [45], suggesting that high cholesterol in middle age and hypocholesterolemia in late life could be a risk factor for AD development.
Studies have shown cholesterol-enriched diets induced AD-like pathology, including increased Aβ, tau phosphorylation, and oxidative stress in rabbits [46]. APPswe/PS1 mice fed with a high-fat diet (HFD) for 4 months also exhibited cognitive deficits, loss of synaptic plasticity, increased body weights, elevated plasma LDL oxidation, and tau phosphorylation [47]. Moreover, cognitive deficits caused by long-term exposure to a HFD were exacerbated by aging [48]. HFD-induced β-amyloid accumulation, tau phosphorylation, and cognitive decline in APP/PSEN1 mice are reversible by the control of fat intake [49]. These animal models show that a high-fat diet is an important risk factor for AD. Several recent FINGER trials, multicenter randomized controlled trials, emphasized multidomain lifestyle intervention (including HFD diet) to improve cognitive outcomes [50,51,52].
It is noteworthy that plasma cholesterol and intracerebral cholesterol are two independent systems due to the existence of the BBB, and the elevation of plasma cholesterol levels does not alter intracerebral cholesterol levels [1]. The pathogenesis of AD caused by hypercholesterolemia needs to be further explored. Considering that serum hypercholesterolemia is associated with elevated levels of 27-hydroxylcholesterol (27-OHC) and 24-OHC in the brain [29], oxysterols may be a bridge between peripheral cholesterol and brain cholesterol, pending further evidence.
Changes in Brain Cholesterol Are Associated with AD
Brain cholesterol is thought to be essential for maintaining cell morphology, neurotransmission, and synaptic formation [53]. Over the past 30 years, cholesterol levels in different brain regions and CSF have been measured in patients with AD. The cholesterol levels in the brain of AD patients either decrease or increase and, in some cases, exhibit no change depending on the region of the brain [54]. For example, the cholesterol level in the superior temporal gyrus of autopsy of AD patients was found to be lower than that in the control group [38]. Also, the cholesterol level of gray matter in the frontal cortex of AD patients with apoE4 genotype was slightly higher (2.65 ± 0.14 mg/g wet tissue weight) than that in the control group (2.04 ± 0.18) [39]. However, there was no difference in cholesterol levels in the hippocampus of AD patients compared with the control group [40]. In the basal ganglia region of AD patients, cholesterol levels are slightly higher than the control [37]. When chemical and enzymatic methods were used to measure total cholesterol in the entire brain, AD patients exhibited nearly 41.3 and 19.37% higher cholesterol levels, respectively, relative to the control group [41]. Compared with the control group, CSF–cholesterol levels in AD patients were significantly lower (about 12%) [30, 33]. In contrast, CSF–cholesterol levels of AD showed no change in an early study [42].
The results of the above experiments showed that the cholesterol levels in the brain of patients with AD varied greatly. Variations in sample selection, preparation, and determination methods could be responsible for the differences between these studies. Also, the total cholesterol level is not a good indicator of the role of cholesterol in biological processes. Besides, the presence and role of cholesterol in different regions of the cell membrane are still unknown [55]. Animal and cell culture studies have shown that membrane cholesterol domains change dramatically in the absence of or with minimal changes in total cholesterol levels. Aging, different APOE genotypes [56, 57], alcohol [57], and statins [46, 58] have been shown to alter the distribution of cholesterol across cell membranes. Also, studies have confirmed that the change in cholesterol distribution in the plasma membrane is related to Aβ production [58]. The biomass membranes isolated from the brains of AD patients at different stages of the condition are rich in cholesterol. Moreover, patients with moderate decline (Braak stage 4) have elevated cholesterol levels in their brains, whereas those with severe decline (Braak stage 6) have significantly higher cholesterol levels than those with mild decline (Braak stage 3). Cholesterol levels on cell membranes increase throughout the clinical stages of the disease [41, 59].
Cholesterol has been found to accumulate around amyloid plaques and abnormal neurites. High cholesterol levels may facilitate the production of toxic amyloid protein. Aβ peptide is produced by the proteolysis of amyloid precursor protein (APP) that can be hydrolyzed by three proteolytic enzymes: α-, β- and, γ-secretases [60]. α-Secretase breaks down APP to produce nonamyloid proteins. β- and γ-secretases lead to Aβ production [61, 62]. In vitro, cholesterol can increase the activity of β- and γ-secretase, as well as Aβ production [41]. α-Secretase is located in the nonlipid raft region, whereas β- and γ-secretases are mainly located in the lipid rafts, a microdomain rich in cholesterol and sphingolipids [63]. APP is modified by some small molecules, such as palmitoylation, to obtain greater hydrophobicity. Subsequently, decorated APP is transferred from the nonlipid rafts to the lipid raft region on the plasmalemma and then internalized into the endosome along with the lipid rafts. β-Secretase in lipid rafts cleaves the APP to produce extracellular soluble fragments and a membrane-binding fragment called C99, which is then cleaved by the γ-secretase to produce the amyloid peptides Aβ-40 and Aβ-42 and the APP intracellular domain (AICD) [41, 63, 64].
Interestingly, the C99 region of the APP is reported to have a cholesterol-binding domain [65], and it is speculated that the combination of APP and cholesterol in lipid rafts may be related to the production of toxic Aβ. This speculation was further confirmed by a previous study, which revealed that an inhibitor of BACE1 on lipid rafts results in decreased levels of Aβ in the hippocampus of an AD mouse model [66]. Contrarily, elevated cholesterol levels can increase the production of the toxic Aβ peptide through the action of β- and γ-secretases on APP [61]. This phenomenon was confirmed by experiments performed in primary neurons and HEK cells [64]. Furthermore, cholesterol has been shown to enhance the phosphorylation of tau through proteasome mediation, but the specific molecular mechanisms remain unknown. Further studies are needed to elucidate the relationship between cholesterol, Aβ, and tau pathology [67]. Based on the current evidence, lowering intracellular cholesterol or changing cholesterol distribution might reduce the formation of Aβ peptide and AD pathology.
GWAS AD-Risk Loci Implicated in Lipid Metabolism
A large genome-wide association (GWAS) meta-analysis of clinically diagnosed late-onset AD (94,437 individuals) confirmed that APOE, CLU (encoding for clusterin/APOJ), ABCA7, and TREM2 were the crucial risk loci [68].
In the brain, APOE is produced by astrocytes, which interact with a variety of lipoprotein receptors to facilitate cholesterol transport and lipid metabolism. There are three common APOE alleles in humans (i.e., APOE-e2, APOE-e3, and APOE-e4), which occur at frequencies of 8.4, 77.9, and 13.7%, respectively. In AD patients, the frequency of APOE-e4 increases by 40% [69]. Genome-wide association studies have demonstrated that the ε4 allele is the most impactful genetic risk factor for AD, whereas the APOE-e2 allele has a protective effect on AD [70,71,72]. Compared with other APOE subtypes, ApoE-e4 is less efficient in membrane lipid circulation and neuronal repair, possibly because structural differences between APOE subtypes determine their ability to bind lipids and receptors [73,74,75]. The APOE genotype, especially APOE-e4, strongly facilitates the deposition of Aβ to form senile plaques [76]. Also, positive amyloid imaging occurs earlier in intact APOE-e4 carriers (about 56 years old) than in noncarriers (76 years old) [77]. These findings suggest that APOE-e4 might increase the incidence of AD by promoting the production and aggregation of Aβ, and impairs its clearance. Also, a truncated segment of APOE-e4, caused by proteolytic cleavage of APOE-e4 after stress or injury, increases tau hyperphosphorylation [78]. At present, the mystery of how apoE-e4 increases AD risk has not been solved.
CLU is viewed as the third greatest genetic risk factor for LOAD after APOE and BIN1. It is mainly synthesized and secreted by astrocytes and participates in various processes including lipid transport, chaperone function, inhibition of the complement system, and regulation of neuronal cell survival and cell death pathways. In the CSF and brain of AD individuals, CLU levels are markedly increased. Moreover, a combined proteomic and neuroimaging approach confirmed that increased plasma CLU is positively related to atrophy of the entorhinal cortex and hippocampus regions, baseline disease severity, and rapid clinical progress in AD individuals [79]. Increased plasma CLU will accelerate cerebral deposition of Abeta and memory impairment, which was further demonstrated in APP/PS1 transgenic mice.
ABCA7 (ATP-binding cassette, subfamily A, member 7) is another novel risk gene of AD. Common single nucleotide polymorphisms (SNPs) of ABCA7 related to AD mainly include intronic SNP rs3764650, missense variant rs3752246, rs4147929, and rs115550680. ABCA7 sentinel SNPs rs3752246 and rs3764650 were significantly associated with increased amyloid deposition. In addition, common ABCA7 risk alleles can increase cortical and hippocampal atrophy at preclinical stages of AD [80].
TREM2 (Triggering receptor expressed on myeloid cells 2) is a lipid and lipoprotein receptor in microglia. GWAS indicated R47H mutation can cause partial loss of TREM2 function and increase the incidence of AD [81]. TREM2-deficient microglia are difficult in clearing myelin cholesterol, finally resulting in CE accumulation. Loss-of-function variants of TREM2 causing cholesterol metabolism disorder can be the reason for higher Alzheimer’s disease risk [82].
