Abstract
BDNF-oxytocin interactions in the brain are implicated in mammalian maternal behavior. We found that BDNF gene expression is increased in the hippocampus of rat mothers that show increased pup licking/grooming (high LG mothers) compared to low LG mothers. High LG mothers also showed increased BDNF protein levels in the nucleus accumbens (nAcc). Immunoneutralization of BDNF in the nAcc eliminated the differences in pup LG between high and low LG mothers. Oxytocin antagonist in the ventral hippocampus significantly decreased the frequency of maternal LG behavior. Oxytocin antagonist significantly prevented the oxytocin-induced BDNF gene expression in primary hippocampal cell cultures. We suggest that oxytocin-induced regulation of BDNF in the nAcc provides a neuroendocrine basis for both individual differences in maternal behavior and resilience to the stress of reproduction in female mammals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The quality of parent-child interactions is linked to parental mental health, which reflects the importance of peripartum mood disorders, with implications for the risk of mental disorders in the offspring [1, 2]. Nevertheless, we have little understanding of the neurobiological responses to the demands of reproduction in females and their effects on maternal care. Despite an astounding physiological challenge and reproductive imperative, the mammalian female maintains a dampened level of stress reactivity, a sustained focus on the care of the offspring and the ability to respond to her own, considerable metabolic demands [3,4,5]. The lactating female mammal thus provides a natural model of stress resilience.
The ability to focus attention on and respond positively to the infant are essential features of mammalian parenting. Infant stimuli activate the same neural, incentive motivational pathways as natural (food, sex) and artificial (drugs of abuse) rewarding stimuli in both rodent and human mothers [6,7,8,9,10]. Pictures of their infants to human mothers activates brain regions within the mesocorticolimbic dopamine pathway [10]. Sequence-based genomic variants in genes that encode for dopamine receptors, notably DRD1, associate with variations in maternal behavior in humans [11]. Rat mothers show activation of the mesocorticolimbic dopamine system in response to pup-related stimuli [12, 13]. Individual differences in maternal behavior in the rat, most notably in pup licking/grooming (LG), correlate with the amplitude of pup-induced increased dopamine release in the nucleus accumbens (nAcc) [13]. Treatments that decrease dopamine release or that block dopamine receptor activation in the nAcc of the rat reduce the quality and frequency of infant-directed maternal behaviors [13].
Oxytocin, a neuropeptide produced from the paraventricular nucleus of the hypothalamus, regulates infant-induced mesolimbic dopamine activity in the maternal rat [14]. Oxytocin is critical for the initiation of maternal behavior, but seemingly becomes less influential over time [15]. Nevertheless, lactating rats continue to exhibit infant-induced increases in dopamine release in the nAcc [7], suggesting a possible sensitization of the mesolimbic system to infant stimuli. Oxytocin facilitates behavioral sensitization to psychostimulants [16] and the mother—offspring bond has been likened to a state of addiction [17]. Repeated exposure to psychostimulants produces a sensitization of behavioral responses [18], which is partially dependent upon brain-derived neurotrophic factor (BDNF) [19,20,21,22], and BDNF regulates behavioral responses to psychostimulants [23,24,25,26] as well as natural rewards [27]. A recent study provides evidence that BDNF-TrkB signaling in oxytocin neurons mediates maternal behavior [28]. Oxytocin has been shown to increase hippocampal BDNF levels [29]. Since the neural systems that mediate the expression of maternal behavior in mammals overlap considerably with the reward circuitry, we wondered whether oxytocin act to regulate BDNF expression, and whether such effects would, in turn, mediate the expression of maternal behavior. We report an evidence for the oxytocin-induced increase in neuronal BDNF expression and BDNF action in the nAcc that regulates maternal behavior in the rat.
Results
Maternal BDNF Expression
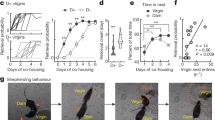
We examined the relation between BDNF mRNA expression and variations in maternal behavior in the rat. We used behavioral observations (see method) to characterize individual differences in the frequency of pup LG of undisturbed mothers, focusing on lactating mothers for which the frequency of pup LG was 1SD greater (i.e., high LG mothers) or 1SD lower (i.e., low LG mothers) than the mean for a cohort of lactating females. A meta-analysis reveals that the scores of maternal LG on day 4 are highly predictive of the scores on postnatal day 6 (Fig. 1a), indicating the highly consistent nature of maternal behavior within the first week of postpartum. We then examined BDNF mRNA levels using an oligonucleotide probe that recognizes the exon IX coding mRNA transcript. In situ hybridization analysis of coronal brain sections obtained from lactating rats on postpartum day 4 revealed significantly higher BDNF mRNA levels in the ventral dentate gyrus (t(8) = 2.453, p = 0.040; n = 4–6/group, Fig. 1b, c) and ventral CA1 (t(8) = 4.553, p = 0.002; n = 4–6/group, Fig. 1b, d) regions of the hippocampus of high compared to low LG mothers, suggesting high LG mothers have increased BDNF gene expression in the hippocampus compared to low LG mothers.
In situ hybridization studies of BDNF mRNA. a Comparing the scores of maternal LG on day 4 and that on postnatal day 6. (n = 388, r = 0.904, p < 0.0001). b In situ hybridization of BDNF in postpartum day 4 high and low LG rat ventral hippocampus. Mean ± SEM relative optical density of BDNF mRNA levels in c the ventral dentate gyrus and d the ventral CA1 regions of the hippocampus in postpartum day 4 high and low LG mothers (n = 4–6/group; *p = 0.040; **p = 0.002)
The incentive motivational pathways including ventral tegmental area (VTA), medial prefrontal cortex (mPFC), nAcc, and hippocampus activated in rodents and human mothers in response to pup stimuli [6, 7, 9]. The amygdala is also activated by infant stimuli, such as cry or smile in humans, to motivate maternal behavior [30]. We, therefore, measured the BDNF exon IX coding transcript in virgin 4-month-old female rats to examine potential differences in BDNF gene expression before pregnancy using qRT-PCR in these brain regions. Analysis of the qRT-PCR data revealed no significant differences in the level of BDNF exon IX mRNA transcript (coding exon) in samples from the mPFC (t(10) = 0.91, p = 0.192, n = 6/group, Fig. 2a), ventral hippocampus (t(10) = 0.47, p = 0.325, n = 6/group, Fig. 2c), and amygdala (t(10) = 1.23, p = 0.123, n = 6/group, Fig. 2e) as well as VTA (data not shown) between the virgin offspring of high and low LG mothers.