Oxysterols Play an Important Role in the Pathogenesis of AD
The levels of plasma 24-OHC are directly related to brain cholesterol levels and are positively correlated with the content of 24-OHC in the brain [83]. As such, when 24-OHC flows from the brain into the peripheral circulation, 27-OHC also flows into the brain. 27-OHC is a product of cholesterol metabolism, catalyzed by cholesterol 27-hydroxylase (CYP27A1) [84]. Both 24-OHC and 27-OHC are physiological inhibitors of brain cholesterol biosynthesis. Increasing the outflow of 24-OHC through the BBB can promote cholesterol synthesis in the brain [85, 86]. A study reported that the 24-OHC levels in CSF and plasma of AD patients were altered compared with the control group [43]. In the brains of AD patients, the quantification of oxysterols showed a significant decrease in 24-OHC content in the later stages of AD, while other oxysterols, such as 27-OHC and 25-OHC, increased significantly. At the same time, in the early stages of AD, the CYP46A1 level significantly decreased, while the CYP27A1 level increased [43]. According to a study in 2012, plasma 24-OHC levels were significantly higher in AD patients (78.82 ng/ml) than in the control group (62.28 ng/ml). However, the CSF level was 2.99 ng/ml and 2.68 ng/ml, respectively, with no significant difference [33]. Patients with AD consistently exhibit lower levels of 24-OHC in all areas of the brain relative to normal individuals [37]. Based on these findings, plasma 24-OHC has been proposed as an early marker for AD [87]. Besides, CYP46A1 and its main metabolic product, 24-OHC, have been studied in different in vitro and in vivo models. Severe deficits in behavioral learning and LTP have been observed in the hippocampus of 24-hydroxylase knockout mice [25]. Moreover, studies in mice overexpressing CYP46A1 found that increased CYP46A1 activity might improve memory. Circulating levels of 24-OHC in mice overexpressing CYP46A1 were found to be six times higher than in wild-type mice [88]. Activation of CYP46A1 was also shown to reduce the production of Aβ [89, 90]. One possible explanation for this is that increased synthesis of 24-OHC might have increased the activity of the α-secretase by lowering cholesterol levels in cell membranes, leading to the degradation of APP into nontoxic peptides [90, 91].
These findings suggest that 24-OHC and CYP46A1 could be protective factors for AD. Therefore, improving the levels of 24-OHC and the activity of CYP46A1 in the brain might be an effective therapeutic strategy for AD. On the contrary, evidence shows that 27-OHC is a risk factor for developing AD (Fig. 2). 27-OHC is the cholesterol metabolite with the highest concentration in plasma and low levels in the brain [37, 92]. 27-OHC was increased in the CSF and plasma of patients with early-onset AD and sporadic AD [37, 93]. Autopsy results of patients with AD revealed low levels of CYP7B1, an enzyme responsible for 27-OHC metabolism. Reduced CYP7B1 activity may be responsible for elevated 27-OHC levels [93, 94]. Furthermore, BBB dysfunction may accelerate peripheral 27-OHC inflow into CSF [37]. Several studies have associated high levels of 27-OHC with memory deficits, AD, and other neurodegenerative processes [95,96,97,98,99,100,101]. Also, hypercholesterolemia and a high-fat diet are often accompanied by an increase in 27-OHC. Subsequently, the release of excess 27-OHC from the circulation into the brain reduces the brain glucose uptake, GLut4 expression, and spatial memory [97, 98]. This may be the mechanism linking hypercholesterolemia and high-fat diet to AD. High levels of 27-OHC may also activate the renin–angiotensin system (RAS) in the brain, thus leading to impaired cognitive function, oxidative stress, and ischemic brain injury. Activation of the RAS can also lead to hypertension and insulin resistance, which are known risk factors for AD [99, 101]. High levels of 27-OHC can increase phosphorylated tau and Aβ by activating β-secretase [102]. These results indicate that 27-OHC may play a significant role in the pathogenesis of AD.
The role of cholesterol metabolites 27-OHC and 24-OHC in Alzheimer’s disease. Cholesterol is catalyzed by enzymes to produce 27-OHC and 24-OHC. 27-OHC can promote the development of Alzheimer’s disease by promoting Aβ production and oxidative stress. Conversely, 24-OHC can activate α-secretase, reduce the production of toxic Aβ, and finally play a protective role in Alzheimer’s disease. Abbreviations: CYP27A1, cholesterol 27-hydroxylase; CYP46A1, cholesterol 24-hydroxylase; 27-OHC, 27-hydroxyl cholesterol; 24-OHC, 24-hydroxycholesterol
Statin Therapy and AD
Statins are a class of drugs commonly used to lower lipid levels in the blood. The drugs have also been reported to lower Aβ levels in vitro. It is thought that statins activate ADAM10 and increase the activity of phospholipid transporters (PLTP), thus resulting in the reduction of p-tau181 (summarized in Table 1) [61, 103,104,105,106,107]. Several studies have demonstrated that statins have anti-inflammatory, antioxidant, and antithrombotic properties.
Although the protective effects of statins in preclinical trials are highly consistent, the results of clinical trials remain controversial (summarized in Table 2). Previous studies have shown that statins can sharply reduce the risk of AD by up to 70% [113, 119]. In contrast, a cohort study involving 2798 individuals found no association between statin therapy and lower risk of dementia [120]. The inconsistencies in the results can be ascribed to differences in the severity of the disease among patients, as well as variations in outcome evaluation indicators. Furthermore, different forms of statins vary in their physical and chemical properties. For example, lipophilic statins, rather than hydrophilic statins, have been shown to attenuate the progression of AD from mild to moderate, possibly because lipophilic statins are more prone to cross the BBB [104]. Lipophilic simvastatin and lovastatin have been extensively used to improve memory and learning, whereas hydrophilic pravastatin has no such effects. These results indicate that statins have the potential to treat AD [114, 115, 121]. However, more evidence from multicenter randomized controlled trials is needed to confirm these findings.
Parkinson’s Disease
PD is the second most common progressive neurodegenerative disease after AD. Currently, the disease affects about 1% of the population over the age of 60 years. Its prevalence increases with age [122]. The pathological features of PD include the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the presence of Lewy bodies (LBs) or Lewy neurites formed by the accumulation of α-synuclein [123]. The clinical features are mainly motor symptoms, including resting tremor, bradykinesia, rigidity, and postural instability [123]. Nonmotor symptoms of AD include sleep disorders, dysautonomia, and depression. PD is caused by several factors [124]. So far, 23 genes or loci have been identified to be associated with PD [125]. There is increasing evidence that cholesterol metabolism may also play a role in the pathogenesis of PD (summarized in Table 3 and Fig. 3).
Cholesterol metabolism in Parkinson’s disease. After APOE-cholesterol particles are endocytosed into neurons, cholesterol is metabolized to 27-OHC and other oxysterols. 27-OHC can increase the level of α-synuclein, downregulate the activity of tyrosine hydroxylase (TH), and cause oxidative stress and apoptosis. In addition, excessive cholesterol and oxysterol can promote the aggregation of α-synuclein, and the aggregated α-synuclein will eventually form Lewy bodies (LBs). A large number of lipid droplets, organelle membranes, and oxysterols are found in the LBs. Abbreviations: TH, tyrosine hydroxylase; LBs, Lewy bodies
Plasma Cholesterol Is Related with PD
Lower levels of LDL and VLDL are risk factors for PD [126, 146]. Notably, low LDL, rather than low total cholesterol, is widely recognized as a risk factor for developing PD [127]. However, the role of total cholesterol in PD has remained controversial. Several case–control studies have found no difference in overall cholesterol levels between PD patients and healthy controls [130, 131]. Conversely, according to some prospective studies, high levels of total cholesterol were even found to be associated with a lower risk of PD [132, 133]. In another study, multivariate analysis indicated that high total cholesterol increased the risk of PD among individuals aged 25–54 years, but the association was not significant after 55 years [134]. There are several possible reasons for these contradictory results. Firstly, these studies are mostly retrospective studies that are prone to bias, especially information bias. Secondly, cholesterol levels decline with age, and people with PD often develop the condition in their 60s. Therefore, observational studies often find a decrease in total cholesterol levels in PD patients. Furthermore, PD patients need continuous exogenous supplementation of dopamine, which can cause depletion of serum cholesterol [147]. Finally, the nonmotor system symptoms of PD, such as constipation, mental problems, and olfaction disorders, can change the lifestyle of PD patients, which can influence their serum cholesterol levels. Based on these findings, high total cholesterol levels in young and middle-aged individuals can promote the development of PD just as much as low levels of LDL can, and this has been confirmed in animal models of high-fat diets. For example, plasma cholesterol was increased threefold, mitochondrial complex I and II activity was reduced in the cerebral cortex, and reactive gliosis, as well as pro-inflammatory media, was overexpressed in the hippocampus of mice on a high-fat diet [148, 149]. A high-fat diet has been associated with increased striatum and substantia nigra dopamine depletion and the presence of oxidative stress in the SN in 6-OHDA-induced Parkinsonian rats [150].
At present, the mechanisms of how high total cholesterol and low LDL affect the pathogenesis of PD are unknown, but several possible hypotheses have been put forward. Firstly, the aggregation of α-synuclein is believed to play a vital role in the pathogenesis of PD [151]. Interestingly, cholesterol treatment can promote the aggregation of α-synuclein in B103 cells that overexpress α-synuclein, whereas statins can reduce the level of α-synuclein. Therefore, we hypothesize that high total plasma cholesterol could promote the aggregation of α-synuclein in the blood. α-Synuclein oligomers and fibrils flow with the blood to different organs of the body, including the brain and digestive tract. In doing so, they deposit in the regions with abundant blood flow, in which they serve as seeds and induce more subsurface aggregation. Secondly, high cholesterol level indicates high levels of oxysterol 27-OHC, 24-OHC, and 25-OHC. These oxidized sterols have toxic effects, as described below.
Brain Cholesterol in PD
No study has reported changes in brain cholesterol levels in PD patients. However, there have been reports on the decline in cholesterol levels in the lipid raft region [152]. The integrity of the lipid raft region is maintained by stable sphingolipids and cholesterol ratio [153]. Furthermore, localized lipid homeostasis helps to maintain normal α-synuclein structure in the cell membrane.
Low Apolipoprotein A-I Is a Strong Risk Factor for PD
The motor symptoms of PD patients are associated with low levels of apolipoprotein A-I (ApoA1), a transporter component of HDL. The risk of PD was reduced by 26% for every ApoA1 tertile increase [128, 129]. This may be attributed to the protective effect of the ApoA1 on dopamine neurons. Higher ApoA1 level confers a defined dopaminergic system. The effect of low plasma ApoA1 on the central nervous system is yet to be identified. However, it is known to be a modifier that exacerbates PD. Evidence on the relationship between ApoA1 and oxygenase 1 (PON1) which has antioxidant effects suggests a potential mechanism. Higher plasma levels of ApoA1 enhanced PON1 activity. Therefore, little cholesterol was converted from LDL to 27-OHC, an oxidized cholesterol metabolite that increases α-synuclein levels [154, 155]. Unlike APOE which is synthesized in astrocytes, ApoA1 is generated from plasma HDL by SR-BI-mediated choroid plexus [156]. The effect of this mechanism on the pathogenesis of PD remains unidentified. Furthermore, it was reported that ApoA1 is co-immunocaptured together with α-synuclein from human plasma [157], suggesting that ApoA1 bridges α-synuclein to the BBB. The α-synuclein accumulates in the CNS as a result of its defective transport by BBB.
Cholesterol Interacts with α-Synuclein
Recent studies have shown that LBs contains a large amount of α-synuclein, organelles, plasma membrane, and lipid droplets [158]. The same phenomenon was observed in transgenic mice that overexpress human α-synuclein [159]. Extensive membranous structures and tubulovesicular architecture were observed in the presynaptic terminals of transgenic mice. The membranous organelles and vesicle-like structures contain high levels of α-synuclein [160]. It has been proved that overexpression of α-synuclein inhibits the transport and the release of intersynaptic vesicles [161], and this affects the entire recycling pool within the synapse. It was reported that α-synuclein has a high affinity to lipids, and they bind to cell membranes or membrane structures and directly affect their kinetics. Besides, isopentenyl diphosphate isomerase, an enzyme mediating cholesterol synthesis, was detected in LBs [162]. This indicates that cholesterol metabolism could potentially have a pathological role in the aggregation of α-synuclein. When cells overexpressing α-synuclein were treated with cholesterol (25 mM, 6 h), more α-synuclein aggregates were found [163]. Moreover, it has been reported that α-synuclein accumulates around lipid droplets saturated with triglycerides and cholesterol [159]. α-Synuclein, therefore, has a similar structure as that of the apolipoproteins [164, 165]. The structure of α-synuclein is made up of 140 amino acids and can be divided into three domains: N-terminal lipid-binding α-helix (residues 1–87), amyloid-binding central domain (residues 61–95) known as NAC, and C-terminal acidic tail (residues 96–140). α-Synuclein is characterized by a tandem repeat in the helical region as in the apolipoprotein. These repeats induce the protein to form a helical structure and, thus, can bind to lipids. The helices prevent the 61–95 sequence from forming a β-sheet that aggregates the protein. The two structural cholesterol-binding domains in α-synuclein give it a strong tendency to bind to the lipid membrane, particularly in cholesterol-rich regions. This suggests that cholesterol can promote the insertion of α-synuclein into lipid rafts through a virus-like fusion mechanism [165]. In addition, α-synuclein promotes the outflow of cholesterol in SH-SY5Y cells [166]. In vivo experiments have shown that the LPR1 receptor may facilitate the outflow α-synuclein from the brain into the peripheral system [167]. Quantitative analysis in a study on the brains of SNCA knockout (KO) mice showed an increase in the levels of cholesterol, cholesterol ester, and triacylglycerol by 1.1 times, 1.6 times, and 1.4 times, respectively [168]. The above observations suggest that α-synuclein may have a role in mediating cholesterol transport. Besides, cholesterol was reported to mediate the interaction between oligomeric α-synuclein and the plasma membrane, which destroys the plasma membrane and eventually leads to cell death [169]. Moreover, at a low concentration of APOE, α-synuclein is more prone to aggregate. A high concentration of APOE inhibits α-synuclein aggregation [170]. It might hint that α-synuclein and APOE were competitively bounded to cholesterol.
Cholesterol Affects Dopamine Transport
Dopamine signaling plays an important role in several processes including motor control, cognition, and emotional processing. Dopamine transporter (DAT) and vesicular monoamine transporter 2 (VMAT2) are the key regulators in dopamine release and signaling dynamics. The crystal structure of the DAT is composed of two conserved cholesterol-like molecules. This suggests that the protein may interact directly with cholesterol. In the absence of cholesterol, it is subjected to conformational changes that initiated synaptic dopamine reuptake. However, in the presence of bounded cholesterol, these conformational changes are inhibited [171]. Cholesterol strengthens the H-bond that attaches dopamine and levodopa to the plasma membrane [172]. Cholesterol strengthens the bond, thereby affecting dopamine metabolism. Methyl-β-cyclodextrin (mβCD) depletes membrane-bound cholesterol and significantly reduces the rate of dopamine reuptake and excretion [173]. Of note, the stability of cholesterol levels is required for dopamine metabolism. Excess amounts of dopamine trigger cholesterol biosynthesis by activating the JNK3/SREBP2 signaling pathway in primary cultured astrocytes [174]. In the end, cholesterol overload causes the dysfunction of DAT and dopaminergic neurons [175]. The dopamine–cholesterol interaction is of interest for future research on PD therapy as it is indicated in the honeymoon phase of levodopa treatment.
Elevated Oxysterols Lead to PD
Oxysterols particularly 24-OHC, 27-OHC, and secosterol are associated with PD. Evidence from a study showed that the level of 24-OHC in CSF in PD patients increased, whereas the 24-OHC level in plasma decreased by 67% [136, 137]. It was observed that 24-OHC in CSF was significantly correlated with the duration of the disease [138]. Based on these findings, 24-OHC was proposed as a PD biomarker. 24-OHC has been found to induce cell injury in SH-SY5Y cells. The lipid droplets and esterified 24-OHC were found to accumulate in cells [155, 176,177,178]. Furthermore, another study established a 10% increase in the CSF 27-OHC level in PD patients [138]. In human dopaminergic neurons, 27-OHC increased the α-synuclein level by inhibiting the proteasome and activating the liver X receptors (LXRs) [179, 180]. Besides, 27-OHC inhibited estrogen receptors to reduce the expression of tyrosine hydroxylase (TH), the speed-limiting enzyme for dopamine synthesis [181]. In the brain tissue of Lewy body diseases, elevated levels of oxidized cholesterol metabolites can accelerate α-synuclein fibrillization, trigger apoptosis, and increase intracellular ROS levels [182, 183].
Statin Therapy and PD
Based on the above findings, cholesterol and oxidized sterols are potential therapeutic targets for PD. Studies have been focusing on the effects of statins on PD over the past decade. Reports from the literature indicate that most statins are linked to the BBB, and chronic treatment is associated with anti-inflammatory effects, inhibition of oxidative stress, and prevention of neuronal apoptosis (summarized in Table 4) [198, 199]. Statin was found to increase nitric oxide bioavailability. It also regulates the inflammatory response by releasing less pro-inflammatory cytokines and inhibits NF-κB activation [198]. Simvastatin treatment inhibited N-methyl-d-aspartic acid receptor 1 (NMDAR1) and attenuated neuroinflammation in 6-hydroxydopamine-treated PC12 cells [184]. Moreover, this anti-inflammatory effect prevented the death of dopamine neurons [185].
Despite several successful preclinical trials of statins for PD, clinical trials have not made gratifying progress (summarized in Table 4). Most observational studies have shown that the use of statins can reduce the risk of PD by 55%, whereas several clinical trials found that statins are harmful to PD patients. Factors including the type and dose of the drugs, disease severity, and outcome measures may be attributed to the unsuccessful clinical trials. Recent clinical researches on the efficacy of statins are mainly observational studies that are considered bias. Therefore, well-designed controlled trials are needed to illustrate the effect of statins on PD.
Conclusions
Cholesterol metabolism in the brain is a delicate and complex process. Cholesterol is required for several physiological functions of the brain, such as synaptic development and synaptic transmission. Therefore, any alteration in its metabolism causes brain dysfunctions. There is an urgent need to perform in-depth studies on the specific role of cholesterol metabolism in neurodegenerative diseases. In addition, the effects of cholesterol/protein and protein/protein interactions may provide insights into the potential pharmacological targets for early clinical intervention of neurodegenerative diseases.