BDNF transcript levels. Mean ± SEM levels of BDNF transcript examined using qRT-PCR analysis and expressed as a ratio of Beta-2 microglobulin (B2M), in samples of mPFC (a) and ventral hippocampus (c) and amygdala (e) from virgin female offspring of High or Low LG mothers (n = 6/group; p > .05), and in samples of mPFC (b) (*p = 0.034), ventral hippocampus (d) (*p = 0.028) and amygdala (f) (p = 0.933) from lactating high or low LG mothers (n = 4–6/group)
We measured the BDNF exon IX coding transcript in a new cohort of 4-month-old P4 lactating mothers to validate the in situ results. Consistent with the in situ hybridization results, analysis of the qRT-PCR data revealed significant differences in the level of BDNF exon IX mRNA transcript in samples from the medial prefrontal cortex (mPFC) (t(7) = 2.15, p = 0.034, n = 4–5/group, Fig. 2b) and ventral hippocampus (t(8) = 2.23, p = 0.028, n = 4–6, Fig. 2d), but no differences in the amygdala (t(9) = 0.09, p = 0.933, n = 5–6, Fig. 2f) in the new cohort animals. Importantly, we were unable to consistently detect BDNF mRNA in the nAcc using qRT-PCR.
BDNF Protein and Activity in nAcc
BDNF is trafficked to projection sites through anterograde transport [31] and both the hippocampus and VTA project directly to the nAcc [32]. We then used ELISA [33] to quantify BDNF protein levels in nAcc samples from a cohort of 4-month-old postpartum day 4 high and low LG mothers. Analysis of BDNF protein levels revealed significantly higher BDNF protein levels in the nAcc (t(11) = 2.29, p = 0.043, n = 6–7/group, Fig. 3a) of high compared to low LG mothers. We used a new cohort of 4-month-old animals to compare BDNF protein levels in the nAcc in lactating high and low LG mothers as well as in 4-month-old virgin females from high and low LG mothers to validate the findings. We again observed increased BDNF protein levels in lactating postpartum day 4 high compared to low LG mothers (t(10) = 2.54, p = 0.029; n = 6/group, figure not shown). In contrast, we found no significant BDNF protein level differences in the nAcc between high and low LG age matched virgin females (t(10) = 0.19, p = 0.85, n = 6/group, Fig. 3b). This finding further suggests that the enhanced BDNF protein levels found in the nAcc of high LG mothers are associated with increased BDNF signaling.
BDNF Signal in nAcc Regulates Maternal Behavior
We then examined the importance of BDNF in the nAcc for individual differences in maternal behavior using immunoneutralization with infusions of an anti-BDNF antibody [26] into the nAcc shell of 6-month-old high and low LG mothers. We used mothers previously characterized as high or low LG mothers to establish the influence of BDNF across the first week of lactation. Individual differences in maternal behavior are highly stable across multiple litters such that the frequency of pup LG for the first litter is strongly correlated with that of the second and third litters [34]. We infused previously characterized High and Low LG mothers with 0.5 μl/side (5.0 μg of BDNF per side) of anti-BDNF or saline/IgG into the nAcc on postpartum days 1 to 4 inclusive. The 5-min infusions of anti-BDNF or saline/IgG control occurred twice a day (10:00 and 17:00 h). Maternal behavior was observed for 2 continuous hours following infusion for total contact time, pup LG and arch back nursing. We verified the infusion sites for all animals using Cresyl violet staining (Fig. 4a). We found a treatment by maternal phenotype interaction effect (F(1,15) = 4.25, p = 0.057, Fig. 4b) and a treatment effect (F(1,15) = 9.217; p = 0.008). Tukey’s multiple comparisons test revealed that among saline-treated animals, the frequency of pup LG was significantly (p < .05) greater in High LG (n = 4) than in low LG mothers (n = 5) over a total of 4 days of maternal observations, confirming the maternal phenotype in the pre-characterized mothers. Intra-nAcc anti-BDNF infusions (high LG, n = 5; low LG, n = 5) significantly attenuated the frequency of pup LG in high LG mothers (p < .05) only. The intra-nAcc anti-BDNF infusions eliminated the differences in LG behavior between high and low LG mothers. The same analysis revealed no effect on maternal contact time (Fig. 4c), suggesting that the effects were specific for LG behavior.
BDNF immunoneutralization and maternal behavior. a Schematic illustration of cannulae placement sites in the nucleus accumbens shell. b Mean ± SEM percentage frequency of total observations of pup licking/grooming (LG) (n = 4–5/group, *p < 0.05) and c percentage time in contact with pups in previously characterized high and low LG lactating females on postpartum day 4 over a 2-h period following an infusion with either saline/IgG or an anti-BDNF antibody into the nAcc shell (n = 3–5/group)
Hippocampal Oxytocin Regulates Maternal Behavior
Individual differences in maternal behavior in the rat associate with differential effects of oxytocin and oxytocin-induced increases in dopamine release in the nAcc [14]. We examined whether such effects reflect an influence of hippocampal oxytocin for maternal behavior by infusing an oxytocin receptor antagonist (OTA) or saline into the ventral hippocampus, a region of the hippocampus with important projections to the nAcc [32], in uncharacterized mothers. We observed maternal behavior for the first 3 days postpartum and infused with 1 μl/side (0.5 μg of OTA per side) of OTA or saline into the ventral hippocampus at approximately 3 pm on postpartum day 4. Immediately after the infusion, maternal behavior was observed for 90 min, and then for 72 min sessions at 1700 and 2000 h. The OTA infusion (n = 9) significantly reduced maternal LG in postnatal day 4 lactating females compared to saline-treated (n = 7) lactating females (t(14) = 3.11, p = 0.008, Fig. 5a). The same OTA infusion had no effect on maternal contact time (t(14) = 0.29, p = 0.78, Fig. 5b), suggesting that the effect is specific to pup LG.
Oxytocin regulates maternal behavior. Mean ± SEM percentage of total observations of pup licking/grooming (LG) (a) or time in contact with pups (b) expressed as a percentage of total observations in postnatal day 4 uncharacterized lactating rats following OTA (n = 9) or saline (SA, n = 7) (**p = 0.008)
Oxytocin Regulates BDNF Gene Expression
We next examined whether the effects of hippocampal oxytocin on maternal behavior mediated by the fluence of oxytocin on BDNF expression. We examined in vitro oxytocin effects on BDNF expression in primary hippocampal neurons based on the high expression of oxytocin receptors in this brain region [35]. BDNF expression was quantified using qRT-PCR for the Exon IX (coding region) in the presence of 50 nM oxytocin or medium alone. This concentration was based on earlier pilot studies showing a maximal effect. One-way ANOVA revealed a treatment effect (F(2,9) = 8.528, p = 0.008). Tukey’s post-hoc analysis revealed that oxytocin treatment significantly increased BDNF exon IX expression (n = 4/group, p = 0.008, Fig. 6a). This effect was abolished by pre-treatment with a 100 nM concentration of the oxytocin receptor antagonist, [β-Mercapto-β,β cyclopentamethylenepropionyl1, O-Me-Tyr2, Orn8]-Oxytocin (OTA) (p = 0.036). Oxytocin effects on hippocampal function are mediated by MAP kinase signaling [36]. We examined the effect of oxytocin on BDNF expression in new cultured hippocampal neurons in the presence of AMP kinase inhibitor, PD98059 (PD, 10 μM concentration). Oxytocin treatment increased BDNF exon IX expression (n = 3) but this effect was also abolished in the presence of a MAP kinase inhibitor (PD 98059, n = 4) and oxytocin antagonist (One-way ANOVA, F(4,14) = 6.53, p = 0.004, n = 4, Fig. 6b). These findings reveal an effect of oxytocin on hippocampal BDNF expression that is likely mediated by a MAP kinase signaling pathway.
Oxytocin regulates hippocampal BDNF expression. a Mean ± SEM BDNF mRNA transcript levels in primary hippocampal cultures treated with either saline, oxytocin (OT; 50 nM, n = 4) or OT with pre-treatment with the OT receptor antagonist (n = 4), OTA (100 nM, n = 4) for 90 min (**p = 0.008, *p = 0.036). b Mean ± SEM BDNF mRNA transcript levels in primary hippocampal cultures treated with either saline (n = 4), oxytocin (OT; 50 nM) (n = 3), OTA (n = 4), or OT with pre-treatment with the OT receptor antagonist, OTA (100 nM, n = 4), or OT with pre-treatment with a MAP kinase inhibitor (PD 98059, PD, 10 μM concentrations, n = 4) for 90 min (Veh vs OT, *p = 0.020; OT vs OT-OTA,*p = 0.037)
Discussion
We report novel findings on the role of nAcc BDNF, a neurotrophic factor that mediates motivational states, on individual differences in maternal behavior in the rat. High LG mothers showed enhanced gene expression of BDNF in the hippocampus compared to low LG mothers. Many brain regions including the mPFC, ventral tegmental area and hippocampus project to the nAcc, a brain region involved in mediating dopamine-induced reward states [37, 38], and there was an increase in the level of BDNF protein in the nAcc of high compared to low LG mothers. We established a functional role for nAcc shell BDNF in mediating individual differences in maternal behavior. BDNF immunoneutralization within the nAcc shell-reduced maternal LG in high LG mothers to levels comparable to those of low LG mothers. These findings imply an important role for nAcc BDNF in the regulation of maternal behavior in the rat.
The differences in the BDNF system between high and low LG female rats were apparent during lactation not in virgin period, which suggests that the differences in BDNF expression emerge in response to hormonal conditions of pregnancy and/or lactation. In support of this idea, we found an evidence implicating oxytocin in the regulation of BDNF expression. We found in vivo evidence for the effect of hippocampal oxytocin; infusion of an oxytocin receptor antagonist directly into the ventral hippocampus, which provides an important projection to the nAcc [32], reduced the frequency of pup LG. We also found that oxytocin-induced the expression of BDNF in hippocampal primary neuronal cultures, an effect that was blocked with a MAP kinase inhibitor, a finding consistent with known oxytocin intra-cellular signaling pathways. These findings suggest that oxytocin-induced changes in BDNF expression mediate the regulation of maternal behavior. Importantly, there is increased hypothalamic expression of oxytocin in high compared to low LG mothers over the first week postpartum [14]. Oxytocin regulates social affiliation, including mother–pup interactions, through effects on the mesolimbic dopamine system [13, 14, 39]. Maternal behavior in the rat relies upon oxytocin projections to the VTA [6, 14] and oxytocin infusion into the VTA increases dopamine release in the nAcc [14], while an oxytocin antagonist infused into the VTA eliminates differences between high and low LG mothers in nAcc dopamine levels during nursing [14]. Likewise, there is increased release of oxytocin in the hippocampus during suckling in the lactating rat [14, 40] and a dense expression of oxytocin receptors in the hippocampus [41]. The hippocampus connects directly to the nAcc [32], a region that regulates responses to rewarding stimuli through D1 receptor-sensitive mechanisms [42]. We propose that oxytocin dynamically regulates both dopamine release and BDNF expression, and the latter effect enhances dopaminergic signaling in high compared to low LG mothers.