Abbreviations
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- HD:
-
Huntington’s disease
- BBB:
-
Blood–brain barrier
- ER:
-
Endoplasmic reticulum
- HMG-CoA:
-
3-Hydroxy-3-methylglutaryl CoA
- DE:
-
Desmosterol
- DHCR24:
-
24-Dehydrocholesterol reductase
- DHCR7:
-
7-Dehydrocholesterol reductase
- LT:
-
Lathosterol
- 7D:
-
7-Dehydrocholesterol
- PM:
-
Plasma membrane
- SREBP-2:
-
Sterol regulatory element-binding protein
- SCAP:
-
SREBP cleavage-activating protein
- SRE:
-
Sterol regulatory elements
- HDL:
-
High-density lipoprotein cholesterol
- LDL:
-
Low-density lipoprotein cholesterol
- VLDL:
-
Very-low-density lipoprotein cholesterol
- APOE:
-
Apolipoprotein E
- ABC transporters:
-
ATP-binding cassette (ABC) transporters
- LDLR:
-
Low-density lipoprotein family receptors
- ACAT1/SOAT1:
-
Acyltransferase 1
- CSF:
-
Cerebrospinal fluid
- 24-OHC:
-
24-Hydroxycholesterol
- Aβ:
-
Amyloid-β
- ACID:
-
APP intracellular domain
- APP:
-
Amyloid precursor protein
- 27-OHC:
-
27-Hydroxylcholesterol
- CYP27A1:
-
Sterol 27-hydroxylase
- RAS:
-
Renin–angiotensin system
- PLTP:
-
Phospholipid transporters
- SNpc:
-
Substantia nigra pars compacta
- LBs:
-
Lewy bodies
- ApoA1:
-
Apolipoprotein A-I
- PON1:
-
Oxygenase 1
- VMAT2:
-
Vesicular monoamine transporter 2
- DAT:
-
Dopamine transporter
- mβCD:
-
Methyl-β-cyclodextrin
- LXRs:
-
Liver X receptors
- TH:
-
Tyrosine hydroxylase
- NMDAR1:
-
N-methyl-d-aspartic acid receptor 1
- TNF-α:
-
Tumor necrosis factor-α
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- NO:
-
Nitric oxide
References
Dietschy JM, Turley SD (2004) Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res 45:1375–1397. https://doi.org/10.1194/jlr.R400004-JLR200
Vance JE (2012) Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative diseases. Dis Model Mech 5:746–755. https://doi.org/10.1242/dmm.010124
Berg JM, Tymoczko JL, Stryer L (2002) The complex regulation of cholesterol biosynthesis takes place at several levels. In: Biochemistry, 5th edn. W.H. Freeman, New York
Nieweg K, Schaller H, Pfrieger FW (2009) Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J Neurochem 109:125–134. https://doi.org/10.1111/j.1471-4159.2009.05917.x
Saher G, Brügger B, Lappe-Siefke C et al (2005) High cholesterol level is essential for myelin membrane growth. Nat Neurosci 8:468–475. https://doi.org/10.1038/nn1426
Quan G, Xie C, Dietschy JM, Turley SD (2003) Ontogenesis and regulation of cholesterol metabolism in the central nervous system of the mouse. Brain Res Dev Brain Res 146:87–98. https://doi.org/10.1016/j.devbrainres.2003.09.015
DeGrella RF, Simoni RD (1982) Intracellular transport of cholesterol to the plasma membrane. J Biol Chem 257:14256–14262
Kaplan MR, Simoni RD (1985) Transport of cholesterol from the endoplasmic reticulum to the plasma membrane. J Cell Biol 101:446–453. https://doi.org/10.1083/jcb.101.2.446
Heino S, Lusa S, Somerharju P et al (2000) Dissecting the role of the Golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc Natl Acad Sci 97:8375–8380. https://doi.org/10.1073/pnas.140218797
Leoni V, Caccia C (2015) The impairment of cholesterol metabolism in Huntington disease. Biochim Biophys Acta 1851:1095–1105. https://doi.org/10.1016/j.bbalip.2014.12.018
Anchisi L, Dessì S, Pani A, Mandas A (2013) Cholesterol homeostasis: a key to prevent or slow down neurodegeneration. Front Physiol 3:486. https://doi.org/10.3389/fphys.2012.00486
Martin MG, Ahmed T, Korovaichuk A et al (2014) Constitutive hippocampal cholesterol loss underlies poor cognition in old rodents. EMBO Mol Med 6:902–917. https://doi.org/10.15252/emmm.201303711
Kim WS, Weickert CS, Garner B (2008) Role of ATP-binding cassette transporters in brain lipid transport and neurological disease. J Neurochem 104:1145–1166. https://doi.org/10.1111/j.1471-4159.2007.05099.x
Herz J (2009) Apolipoprotein E receptors in the nervous system. Curr Opin Lipidol 20:190–196. https://doi.org/10.1097/MOL.0b013e32832d3a10
Pottier C, Hannequin D, Coutant S et al (2012) High frequency of potentially pathogenic SORL1 mutations in autosomal dominant early-onset Alzheimer disease. Mol Psychiatry 17:875–879. https://doi.org/10.1038/mp.2012.15
Rushworth JV, Griffiths HH, Watt NT, Hooper NM (2013) Prion protein-mediated toxicity of amyloid-β oligomers requires lipid rafts and the transmembrane LRP1. J Biol Chem 288:8935–8951. https://doi.org/10.1074/jbc.M112.400358
Rensen P, Jong MC, Vark LC et al (2000) Internalization of apolipoprotein E by hepatocytes via the LDL receptor is coupled to retroendocytosis. Atherosclerosis 151:239. https://doi.org/10.1016/S0021-9150(00)81081-3
Vance JE, Karten B (2014) Niemann-Pick C disease and mobilization of lysosomal cholesterol by cyclodextrin. J Lipid Res 55:1609–1621. https://doi.org/10.1194/jlr.R047837
Bryleva EY, Rogers MA, Chang CCY et al (2010) ACAT1 gene ablation increases 24(S)-hydroxycholesterol content in the brain and ameliorates amyloid pathology in mice with AD. Proc Natl Acad Sci 107:3081–3086. https://doi.org/10.1073/pnas.0913828107
Liu B, Turley SD, Burns DK et al (2009) Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1-/- mouse. Proc Natl Acad Sci 106:2377–2382. https://doi.org/10.1073/pnas.0810895106
Panzenboeck U et al (2002) ABCA1 and SR-BI are modulators of reverse sterol transport at an in vitro blood-brain barrier constituted of porcine brain capillary endothelial cells. J Biol Chem 277:42781–42789
Lange Y, Ye J, Strebel F (1995) Movement of 25-hydroxycholesterol from the plasma membrane to the rough endoplasmic reticulum in cultured hepatoma cells. J Lipid Res 36:1092–1097
Meaney S, Bodin K, Diczfalusy U, Björkhem I (2002) On the rate of translocation in vitro and kinetics in vivo of the major oxysterols in human circulation: critical importance of the position of the oxygen function. J Lipid Res 43:2130–2135. https://doi.org/10.1194/jlr.M200293-JLR200
Lund EG, Xie C, Kotti T et al (2003) Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J Biol Chem 278:22980–22988. https://doi.org/10.1074/jbc.M303415200
Ramirez DMO, Andersson S, Russell DW (2010) Neuronal expression and subcellular localization of cholesterol 24-hydroxylase in the mouse brain. J Comp Neurol 507(5):1676–1693
Goedert M, Spillantini MG (2006) A century of Alzheimer’s disease. Science 314:777–781. https://doi.org/10.1126/science.1132814
Kivipelto M, Solomon A (2006) Cholesterol as a risk factor for Alzheimer’s disease - epidemiological evidence. Acta Neurol Scand Suppl 185:50–57. https://doi.org/10.1111/j.1600-0404.2006.00685.x
Solomon A, Kåreholt I, Ngandu T et al (2007) Serum cholesterol changes after midlife and late-life cognition: twenty-one-year follow-up study. Neurology 68:751–756. https://doi.org/10.1212/01.wnl.0000256368.57375.b7
Reed B, Villeneuve S, Mack W et al (2014) Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol 71:195. https://doi.org/10.1001/jamaneurol.2013.5390
Popp J, Meichsner S, Kölsch H et al (2013) Cerebral and extracerebral cholesterol metabolism and CSF markers of Alzheimer’s disease. Biochem Pharmacol 86:37–42. https://doi.org/10.1016/j.bcp.2012.12.007
Kivipelto M, Ngandu T, Fratiglioni L et al (2005) Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 62:1556–1560. https://doi.org/10.1001/archneur.62.10.1556
Kuo YM, Emmerling MR, Bisgaier CL et al (1998) Elevated low-density lipoprotein in Alzheimer’s disease correlates with brain abeta 1-42 levels. Biochem Biophys Res Commun 252:711–715. https://doi.org/10.1006/bbrc.1998.9652
Popp J, Lewczuk P, Kölsch H et al (2012) Cholesterol metabolism is associated with soluble amyloid precursor protein production in Alzheimer’s disease. J Neurochem 123:310–316. https://doi.org/10.1111/j.1471-4159.2012.07893.x
Reitz C, Tang M-X, Manly J et al (2008) Plasma lipid levels in the elderly are not associated with the risk of mild cognitive impairment. Dement Geriatr Cogn Disord 25:232–237. https://doi.org/10.1159/000115847
Mielke MM, Zandi PP, Sjögren M et al (2005) High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology 64:1689–1695. https://doi.org/10.1212/01.WNL.0000161870.78572.A5
Anstey KJ, Ashby-Mitchell K, Peters R (2017) Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis. J Alzheimers Dis 56:215–228. https://doi.org/10.3233/JAD-160826
Heverin M, Bogdanovic N, Lütjohann D et al (2004) Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer’s disease. J Lipid Res 45:186–193. https://doi.org/10.1194/jlr.M300320-JLR200
Mason RP, Shoemaker WJ, Shajenko L et al (1992) Evidence for changes in the Alzheimer’s disease brain cortical membrane structure mediated by cholesterol. Neurobiol Aging 13:413–419. https://doi.org/10.1016/0197-4580(92)90116-f
Sparks DL (1997) Coronary artery disease, hypertension, ApoE, and cholesterol: a link to Alzheimer’s disease? Ann N Y Acad Sci 826:128–146. https://doi.org/10.1111/j.1749-6632.1997.tb48466.x
Eckert GP, Cairns NJ, Maras A et al (2000) Cholesterol modulates the membrane-disordering effects of beta-amyloid peptides in the hippocampus: specific changes in Alzheimer’s disease. Dement Geriatr Cogn Disord 11:181–186. https://doi.org/10.1159/000017234
Xiong H, Callaghan D, Jones A et al (2008) Cholesterol retention in Alzheimer’s brain is responsible for high β- and γ-secretase activities and Aβ production. Neurobiol Dis 29:422–437. https://doi.org/10.1016/j.nbd.2007.10.005
Papassotiropoulos A, Lütjohann D, Bagli M et al (2002) 24S-hydroxycholesterol in cerebrospinal fluid is elevated in early stages of dementia. J Psychiatr Res 36:27–32. https://doi.org/10.1016/s0022-3956(01)00050-4
Testa G, Staurenghi E, Zerbinati C et al (2016) Changes in brain oxysterols at different stages of Alzheimer’s disease: their involvement in neuroinflammation. Redox Biol 10:24–33. https://doi.org/10.1016/j.redox.2016.09.001
Yu A, Zhang X, Wang Y et al (2019) Longitudinal and nonlinear relations of dietary and serum cholesterol in midlife with cognitive decline: results from EMCOA study. Mol Neurodegener 14:51. https://doi.org/10.1186/s13024-019-0353-1
Whitmer RA, Sidney S, Selby J et al (2005) Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64:277–281. https://doi.org/10.1212/01.WNL.0000149519.47454.F2
Kirsch C, Eckert GP, Koudinov AR, Müller WE (2003) Brain cholesterol, statins and Alzheimer’s disease. Pharmacopsychiatry 36(Suppl 2):S113–S119. https://doi.org/10.1055/s-2003-43058
Thériault P, ElAli A, Rivest S (2016) High fat diet exacerbates Alzheimer’s disease-related pathology in APPswe/PS1 mice. Oncotarget 7:67808–67827. https://doi.org/10.18632/oncotarget.12179
Ledreux A, Wang X, Schultzberg M et al (2016) Detrimental effects of a high fat/high cholesterol diet on memory and hippocampal markers in aged rats. Behav Brain Res 312:294–304. https://doi.org/10.1016/j.bbr.2016.06.012
Walker JM, Dixit S, Saulsberry AC et al (2017) Reversal of high fat diet-induced obesity improves glucose tolerance, inflammatory response, β-amyloid accumulation and cognitive decline in the APP/PSEN1 mouse model of Alzheimer’s disease. Neurobiol Dis 100:87–98. https://doi.org/10.1016/j.nbd.2017.01.004
Lehtisalo J, Levälahti E, Lindström J et al (2019) Dietary changes and cognition over 2 years within a multidomain intervention trial—the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). Alzheimers Dement 15:410–417. https://doi.org/10.1016/j.jalz.2018.10.001
Ngandu T, Lehtisalo J, Solomon A et al (2015) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385:2255–2263. https://doi.org/10.1016/S0140-6736(15)60461-5
Rosenberg A, Ngandu T, Rusanen M et al (2018) Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: the FINGER trial. Alzheimers Dement J Alzheimers Assoc 14:263–270. https://doi.org/10.1016/j.jalz.2017.09.006
Pfrieger FW (2003) Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci CMLS 60:1158–1171. https://doi.org/10.1007/s00018-003-3018-7
Wood WG, Igbavboa U, Eckert GP et al (2005) Is hypercholesterolemia a risk factor for Alzheimer’s disease? Mol Neurobiol 31:185–192. https://doi.org/10.1385/MN:31:1-3:185
Sonnino S, Prinetti A (2013) Membrane domains and the “lipid raft” concept. Curr Med Chem 20:4–21
Hayashi H, Igbavboa U, Hamanaka H et al (2002) Cholesterol is increased in the exofacial leaflet of synaptic plasma membranes of human apolipoprotein E4 knock-in mice. Neuroreport 13:383–386. https://doi.org/10.1097/00001756-200203250-00004
Igbavboa U, Avdulov NA, Schroeder F, Wood WG (1996) Increasing age alters transbilayer fluidity and cholesterol asymmetry in synaptic plasma membranes of mice. J Neurochem 66:1717–1725. https://doi.org/10.1046/j.1471-4159.1996.66041717.x
Burns MP, Igbavboa U, Wang L et al (2006) Cholesterol distribution, not total levels, correlate with altered amyloid precursor protein processing in statin-treated mice. NeuroMolecular Med 8:319–328. https://doi.org/10.1385/nmm:8:3:319
Cutler RG, Kelly J, Storie K et al (2004) Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci U S A 101:2070–2075. https://doi.org/10.1073/pnas.0305799101
Kang J, Lemaire HG, Unterbeck A et al (1987) The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325:733–736. https://doi.org/10.1038/325733a0
Kojro E, Gimpl G, Lammich S et al (2001) Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the α-secretase ADAM 10. Proc Natl Acad Sci U S A 98:5815–5820. https://doi.org/10.1073/pnas.081612998
Seubert P, Oltersdorf T, Lee MG et al (1993) Secretion of beta-amyloid precursor protein cleaved at the amino terminus of the beta-amyloid peptide. Nature 361:260–263. https://doi.org/10.1038/361260a0
Vetrivel KS, Thinakaran G (2010) Membrane rafts in Alzheimer’s disease beta-amyloid production. Biochim Biophys Acta 1801:860–867. https://doi.org/10.1016/j.bbalip.2010.03.007
Marquer C, Devauges V, Cossec J-C et al (2011) Local cholesterol increase triggers amyloid precursor protein-Bace1 clustering in lipid rafts and rapid endocytosis. FASEB J Off Publ Fed Am Soc Exp Biol 25:1295–1305. https://doi.org/10.1096/fj.10-168633
Barrett PJ, Song Y, Van Horn WD et al (2012) The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science 336:1168–1171. https://doi.org/10.1126/science.1219988
Makarov M, Grossard M (2008) Corrections and clarifications: efficient inhibition of the Alzheimer’s disease β-secretase by membrane targeting. Science 321:912–912
van der Kant R, Langness VF, Herrera CM et al (2019) Cholesterol metabolism is a druggable axis that independently regulates tau and amyloid-β in iPSC-derived Alzheimer’s disease neurons. Cell Stem Cell 24:363-375.e9. https://doi.org/10.1016/j.stem.2018.12.013
Kunkle BW, Grenier-Boley B, Sims R et al (2019) Author correction: genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet 51:1423–1424. https://doi.org/10.1038/s41588-019-0495-7
Liu C-C, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nat Rev Neurol 9:106–118. https://doi.org/10.1038/nrneurol.2012.263
Harold D, Abraham R, Hollingworth P et al (2009) Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet 41(10):1088–1093
Lambert J-C, Heath S, Even G et al (2009) Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet 41:1094–1099. https://doi.org/10.1038/ng.439
Corder EH, Saunders AM, Risch NJ et al (1994) Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 7:180–184. https://doi.org/10.1038/ng0694-180
Poirier J (1994) Apolipoprotein E in animal models of CNS injury and in Alzheimer’s disease. Trends Neurosci 17:525–530. https://doi.org/10.1016/0166-2236(94)90156-2
Rapp A, Gmeiner B, Hüttinger M (2006) Implication of apoE isoforms in cholesterol metabolism by primary rat hippocampal neurons and astrocytes. Biochimie 88:473–483. https://doi.org/10.1016/j.biochi.2005.10.007
Gong J-S, Kobayashi M, Hayashi H et al (2002) Apolipoprotein E (ApoE) isoform-dependent lipid release from astrocytes prepared from human ApoE3 and ApoE4 knock-in mice. J Biol Chem 277:29919–29926. https://doi.org/10.1074/jbc.M203934200
Ellis RJ, Olichney JM, Thal LJ et al (1996) Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience, part XV. Neurology 46:1592–1596. https://doi.org/10.1212/wnl.46.6.1592
Fleisher AS, Chen K, Liu X et al (2013) Apolipoprotein E ε4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol Aging 34:1–12. https://doi.org/10.1016/j.neurobiolaging.2012.04.017
Brecht WJ, Harris FM, Chang S et al (2004) Neuron-specific apolipoprotein E4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci 24:2527–2534. https://doi.org/10.1523/JNEUROSCI.4315-03.2004
Thambisetty M, Simmons A, Velayudhan L et al (2010) Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry 67:739–748. https://doi.org/10.1001/archgenpsychiatry.2010.78