BDNF acts through TrkB receptors [43] to regulate behavioral responses to both natural and artificial rewards through effects on the mesolimbic dopamine system [21,22,23,24,25,26,27]. BDNF is highly expressed in the VTA, co-localizes with tyrosine hydroxylase in dopamine neurons and binds with a high affinity to TrkB receptor, which is expressed throughout the VTA–nAcc pathway [44]. There is anterograde transport of BDNF from the VTA to the nAcc [31], and BDNF acts on TrkB receptors in the nAcc to regulate behavioral responses to rewarding stimuli [26, 43]. siRNA-mediated decreases in TrkB in the nAcc impair the establishment of a conditioned place preference for cocaine [26, 43]. BDNF also increases the expression of D1 and D3 dopamine receptors [20, 45]. Interestingly, dopamine signal in the nAcc during pup LG as well as both D1 and D3 receptor binding in the nAcc are enhanced in high compared with low LG mothers [13]. The activation of D1 receptors regulates the expression of maternal behavior [6]. The present findings suggest that BDNF activity in the nAcc is associated with responding to natural rewards, in this instance, the response of lactating rats towards pups.
BDNF immunoneutralization in the nAcc abolished the difference in pup LG between high and low LG mothers, but did not completely eliminate pup LG nor did it affect the nursing behaviors of the mothers (Fig. 4b, c). Disruption of oxytocin signaling in lactating mothers produces a comparable effect [46]. A ventral hippocampal infusion of an oxytocin antagonist on days 3–4 postpartum significantly reduced the maternal LG (Fig. 5a) but a low level of pup LG continues to occur and nursing behaviors and contact time with pups were unaffected (Fig. 5b) [46]. Thus, neither oxytocin nor BDNF is essential for maintaining maternal behavior once established, but oxytocin and BDNF rather act as neuromodulators regulating the intensity of the behavior and determining individual differences in mother–pup interactions.
The importance of BDNF activity within the nAcc in mediating appetitive responses to rewarding stimuli stands in apparent contrast to the results of studies revealing that increased activity within this same system enhances “depression-like” behavior [47, 48] following social defeat [49]. Social defeat increases BDNF expression in the VTA and associates with sustained social avoidance as well as anhedonia in male mice. These findings contrast with those showing that enhanced BDNF signaling in the nAcc supports positive incentive motivational states, such as maternal behavior. In each condition, BDNF activity targets nAcc dopamine projections that are sensitive to both rewarding and aversive stimuli. Aversive and rewarding stimuli produce opposing effects on behavior (approach vs. withdrawal) and affective states. There are at least two features that distinguish these states. First, the depressive-like effects of increased BDNF signaling in the nAcc following social defeat require increased corticotropin-releasing factor signaling [50]. Second, while both social defeat stress and exposure to pups for high LG dams associate with an increase in BDNF expression in the VTA, their opposing effects on BDNF expression are observed in the hippocampus. Stress decreases BDNF expression in the hippocampus [47], while exposure to pups in high LG mothers associates with an increase in hippocampal BDNF expression. Increased hippocampal BDNF produces antidepressant-like effects [51, 52]. Site-specific reduction of BDNF in the hippocampus induces depression-like behaviors [47], and overexpression of BDNF in the hippocampus blocks stress-induced anhedonia [53]. We propose that environmental conditions associated with both BDNF-mediated increased mesolimbic dopamine activity and increased hippocampal BDNF associate with enhanced approach behavior commonly associated with affectively positive states; this is well characterized by the maternal behavior of high LG mothers. In contrast, conditions that increase BDNF activity within the mesolimbic dopamine system and decrease hippocampal BDNF expression associate with “depression-like” states of withdrawal that are more representative of maternal behavior of low LG mothers. This proposal is consistent with the evidence showing that antidepressant medications block the effect of stress on hippocampal BDNF [51]. Nevertheless, we cannot exclude the potential protective effect of other hormonal factors related to lactation on protecting dams from the depressogenic effects of mesolimbic BDNF.
These findings suggest that the enhanced BDNF expression in limbic regions that project to the nAcc in high LG mothers may serve to maintain a strong maternal motivational state in the presence of the pups, despite the imposing metabolic demands of lactation. While many human mothers experience maternity as a period of emotional well-being, a significant percentage experience dysphoria (i.e., postpartum depression) [54, 55]. The current findings regarding the role of BDNF in mediating individual differences in maternal behavior in the rat relate to the human condition, where peripartum depression and the accompanying decrease in the quality of mother–child interactions reflect a state of decreased resilience of profound importance for the mother–child dyad [1, 2]. Understanding the neurobiological basis for such individual variations may help to identify the biological basis of individual differences in vulnerability/resistance to the effects of increased environmental demand on motivational/emotional states.
Limitations
There are important challenges involved in examining the direct link between oxytocin-induced BDNF in the ventral hippocampus and its transportation to the nucleus accumbens in vivo in mediating maternal behavior. Although we demonstrated the effect of oxytocin on regulation of BDNF gene expression in vitro, it is challenging to identify changes in BDNF levels in the nucleus accumbens after acute infusion of an oxytocin antagonist into the ventral hippocampus since there are multiple sources of BDNF occurring in different brain regions. For example, frontal cortex BDNF also regulates nucleus accumbens BDNF levels. Future studies should find a way to directly examine the transportation of BDNF from the ventral hippocampus to the nucleus accumbens and examine the detailed BDNF-TrkB signaling pathways that are involved in maternal behavior. In addition, the BDNF level between high and low LG mothers after weaning needs to be further studied to validate whether the difference is apparent only during lactation.