De Roeck A, Van Broeckhoven C, Sleegers K (2019) The role of ABCA7 in Alzheimer’s disease: evidence from genomics, transcriptomics and methylomics. Acta Neuropathol (Berl) 138:201–220. https://doi.org/10.1007/s00401-019-01994-1
Kunkle BW, Grenier-Boley B, Sims R et al (2019) Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet 51:414–430. https://doi.org/10.1038/s41588-019-0358-2
Nugent AA, Lin K, van Lengerich B et al (2020) TREM2 regulates microglial cholesterol metabolism upon chronic phagocytic challenge. Neuron 105:837-854.e9. https://doi.org/10.1016/j.neuron.2019.12.007
Björkhem I (2006) Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med 260:493–508. https://doi.org/10.1111/j.1365-2796.2006.01725.x
Gamba P, Testa G, Gargiulo S et al (2015) Oxidized cholesterol as the driving force behind the development of Alzheimer’s disease. Front Aging Neurosci 7:119. https://doi.org/10.3389/fnagi.2015.00119
Ali Z, Heverin M, Olin M et al (2013) On the regulatory role of side-chain hydroxylated oxysterols in the brain. Lessons from CYP27A1 transgenic and Cyp27a1−/− mice. J Lipid Res 54:1033–1043. https://doi.org/10.1194/jlr.M034124
Saeed AA, Genové G, Li T et al (2014) Effects of a disrupted blood-brain barrier on cholesterol homeostasis in the brain. J Biol Chem 289:23712–23722. https://doi.org/10.1074/jbc.M114.556159
Lütjohann D, von Bergmann K (2003) 24S-hydroxycholesterol: a marker of brain cholesterol metabolism. Pharmacopsychiatry 36(Suppl 2):S102–S106. https://doi.org/10.1055/s-2003-43053
Maioli S, Båvner A, Ali Z et al (2013) Is it possible to improve memory function by upregulation of the cholesterol 24S-hydroxylase (CYP46A1) in the brain? PLoS One 8:e68534. https://doi.org/10.1371/journal.pone.0068534
Brown J, Theisler C, Silberman S et al (2004) Differential expression of cholesterol hydroxylases in Alzheimer’s disease. J Biol Chem 279:34674–34681. https://doi.org/10.1074/jbc.M402324200
Famer D, Meaney S, Mousavi M et al (2007) Regulation of alpha- and beta-secretase activity by oxysterols: cerebrosterol stimulates processing of APP via the alpha-secretase pathway. Biochem Biophys Res Commun 359:46–50. https://doi.org/10.1016/j.bbrc.2007.05.033
Simons K, Ehehalt R (2002) Cholesterol, lipid rafts, and disease. J Clin Invest 110:597–603. https://doi.org/10.1172/JCI16390
Meaney S, Heverin M, Panzenboeck U et al (2007) Novel route for elimination of brain oxysterols across the blood-brain barrier: conversion into 7alpha-hydroxy-3-oxo-4-cholestenoic acid. J Lipid Res 48:944–951. https://doi.org/10.1194/jlr.M600529-JLR200
Shafaati M, Marutle A, Pettersson H et al (2011) Marked accumulation of 27-hydroxycholesterol in the brains of Alzheimer’s patients with the Swedish APP 670/671 mutation. J Lipid Res 52:1004–1010. https://doi.org/10.1194/jlr.M014548
Yau JLW, Rasmuson S, Andrew R et al (2003) Dehydroepiandrosterone 7-hydroxylase CYP7B: predominant expression in primate hippocampus and reduced expression in Alzheimer’s disease. Neuroscience 121:307–314. https://doi.org/10.1016/s0306-4522(03)00438-x
Mateos L, Akterin S, Gil-Bea F-J et al (2009) Activity-regulated cytoskeleton-associated protein in rodent brain is down-regulated by high fat diet in vivo and by 27-hydroxycholesterol in vitro. Brain Pathol Zurich Switz 19:69–80. https://doi.org/10.1111/j.1750-3639.2008.00174.x
Björkhem I, Cedazo-Minguez A, Leoni V, Meaney S (2009) Oxysterols and neurodegenerative diseases. Mol Asp Med 30:171–179. https://doi.org/10.1016/j.mam.2009.02.001
Ismail M-A-M, Mateos L, Maioli S et al (2017) 27-Hydroxycholesterol impairs neuronal glucose uptake through an IRAP/GLUT4 system dysregulation. J Exp Med 214:699–717. https://doi.org/10.1084/jem.20160534
Heverin M, Maioli S, Pham T et al (2015) 27-Hydroxycholesterol mediates negative effects of dietary cholesterol on cognition in mice. Behav Brain Res 278:356–359. https://doi.org/10.1016/j.bbr.2014.10.018
Mateos L, Ismail M-A-M, Gil-Bea F-J et al (2011) Upregulation of brain renin angiotensin system by 27-hydroxycholesterol in Alzheimer’s disease. J Alzheimers Dis JAD 24:669–679. https://doi.org/10.3233/JAD-2011-101512
Cedazo-Mínguez A, Ismail M-A-M, Mateos L (2011) Plasma cholesterol and risk for late-onset Alzheimer’s disease. Expert Rev Neurother 11:495–498. https://doi.org/10.1586/ern.11.36
Mateos L, Ismail M-A-M, Gil-Bea F-J et al (2011) Side chain-oxidized oxysterols regulate the brain renin-angiotensin system through a liver X receptor-dependent mechanism. J Biol Chem 286:25574–25585. https://doi.org/10.1074/jbc.M111.236877
Marwarha G, Dasari B, Prasanthi JRP et al (2010) Leptin reduces the accumulation of Aβ and phosphorylated tau induced by 27-hydroxycholesterol in rabbit organotypic slices. J Alzheimers Dis JAD 19:1007–1019. https://doi.org/10.3233/JAD-2010-1298
Fassbender K, Simons M, Bergmann C et al (2001) Simvastatin strongly reduces levels of Alzheimer’s disease β-amyloid peptides Aβ42 and Aβ40 in vitro and in vivo. Proc Natl Acad Sci U S A 98:5856–5861. https://doi.org/10.1073/pnas.081620098
Lin F-C, Chuang Y-S, Hsieh H-M et al (2015) Early statin use and the progression of Alzheimer disease. Medicine (Baltimore) 94:47. https://doi.org/10.1097/MD.0000000000002143
Kurinami H, Sato N, Shinohara M et al (2008) Prevention of amyloid beta-induced memory impairment by fluvastatin, associated with the decrease in amyloid beta accumulation and oxidative stress in amyloid beta injection mouse model. Int J Mol Med 21:531–537
Li L, Cao D, Kim H et al (2006) Simvastatin enhances learning and memory independent of amyloid load in mice. Ann Neurol 60:729–739. https://doi.org/10.1002/ana.21053
Petanceska SS, DeRosa S, Olm V et al (2002) Statin therapy for Alzheimer’s disease: will it work? J Mol Neurosci MN 19:155–161. https://doi.org/10.1007/s12031-002-0026-2
Chauhan NB, Siegel GJ, Feinstein DL (2004) Effects of lovastatin and pravastatin on amyloid processing and inflammatory response in TgCRND8 brain. Neurochem Res 29:1897–1911. https://doi.org/10.1023/b:nere.0000042217.90204.8d
Paris D, Townsend KP, Humphrey J et al (2002) Statins inhibit A beta-neurotoxicity in vitro and A beta-induced vasoconstriction and inflammation in rat aortae. Atherosclerosis 161:293–299. https://doi.org/10.1016/s0021-9150(01)00660-8
Abad-Rodriguez J, Ledesma MD, Craessaerts K et al (2004) Neuronal membrane cholesterol loss enhances amyloid peptide generation. J Cell Biol 167:953–960. https://doi.org/10.1083/jcb.200404149
Puglielli L, Konopka G, Pack-Chung E et al (2001) Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nat Cell Biol 3:905–912. https://doi.org/10.1038/ncb1001-905
Hutter-Paier B, Huttunen HJ, Puglielli L et al (2004) The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer’s disease. Neuron 44:227–238. https://doi.org/10.1016/j.neuron.2004.08.043
Wolozin B, Kellman W, Ruosseau P et al (2000) Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol 57:1439–1443. https://doi.org/10.1001/archneur.57.10.1439
Wolozin B, Wang SW, Li N-C et al (2007) Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med 5:20. https://doi.org/10.1186/1741-7015-5-20
Haag MDM, Hofman A, Koudstaal PJ et al (2009) Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry 80:13–17. https://doi.org/10.1136/jnnp.2008.150433
Feldman HH, Doody RS, Kivipelto M et al (2010) Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology 74:956–964. https://doi.org/10.1212/WNL.0b013e3181d6476a
Sparks DL, Sabbagh MN, Connor DJ et al (2005) Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol 62:753–757. https://doi.org/10.1001/archneur.62.5.753
Friedhoff LT, Cullen EI, Geoghagen NS, Buxbaum JD (2001) Treatment with controlled-release lovastatin decreases serum concentrations of human beta-amyloid (A beta) peptide. Int J Neuropsychopharmacol 4:127–130. https://doi.org/10.1017/S1461145701002310
Rockwood K, Kirkland S, Hogan DB et al (2002) Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch Neurol 59:223–227. https://doi.org/10.1001/archneur.59.2.223
Rea TD, Breitner JC, Psaty BM et al (2005) Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol 62:1047–1051. https://doi.org/10.1001/archneur.62.7.1047
Wahner AD, Bronstein JM, Bordelon YM, Ritz B (2008) Statin use and the risk of Parkinson’s disease. Neurology 70:1418–1422. https://doi.org/10.1212/01.wnl.0000286942.14552.51
Tysnes O-B, Storstein A (2017) Epidemiology of Parkinson’s disease. J Neural Transm Vienna Austria 1996 124:901–905. https://doi.org/10.1007/s00702-017-1686-y
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet Lond Engl 386:896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
Sweeney P, Park H et al (2017) Protein misfolding in neurodegenerative diseases: implications and strategies. Transl Neurodegener 6:6. https://doi.org/10.1186/s40035-017-0077-5
Karimi-Moghadam A, Charsouei S, Bell B, Jabalameli MR (2018) Parkinson disease from Mendelian forms to genetic susceptibility: new molecular insights into the neurodegeneration process. Cell Mol Neurobiol 38:1153–1178. https://doi.org/10.1007/s10571-018-0587-4
Wei Q, Wang H, Tian Y et al (2013) Reduced serum levels of triglyceride, very low density lipoprotein cholesterol and apolipoprotein B in Parkinson’s disease patients. PLoS ONE 8. https://doi.org/10.1371/journal.pone.0075743
Huang X, Chen H, Miller WC et al (2007) Lower low density lipid cholesterol levels are associated with Parkinson’s disease. Mov Disord Off J Mov Disord Soc 22:377–381. https://doi.org/10.1002/mds.21290
Swanson CR, Berlyand Y, Xie SX et al (2015) Plasma ApoA1 associates with age at onset and motor severity in early Parkinson disease patients. Mov Disord Off J Mov Disord Soc 30:1648–1656. https://doi.org/10.1002/mds.26290
Qiang JK, Wong YC, Siderowf A et al (2013) Plasma apolipoprotein A1 as a biomarker for Parkinson’s disease. Ann Neurol 74:119–127. https://doi.org/10.1002/ana.23872
Teunissen CE, Lütjohann D, Bergmann KV et al (2003) Combination of serum markers related to several mechanisms in Alzheimer’s disease. Neurobiol Aging 24:893–902. https://doi.org/10.1016/S0197-4580(03)00005-8
Sohmiya M, Tanaka M, Tak NW et al (2004) Redox status of plasma coenzyme Q10 indicates elevated systemic oxidative stress in Parkinson’s disease. J Neurol Sci 223:161–166. https://doi.org/10.1016/j.jns.2004.05.007
de Lau LML, Koudstaal PJ, Albert H, Breteler MMB (2006) Serum cholesterol levels and the risk of Parkinson’s disease. Am J Epidemiol 10. https://doi.org/10.1093/aje/kwj283
Rozani V, Gurevich T, Giladi N et al (2018) Higher serum cholesterol and decreased Parkinson’s disease risk: a statin-free cohort study. Mov Disord. https://doi.org/10.1002/mds.27413
Hu G, Antikainen R, Jousilahti P et al (2008) Total cholesterol and the risk of Parkinson disease. Neurology 70:1972–1979. https://doi.org/10.1212/01.wnl.0000312511.62699.a8
Hu G (2009) Total cholesterol and the risk of Parkinson’s disease: a review for some new findings. Park Dis 2010:836962. https://doi.org/10.4061/2010/836962
Peter S et al (2002) Cerebrospinal fluid 24S-hydroxycholesterol is increased in patients with Alzheimer’s disease compared to healthy controls. Neurosci Lett. https://doi.org/10.1016/S0304-3940(02)00164-7
Lee C-YJ, Seet RCS, Huang SH et al (2009) Different patterns of oxidized lipid products in plasma and urine of dengue fever, stroke, and Parkinson’s disease patients: cautions in the use of biomarkers of oxidative stress. Antioxid Redox Signal 11:407–420. https://doi.org/10.1089/ars.2008.2179
Björkhem I, Lövgren-Sandblom A, Leoni V et al (2013) Oxysterols and Parkinson’s disease: evidence that levels of 24S-hydroxycholesterol in cerebrospinal fluid correlates with the duration of the disease. Neurosci Lett 555:102–105. https://doi.org/10.1016/j.neulet.2013.09.003
Huang X, Chen PC, Poole C (2004) APOE-[epsilon]2 allele associated with higher prevalence of sporadic Parkinson disease. Neurology 62:2198–2202. https://doi.org/10.1212/01.wnl.0000130159.28215.6a
Wakabayashi K, Kakita A, Hayashi S et al (1998) Apolipoprotein E epsilon4 allele and progression of cortical Lewy body pathology in Parkinson’s disease. Acta Neuropathol (Berl) 95:450–454. https://doi.org/10.1007/s004010050824
Huang X, Chen P, Kaufer DI et al (2006) Apolipoprotein E and dementia in Parkinson disease: a meta-analysis. Arch Neurol 63:189–193. https://doi.org/10.1001/archneur.63.2.189
Williams-Gray CH, Goris A, Saiki M et al (2009) Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson’s disease. J Neurol 256:493–498. https://doi.org/10.1007/s00415-009-0119-8
Gao J, Huang X, Park Y et al (2011) Apolipoprotein E genotypes and the risk of Parkinson disease. Neurobiol Aging 32:2106.e1-2106.e6. https://doi.org/10.1016/j.neurobiolaging.2011.05.016
Federoff M, Jimenez-Rolando B, Nalls MA, Singleton AB (2012) A large study reveals no association between APOE and Parkinson’s disease. Neurobiol Dis 46:389–392. https://doi.org/10.1016/j.nbd.2012.02.002
Marwarha G, Ghribi O (2015) Does the oxysterol 27-hydroxycholesterol underlie Alzheimer’s disease-Parkinson’s disease overlap? Exp Gerontol 68:13–18. https://doi.org/10.1016/j.exger.2014.09.013
Jeong S-M, Jang W, Shin DW (2019) Association of statin use with Parkinson’s disease: dose-response relationship. Mov Disord Off J Mov Disord Soc 34:1014–1021. https://doi.org/10.1002/mds.27681
Kimura T, Hasegawa M, Takano O (2002) The effect of dopamine on serum lipid concentration after propofol administration. Masui 51:286–288
de Oliveira J, Moreira ELG, Mancini G et al (2013) Diphenyl diselenide prevents cortico-cerebral mitochondrial dysfunction and oxidative stress induced by hypercholesterolemia in LDL receptor knockout mice. Neurochem Res 38:2028–2036. https://doi.org/10.1007/s11064-013-1110-4
Thirumangalakudi L, Prakasam A, Zhang R et al (2008) High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem 106:475–485. https://doi.org/10.1111/j.1471-4159.2008.05415.x
Morris JK, Bomhoff GL, Stanford JA, Geiger PC (2010) Neurodegeneration in an animal model of Parkinson’s disease is exacerbated by a high-fat diet. Am J Physiol - Regul Integr Comp Physiol 299:R1082–R1090. https://doi.org/10.1152/ajpregu.00449.2010
Visanji NP, Lang AE, Kovacs GG (2019) Beyond the synucleinopathies: alpha synuclein as a driving force in neurodegenerative comorbidities. Transl Neurodegener 8:28. https://doi.org/10.1186/s40035-019-0172-x
Fabelo N, Martín V, Santpere G et al (2011) Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Mol Med 17:1107–1118. https://doi.org/10.2119/molmed.2011.00119
Ida E, Nath S et al (2017) Impact of high cholesterol in a Parkinson’s disease model: prevention of lysosomal leakage versus stimulation of α-synuclein aggregation. Eur J Cell Biol. https://doi.org/10.1016/j.ejcb.2017.01.002
Abbott CA, Mackness MI, Kumar S et al (1995) Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler Thromb Vasc Biol 15:1812–1818. https://doi.org/10.1161/01.atv.15.11.1812
Prasanthi JR, Huls A, Thomasson S et al (2009) Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on β-amyloid precursor protein levels and processing in human neuroblastoma SH-SY5Y cells. Mol Neurodegener 4:1. https://doi.org/10.1186/1750-1326-4-1
Balazs Z, Panzenboeck U, Hammer A et al (2004) Uptake and transport of high-density lipoprotein (HDL) and HDL-associated alpha-tocopherol by an in vitro blood-brain barrier model. J Neurochem 89:939–950. https://doi.org/10.1111/j.1471-4159.2004.02373.x
Emamzadeh FN, Allsop D (2017) α-Synuclein interacts with lipoproteins in plasma. J Mol Neurosci 63:165. https://doi.org/10.1007/s12031-017-0967-0
Shahmoradian SH, Genoud C, Graffmeyer A et al (2017) Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat Neurosci 22:1099–1109. https://doi.org/10.1038/s41593-019-0423-2
Halliday GM, Ophof A, Broe M et al (2005) Alpha-synuclein redistributes to neuromelanin lipid in the substantia nigra early in Parkinson’s disease. Brain J Neurol 128:2654–2664. https://doi.org/10.1093/brain/awh584
Boassa D, Berlanga ML, Yang MA et al (2013) Mapping the subcellular distribution of α-synuclein in neurons using genetically encoded probes for correlated light and electron microscopy: implications for Parkinson’s disease pathogenesis. J Neurosci 33:2605–2615. https://doi.org/10.1523/JNEUROSCI.2898-12.2013
Scott D, Roy S (2012) α-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J Neurosci 32(30):10129–10135. https://doi.org/10.1523/JNEUROSCI.0535-12.2012
Nakamura K, Mori F, Tanji K et al (2015) Isopentenyl diphosphate isomerase, a cholesterol synthesizing enzyme, is localized in Lewy bodies. Neuropathol Off J Jpn Soc Neuropathol 35:432–440. https://doi.org/10.1111/neup.12204