Materials and Methods
Animals
The animals were out-bred Long-Evans, hooded rats born in our colony from females obtained from Charles River Canada (St. Constant, QC). Food and water were provided ad libitum. The colony was maintained on a 12:12 light/dark schedule with lights on at 09:00 h. Rats were singly housed for 2 weeks following mating. Females gave birth and were left undisturbed with their pups until weaning, with the exception of regular cage maintenance. All procedures were performed according to the guidelines from the Canadian Council on Animal Care and approved by the McGill University Animal Care Committee.
Maternal Behavior
Maternal behavior was observed for five, 75-min periods/day for the first 4 days or 6 days postpartum based on the experiment as previously described [14, 34]. High LG mothers were defined as females for which the mean frequency scores for LG over the first 6 days postpartum were > 1 SD above the cohort mean. Low LG mothers were females for which the mean frequency scores for LG were < 1 SD below the cohort mean.
BDNF In situ Hybridization
A meta-analysis was performed to study whether the licking grooming scores on postnatal day 4 can predict the scores on postnatal day 6 using 6 cohorts containing 964 animals. We analyzed the correlation between the LG score on day 4 and that on day 6 using linear regression. The meta-analysis revealed that maternal LG scores on day 4 are highly predictive of the scores on postnatal day 6 (Fig. 1a). A new cohort lactating mothers were observed, from which 4 high and 5 low LG mothers were selected on postpartum day 4 and their brains were dissected following rapid decapitation (less than 1 min). The brains were snap frozen in isopentane and stored at − 80 °C. Coronal sections of the brain were sliced at 15 μm and were thaw-mounted on poly-L-lysine-coated slides under RNase-free conditions and stored at − 80 °C. Rat brain sections were prefixed in a 4% paraformaldehyde solution for 10 min in preparation for the hybridization experiments. The sections were rinsed in 2 × SSC for 10 min and in 0.25% acetic anhydride and 0.1 M triethanolamine solution (pH 8.0; 1× 10 min). Sections were then dehydrated using a 50–100% ethanol gradient, placed in chloroform for 10 min, followed by rehydration in 95% ethanol. The sections were then incubated overnight at 37 °C with 150 μl/slide of hybridization buffer containing 50% deionized formamide, 10 mM dithiothreitol, 10 mM Tris (pH 7.5), 600 mM sodium chloride, 1 mM EDTA, 10% dextran sulfate, 1 × Denhardt’s solution, 100 g salmon sperm DNA, 100 μg/ml yeast tRNA, and [35S] ddATP-labeled oligonucleotide probe. The oligonucleotide sequence for BDNF messenger RNA (5′AGTTCCAGTGCC TTTTGTCTATGCCCCTGCAGCCTTCCTTCGTGTAACCC3′) was labeled using a DNA 3′-end labeling kit (Roche, Quebec, Canada). Following hybridization, slides were washed in 1 × SSC buffer (1 × 15 min at RT), 2 × SSC buffer (1 × 30 min at 55 °C), 0.5 × SCC buffer (1 × 15 min at RT), rinsed briefly in water, dried, and apposed to Hyperfilm for 10 days. The hybridization signals within various brain regions including dorsal CA1, dorsal dentate gyrus, ventral CA1, ventral dentate gyrus (vDG) of hippocampus, and the ventral tegmental area (VTA) were quantified using densitometry with an image analysis system (MCID, St. Catharines, Ontario). The data from the ventral CA1 and ventral dentate gyrus (vDG) are presented as arbitrary optical density (absorbance) units following correction by subtraction of background and are the averages drawn from three sections per brain region/per animal. The anatomical regions used for analyses were verified using Nissl-staining of slide sections and the rat brain atlas of Paxinos and Watson [56].
Quantitative Real-time Reverse Transcription-PCR (qRT-PCR) Analysis for BDNF Gene Expression
Whole brains were removed from 4-month old, postpartum day 4 high (n = 5) and low LG mothers (n = 6) and the age matched 4-month-old virgin female offspring of high (n = 6) and low LG mothers (n = 6) by rapid decapitation, snap frozen on dry ice using ice-cold isopentane, and stored at − 80 °C. The brains were then sliced at 200 μm thickness in a cryostat at − 18 °C. Brain regions including the mPFC, nAcc, dorsal and ventral hippocampus, and amygdala were excised using a 0.8-mm diameter tissue puncher. RNA was isolated with the Trizol reagent method (Invitrogen, Burlington, ON), followed by treatment with DNase I to eliminate any remaining genomic DNA. cDNA was synthesized using 2 μg of total RNA, random hexamer primers and Avian Myeloblastosis Virus reverse transcriptase (Fermentas, Burlington, ON). A non-template negative control as well as control samples without reverse transcriptase were used within the cDNA samples. To control for equal loading, the rat Beta-2 microglobulin (B2M) gene (GenBank accession number NM_012512) was also subjected to PCR amplification (forward primer: 5′-CCGTGATCTTTCTGGTGCTT-3′; reverse primer: 5′ AAGTTGGGCTTCCCATTCTC-3′). BDNF exonIX (forward primer: 5′-GAGAAGAGTGATGACCATCCT-3′; reverse primer: 5′-TCACGTGCTCAAAAGTGTCAG-3′) and B2M amplification were performed in parallel in a real-time thermocycler (LightCycler 480; Roche Applied Science). Relative gene expression levels were calculated using the normal delta-Ct method. The specificity of the amplified PCR products was assessed by performing a melting curve analysis following PCR amplification. No primer–dimers that interfered with the quantification of the PCR products were detected.