Baron P, Crews L, Koob AO et al (2008) Statins reduce neuronal alpha-synuclein aggregation in in vitro models of Parkinson’s disease. J Neurochem 105:1656–1667. https://doi.org/10.1111/j.1471-4159.2008.05254.x
Krüger R, Vieira-Saecker AM, Kuhn W et al (1999) Increased susceptibility to sporadic Parkinson’s disease by a certain combined alpha-synuclein/apolipoprotein E genotype. Ann Neurol 45:611–617. https://doi.org/10.1002/1531-8249(199905)45:5<611::aid-ana9>3.0.co;2-x
Fantini J, Carlus D, Yahi N (2011) The fusogenic tilted peptide (67-78) of α-synuclein is a cholesterol binding domain. Biochim Biophys Acta 1808:2343–2351. https://doi.org/10.1016/j.bbamem.2011.06.017
Hsiao J-HT, Halliday GM et al (2017) α-Synuclein regulates neuronal cholesterol efflux. Molecules 22(10):1769
Sui Y-T, Bullock KM, Erickson MA et al (2014) Alpha synuclein is transported into and out of the brain by the blood-brain barrier. Peptides 62:197–202. https://doi.org/10.1016/j.peptides.2014.09.018
Barceló-Coblijn G et al (2007) Brain neutral lipids mass is increased in α-synuclein gene-ablated mice. J Neurochem 110(1):132–141. https://doi.org/10.1111/j.1471-4159.2006.04348.x
van Maarschalkerweerd A, Vetri V, Vestergaard B (2015) Cholesterol facilitates interactions between α-synuclein oligomers and charge-neutral membranes. FEBS Lett 589:2661–2667. https://doi.org/10.1016/j.febslet.2015.08.013
Emamzadeh FN, Aojula H, McHugh PC, Allsop D (2016) Effects of different isoforms of apoE on aggregation of the α-synuclein protein implicated in Parkinson’s disease. Neurosci Lett 618:146–151. https://doi.org/10.1016/j.neulet.2016.02.042
Zeppelin T, Ladefoged LK, Sinning S et al (2018) A direct interaction of cholesterol with the dopamine transporter prevents its out-to-inward transition. PLoS Comput Biol 14(1):e1005907. https://doi.org/10.1371/journal.pcbi.1005907
Orłowski A, Grzybek M, Bunker A et al (2012) Strong preferences of dopamine and l-dopa towards lipid head group: importance of lipid composition and implication for neurotransmitter metabolism. J Neurochem 122:681–690. https://doi.org/10.1111/j.1471-4159.2012.07813.x
Jones KT, Zhen J, Reith MEA (2012) Importance of cholesterol in dopamine transporter function. J Neurochem 123:700–715. https://doi.org/10.1111/jnc.12007
Zhuge W, Wen F, Ni Z et al (2019) Dopamine burden triggers cholesterol overload following disruption of synaptogenesis in minimal hepatic encephalopathy. Neuroscience 410:1–15. https://doi.org/10.1016/j.neuroscience.2019.04.056
Raju A, Jaisankar P, Borah A, Mohanakumar KP (2017) 1-Methyl-4-phenylpyridinium-induced death of differentiated SH-SY5Y neurons is potentiated by cholesterol. Ann Neurosci 24:243–251. https://doi.org/10.1159/000481551
Kölsch H, Lütjohann D, Tulke A et al (1999) The neurotoxic effect of 24-hydroxycholesterol on SH-SY5Y human neuroblastoma cells. Brain Res 818:171–175. https://doi.org/10.1016/s0006-8993(98)01274-8
Yamanaka K, Saito Y, Yamamori T et al (2011) 24(S)-hydroxycholesterol induces neuronal cell death through necroptosis, a form of programmed necrosis. J Biol Chem 286:24666–24673. https://doi.org/10.1074/jbc.M111.236273
Yamanaka K, Urano Y, Takabe W et al (2014) Induction of apoptosis and necroptosis by 24(S)-hydroxycholesterol is dependent on activity of acyl-CoA:cholesterol acyltransferase 1. Cell Death Dis 5:e990. https://doi.org/10.1038/cddis.2013.524
Cheng D, Kim WS, Garner B (2008) Regulation of alpha-synuclein expression by liver X receptor ligands in vitro. Neuroreport 19:1685–1689. https://doi.org/10.1097/WNR.0b013e32831578b2
Schommer J, Marwarha G, Schommer T et al (2018) 27-Hydroxycholesterol increases α-synuclein protein levels through proteasomal inhibition in human dopaminergic neurons. BMC Neurosci 19(1):17. https://doi.org/10.1186/s12868-018-0420-5
Marwarha G, Rhen T, Schommer T, Ghribi O (2008) The oxysterol 27-hydroxycholesterol regulates α-synuclein and tyrosine hydroxylase expression levels in human neuroblastoma cells through modulation of liver X receptors and estrogen receptors-relevance to Parkinson’s disease. J Neurochem 107:1722–1729. https://doi.org/10.1111/j.1471-4159.2008.05736.x
Bosco DA, Fowler DM, Zhang Q et al (2006) Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat Chem Biol 2:249–253. https://doi.org/10.1038/nchembio782
Bieschke J, Zhang Q, Bosco DA et al (2006) Small molecule oxidation products trigger disease-associated protein misfolding. Acc Chem Res 39:611–619. https://doi.org/10.1021/ar0500766
Yan J, Sun J, Huang L et al (2014) Simvastatin prevents neuroinflammation by inhibiting N-methyl-D-aspartic acid receptor 1 in 6-hydroxydopamine-treated PC12 cells. J Neurosci Res 92:634–640. https://doi.org/10.1002/jnr.23329
Yan J, Xu Y, Zhu C et al (2011) Simvastatin prevents dopaminergic neurodegeneration in experimental parkinsonian models: the association with anti-inflammatory responses. PLoS One 6:6. https://doi.org/10.1371/journal.pone.0020945
Xu Y, Long L, Yan J et al (2012) Simvastatin induces neuroprotection in 6-OHDA-lesioned PC12 via the PI3K/AKT/caspase 3 pathway and anti-inflammatory responses. CNS Neurosci Ther 19:170–177. https://doi.org/10.1111/cns.12053
Selley ML (2005) Simvastatin prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced striatal dopamine depletion and protein tyrosine nitration in mice. Brain Res 1037:1–6. https://doi.org/10.1016/j.brainres.2004.02.083
Kumar A, Sharma N, Gupta A et al (2012) Neuroprotective potential of atorvastatin and simvastatin (HMG-CoA reductase inhibitors) against 6-hydroxydopamine (6-OHDA) induced Parkinson-like symptoms. Brain Res 1471:13–22. https://doi.org/10.1016/j.brainres.2012.06.050
Yan J-Q, Ma Y-J, Sun J-C et al (2014) Neuroprotective effect of lovastatin by inhibiting NMDA receptor1 in 6-hydroxydopamine treated PC12 cells. Int J Clin Exp Med 7:3313–3319
Koob AO, Ubhi K, Paulsson JF et al (2010) Lovastatin ameliorates α-synuclein accumulation and oxidation in transgenic mouse models of α-synucleinopathies. Exp Neurol 221:267–274. https://doi.org/10.1016/j.expneurol.2009.11.015
Lee Y-C, Lin C-H, Wu R-M et al (2013) Discontinuation of statin therapy associates with Parkinson disease: a population-based study. Neurology 81:410–416. https://doi.org/10.1212/WNL.0b013e31829d873c
Huang X, Alonso A, Guo X et al (2015) Statins, plasma cholesterol and risk of Parkinson’s disease: a prospective study. Mov Disord Off J Mov Disord Soc 30:552–559. https://doi.org/10.1002/mds.26152
Liu G, Sterling NW, Kong L et al (2017) Statins may facilitate Parkinson’s disease: insight gained from a large, national claims database. Mov Disord Off J Mov Disord Soc 32:913–917. https://doi.org/10.1002/mds.27006
Rozani V, Giladi N, El-Ad B et al (2017) Statin adherence and the risk of Parkinson’s disease: a population-based cohort study. PLoS One 12:e0175054. https://doi.org/10.1371/journal.pone.0175054
Yan J, Qiao L, Tian J et al (2019) Effect of statins on Parkinson’s disease: a systematic review and meta-analysis. Medicine (Baltimore) 98:e14852. https://doi.org/10.1097/MD.0000000000014852
Bai S, Song Y, Huang X et al (2016) Statin use and the risk of Parkinson’s disease: an updated meta-analysis. PLoS One 11:3. https://doi.org/10.1371/journal.pone.0152564
Sheng Z, Jia X, Kang M (2016) Statin use and risk of Parkinson’s disease: a meta-analysis. Behav Brain Res 309:29–34. https://doi.org/10.1016/j.bbr.2016.04.046
Sierra S, Ramos MC, Molina P et al (2011) Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J Alzheimers Dis JAD 23:307–318. https://doi.org/10.3233/JAD-2010-101179
van der Most PJ, Dolga AM, Nijholt IM et al (2009) Statins: mechanisms of neuroprotection. Prog Neurobiol 88:64–75. https://doi.org/10.1016/j.pneurobio.2009.02.002
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81822016 and 81771382) and the Natural Science Foundation of Hubei Province (No. 2018CFA036) to Z. Zhang.
Author information
Authors and Affiliations
Contributions
LJD conceived and drafted the manuscript. LZ helped to draw the schematics. The final manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dai, L., Zou, L., Meng, L. et al. Cholesterol Metabolism in Neurodegenerative Diseases: Molecular Mechanisms and Therapeutic Targets. Mol Neurobiol 58, 2183–2201 (2021). https://doi.org/10.1007/s12035-020-02232-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02232-6