Enzyme-Linked Immunosorbent Assay (ELISA)
Whole brains from postpartum day 4 4-month-old high (n = 6) and low LG (n = 7) female rats or 4-month-old virgin female rats (n = 6/group) were rapidly removed after decapitation and flash frozen between 9:00 am and 12:00 pm. Bilateral tissue punches of the nAcc (core and shell) were obtained from 200 μm coronal sections sliced on a cryostat at −18 °C. The coronal sections were approximately + 1.8, + 0.26 mm from bregma for the nAcc [57]. The punched samples were snap frozen on dry ice and stored at − 80 °C. Frozen tissue punches were sonicated in 300 μl of lysis buffer (137 mM NaCl, 20 mM Tris, 1% NP-40, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 1 μg/ml leupeptin, and 0.5 mM sodium vanadate). The homogenates were incubated at 4 °C for 30 min and centrifuged at 12,000×g for 10 min. The protein concentrations from the supernatants were determined using the Micro-BCA assay kit (Pierce, Rockford, IL). ELISAs were performed using the BDNF ELISA kit (Cat # CYT306, Millipore, Temecula CA) according to the manufacturer’s instructions. Using the ChemiKine BDNF assay system, rabbit polyclonal antibodies generated against human BDNF were coated onto a microplate and used to capture BDNF from tissue. BDNF-specific, biotin conjugated, mouse monoclonal antibody (No. 60583) was used to detect the captured BDNF. BDNF content was interpolated from standard curve runs for each plate (linear range of 7.8–500 pg/ml for BDNF). BDNF protein content was divided by total protein in each sample to determine the picograms of peptide per microgram of total protein. We repeated the ELISA experiment to validate the previous result by using a new cohort of high (n = 6) and low LG (n = 6) mothers.
BDNF Immunoneutralization
We based our protocol on the study of Graham et al. (2007) [26] that used anti-BDNF infusions into the nAcc shell to reveal the role of nAcc BDNF in cocaine-seeking behavior. On gestational day, 14–15 female rats were anesthetized and bilateral guide cannulae (23-gauge, Plastic One, Roanoke, VA) were implanted in the nAcc shell (1.2 mm anterior to bregma, ± 0.8 mm lateral to the midline, and − 7.0 mm ventral to the surface of the cortex). These animals were 6 months old previously characterized as high and low LG mothers. The assembly was secured with acrylic dental cement. Animals were allowed to recover for 7–8 days and give birth. We infused mothers twice per day at 10:00 and 17:00 based on meta-analyses data obtained from 3 cohorts showing that pup LG occurred at its highest frequency during these hours. On postpartum days 1, 2, 3, and 4, bilateral infusions of 0.5 μl/side anti-BDNF (Chemicon, Millipore, Etobicoke, ON. Cat# AB1513P) or saline/IgG were infused into the nAcc shell of high (saline, n = 4; anti-BDNF, n = 5) and low LG (saline, n = 5; anti-BDNF, n = 5) rat mothers. Each infusion lasted 5 min and involved an anti-BDNF concentration of 5 μg per 0.5 μl/side. The concentration used for the anti-BDNF infusions is based on previous studies showing that this dose effectively reduces BDNF levels in the nAcc [26]. Maternal behaviors were observed for 120 min after each infusion.
Oxytocin Receptor Antagonist (OTA) Infusions
We examined the importance of oxytocin in the ventral hippocampus, the main hippocampal sub-region projecting to the nAcc [32], for maternal behavior by infusing an oxytocin receptor antagonist [β-Mercapto-β,β cyclopentamethylenepropionyl1, O-Me-Tyr2, Orn8]-Oxytocin into the ventral hippocampus of dams. We used uncharacterized 4-month-old mothers to determine the effect of hippocampal oxytocin on maternal behavior. On pregnancy day 14–15, female rats were anesthetized and bilateral guide cannulae (23-gauge, Plastic One, Roanoke, VA) were implanted in the ventral hippocampus (− 6.1 mm anterior to bregma, ± 4.5 mm lateral to the midline, and − 6.7 mm ventral to the surface of the cortex). The assembly was secured with acrylic dental cement. Animals were allowed to recover for 7–8 days and give birth. Lactating mothers were observed for the first 3 days postpartum, and on postpartum day 4 were infused with 1 μl/side (0.5 μg OTA per side) of OTA (n = 9) or saline (n = 7) at approximately 3 pm. For each infusion session, dams were briefly taken out of their home cages, handled and a bilateral infuser was inserted into the guide cannula. As soon as the infuser was in place, dams were returned to their home cage and infused with either OTA or saline/IgG using a 1 μl Hamilton syringe. Immediately after the infusion, maternal behavior was observed for 90 min and for two 72-min sessions at 17:00 and 20:00 h. Histological verification confirmed the infusion site for all animals.
Hippocampal Cell Cultures
Primary hippocampal cell cultures were prepared from hippocampi dissected from male and female rats on embryonic day 20, incubated 15 min in 0.25% trypsin, dissociated, and plated at a density of 3 × 108 cells/cm2 as previously described [58]. MEM Alpha media (Life technologies, Cat#12561-056) supplemented with 10% fetal bovine serum, 15 mM HEPES, 20 mM potassium chloride, 55 mM glucose and 0.1% penicillin/streptomycin was changed the day after plating and every 3 days thereafter. One day after seeding, media was supplemented with 20 mM each of uridine and 5-fluorodeoxyuridine to prevent proliferation of glial cells. Cells were maintained for 7 days at 37 °C in a humid atmosphere with 5% carbon dioxide. The cells were cultured for 7 days in vitro since sex differentiation has not occurred yet at this stage [59]; therefore, the neurons from both sexes can still be studied as one group. Previous characterizations of cultures generated with this protocol have shown them to be over 95% neuronal [60]. MEM alpha media was changed to neurobasal Medium (life technologies, Cat# 12348-017) without fetal bovine serum, followed by adding 0.5 mM GlutaMax supplement (Life technologies, Cat# 35050-061) 2 h before treatments with specific drugs (i.e., oxytocin, Sigma-Aldrich, Cat# O6379; oxytocin antagonist (OTA), [β-Mercapto-β,β cyclopentamethylenepropionyl1, O-Me-Tyr2, Orn8]- Oxytocin, Sigma-Aldrich, St. Louis, MO, Cat #O6887; MAPK kinase inhibitor PD98059, Thermo fisher scientific, Cat# PHZ1164). Cells were collected and RNA was isolated at different time points after drug treatment using a Nucleospin RNA XS kit (Macherey-nagel, Cat# 740902.50). On column DNase was treated to remove genomic DNA residue according to the kit protocol. cDNA conversion was then performed using 0.5 μg RNA and Maxima reverse transcriptase (Thermo scientific, Cat# EP0743) for qRT-PCR analysis of BDNF exon IX mRNA.
Cresyl Violet Staining
Rat brains with cannula implantation were collected and sliced in 40 μm coronal sections. Brain sections were fixed using 4% paraformaldehyde in 1 X phosphate-buffered saline (PBS) for 20 min. Tissues were rinsed in 1 X PBS then incubated in 1:1 alcohol/chloroform overnight to reduce background from fat staining. Tissues were then rehydrated and stained in 0.1% cresyl violet solution at 37 °C for 10 min. Tissues were rinsed in distilled water and dehydrated in alcohol and then cover slipped using permanent mounting medium.
Statistical Analyses
BDNF mRNA levels in hippocampal cell culture were analyzed using one-way analysis of variance (ANOVA). Maternal behaviors, BDNF mRNA, and BDNF protein expression were analyzed using student unpaired, two-tailed t tests. Maternal behavior in the BDNF immunoneutralization study was analyzed using one-way ANOVA followed by Bonferroni post-hoc analyses when a significant interaction was reached.
Abbreviations
- BDNF:
-
Brain-derived neurotrophic factor
- B2M:
-
Beta-2 microglobulin
- FBS:
-
Fetal bovine serum
- LG:
-
Licking/grooming
- nAcc:
-
Nucleus accumbens
- OT:
-
Oxytocin
- OTA:
-
[β-Mercapto-β,β cyclopentamethylenepropionyl1, O-Me-Tyr2, Orn8]- Oxytocin
- PBS:
-
Phosphate buffer saline
- SD:
-
Standard deviation
- SEM:
-
Standard error of the mean
- Veh:
-
Vehicle
References
Repetti RL, Taylor SE, Seeman TE (2002) Risky families: family social environments and the mental and physical health of offspring. Psychol Bull 128:330–366
Belsky J, de Haan M (2011) Annual research review: parenting and children’s brain development: the end of the beginning. J Child Psychol Psychiatry 52:409–428
Fischer D, Patchev VK, Hellbach S, Hassan AH, Almeida OF (1995) Lactation as a model for naturally reversible hypercorticalism plasticity in the mechanisms governing hypothalamo-pituitary- adrenocortical activity in rats. J Clin Invest 96:1208–1215
Walker CD, Lightman SL, Steele MK, Dallman MF (1992) Suckling is a persistent stimulus to the adrenocortical system of the rat. Endocrinology 130:115–125
Neumann ID (2009) The advantage of social living: brain neuropeptides mediate the beneficial consequences of sex and motherhood. Front Neuroendocrinol 30:483–496
Numan M, Stolzenberg DS (2009) Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol 30:46–64
Barrett J, Fleming AS (2011) Annual research review: all mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. J Child Psychol Psychiatry 52:368–397
Ferris CF, Kulkarni P, Sullivan JM Jr et al (2005) Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci 25:149–156
Pereira M, Morrell JI (2011) Functional mapping of the neural circuitry of rat maternal motivation: effects of site-specific transient neural inactivation. J Neuroendocrinol 23:1020–1035
Swain JE (2011) The human parental brain: in vivo neuroimaging. Prog Neuro-Psychopharmacol Biol Psychiatry 35:1242–1254
Mileva-Seitz V, Fleming AS, Meaney MJ, Mastroianni A, Sinnwell JP, Steiner M, Atkinson L, Levitan RD et al (2012) Dopamine receptors D1 and D2 are related to observed maternal behavior. Genes Brain Behav 11:684–694
Hansen S, Bergvall AH, Nyiredi S (1993) Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav 45:673–676
Champagne FA (2004) Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci 24:4113–4123
Shahrokh DK, Zhang T-Y, Diorio J, Gratton A, Meaney MJ (2010) Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology 151:2276–2286
Pedersen CA (1997) Oxytocin control of maternal behavior. Regulation by sex steroids and offspring stimuli. Ann N Y Acad Sci 807:126–145
Sarnyai Z (1999) Oxytocin and neuroadaptation to cocaine. Prog Brain Res 119:449–466
Insel TR (2003) Is social attachment an addictive disorder? Physiol Behav 79:351–357
Kalivas PW, Stewart J (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev 16:223–244
Thomas MJ, Kalivas PW, Shaham Y (2008) Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol 154:327–342
Pu L, Liu Q-S, Poo M-M (2006) BDNF-dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. Nat Neurosci 9:605–607
Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P (2001) BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 411:86–89
Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L (2010) Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev 35:157–171
Bahi A, Boyer F, Dreyer J-L (2008) Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned-place preference, and reinstatement in rats. Psychopharmacology 199:169–182
Hall FS, Drgonova J, Goeb M, Uhl GR (2003) Reduced behavioral effects of cocaine in heterozygous brain-derived neurotrophic factor (BDNF) knockout mice. Neuropsychopharmacology 28:1485–1490
Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR (1999) Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci 19:4110–4122
Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW (2007) Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci 10:1029–1037
Cordeira JW, Frank L, Sena-Esteves M, Pothos EN, Rios M (2010) Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J Neurosci 30:2533–2541
Maynard KR, Hobbs JW, Phan BN, et al (2018) BDNF-TrkB signaling in oxytocin neurons contributes to maternal behavior. Elife 7. doi https://doi.org/10.7554/eLife.33676
Havranek T, Zatkova M, Lestanova Z, Bacova Z, Mravec B, Hodosy J, Strbak V, Bakos J (2015) Intracerebroventricular oxytocin administration in rats enhances object recognition and increases expression of neurotrophins, microtubule-associated protein 2, and synapsin I. J Neurosci Res 93:893–901
Barrett J, Wonch KE, Gonzalez A, Ali N, Steiner M, Hall GB, Fleming AS (2012) Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Soc Neurosci 7:252–268
Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ (1997) Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 389:856–860
Hansen N, Manahan-Vaughan D (2014) Dopamine D1/D5 receptors mediate informational saliency that promotes persistent hippocampal long-term plasticity. Cereb Cortex 24:845–858
Greisen MH, Altar CA, Bolwig TG, Whitehead R, Wörtwein G (2005) Increased adult hippocampal brain-derived neurotrophic factor and normal levels of neurogenesis in maternal separation rats. J Neurosci Res 79:772–778
Champagne FA, Francis DD, Mar A, Meaney MJ (2003) Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav 79:359–371
Vaccari C, Lolait SJ, Ostrowski NL (1998) Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology 139:5015–5033
Tomizawa K, Iga N, Lu Y-F, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T et al (2003) Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci 6:384–390
Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28:309–369
Wise RA, Rompre PP (1989) Brain dopamine and reward. Annu Rev Psychol 40:191–225
Ross HE, Young LJ (2009) Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol 30:534–547
Neumann I, Landgraf R (1989) Septal and hippocampal release of oxytocin, but not vasopressin, in the conscious lactating rat during suckling. J Neuroendocrinol 1:305–308
Mühlethaler M, Sawyer WH, Manning MM, Dreifuss JJ (1983) Characterization of a uterine-type oxytocin receptor in the rat hippocampus. Proc Natl Acad Sci U S A 80:6713–6717
Goto Y, Grace AA (2008) Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci 31:552–558
Graham DL, Krishnan V, Larson EB, Graham A, Edwards S, Bachtell RK, Simmons D, Gent LM et al (2009) Tropomyosin-related kinase B in the mesolimbic dopamine system: region-specific effects on cocaine reward. Biol Psychiatry 65:696–701
Yan Q, Matheson C, Sun J, Radeke MJ, Feinstein SC, Miller JA (1994) Distribution of intracerebral ventricularly administered neurotrophins in rat brain and its correlation with Trk receptor expression. Exp Neurol 127:23–36
Do T, Kerr B, Kuzhikandathil EV (2007) Brain-derived neurotrophic factor regulates the expression of D1 dopamine receptors. J Neurochem 100:416–428
Champagne F, Diorio J, Sharma S, Meaney MJ (2001) Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci 98:12736–12741
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM et al (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868
Eisch AJ, Bolaños CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ (2003) Brain-derived neurotrophic factor in the ventral midbrain–nucleus accumbens pathway: a role in depression. Biol Psychiatry 54:994–1005
Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, LaPlant Q, Graham A et al (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131:391–404
Walsh JJ, Friedman AK, Sun H et al (2013) Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci 17:27–29
Shirayama Y, Chen AC-H, Nakagawa S, Russell DS, Duman RS (2002) Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22:3251–3261
Deltheil T, Tanaka K, Reperant C, Hen R, David DJ, Gardier AM (2009) Synergistic neurochemical and behavioural effects of acute intrahippocampal injection of brain-derived neurotrophic factor and antidepressants in adult mice. Int J Neuropsychopharmacol 12:905–915
Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A (2011) Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J Neurosci 31:4475–4483
Mileva-Seitz V, Steiner M, Atkinson L, Meaney MJ, Levitan R, Kennedy JL, Sokolowski MB, Fleming AS (2013) Interaction between oxytocin genotypes and early experience predicts quality of mothering and postpartum mood. PLoS One 8:e61443
Green CG, Babineau V, Jolicoeur-Martineau A, Bouvette-Turcot AA, Minde K, Sassi R, St-André M, Carrey N et al (2017) Prenatal maternal depression and child serotonin transporter linked polymorphic region (5-HTTLPR) and dopamine receptor D4 (DRD4) genotype predict negative emotionality from 3 to 36 months. Dev Psychopathol 29:901–917
Paxinos G, Watson C (2006) The rat brain in stereotaxic coordinates: hard cover edition. Elsevier, Amsterdam
Herman JP, Watson SJ (1987) The rat brain in stereotaxic coordinates (2nd edn). Trends Neurosci 10:439
Hellstrom IC, Dhir SK, Diorio JC, Meaney MJ (2012) Maternal licking regulates hippocampal glucocorticoid receptor transcription through a thyroid hormone-serotonin-NGFI-A signalling cascade. Philos Trans R Soc Lond Ser B Biol Sci 367:2495–2510
Reisert I, Lieb K, Beyer C, Pilgrim C (1996) Sex differentiation of rat hippocampal GABAergic neurons. Eur J Neurosci 8:1718–1724
Mitchell JB, Rowe W, Boksa P, Meaney MJ (1990) Serotonin regulates type II corticosteroid receptor binding in hippocampal cell cultures. J Neurosci 10:1745–1752
Funding
This research was supported by grants from the Hope for Depression Research Foundation and the Canadian Institutes for Health Research to MJM, by grants from Natural Sciences and Engineering Research Council of Canada to TYZ.
Author information
Authors and Affiliations
Contributions
TYZ, DS, ICH, XLW, JD, and CC performed all experiments, which were designed by TYZ, DS, ICH, JD, and MJM. TYZ, DS, ICH, and MJM analyzed the data and prepared the manuscript.
Corresponding authors
Ethics declarations
All procedures were performed according to the guidelines from the Canadian Council on Animal Care and approved by the McGill University Animal Care Committee.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, TY., Shahrokh, D., Hellstrom, I.C. et al. Brain-Derived Neurotrophic Factor in the Nucleus Accumbens Mediates Individual Differences in Behavioral Responses to a Natural, Social Reward. Mol Neurobiol 57, 290–301 (2020). https://doi.org/10.1007/s12035-019-01699-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01699-2