Abstract
Depression is the leading cause of disability worldwide with global distribution of 322 million people suffering from the disease. While much is understood about depression, the underlying pathophysiology is yet to be fully characterized. Recently, the unfolded protein response (UPR) has been shown to be involved in regulating key aspects like inflammation, cell death, and behavioral depression. The UPR is an evolutionarily conserved ancient response system that reacts to the stressful environmental impact on a cell; the net effect of stress to a cell is that the quality of protein folding is diminished. The UPR responds by repairing and removing misfolded proteins and, if necessary, initiates apoptosis. Here, we demonstrate that the UPR is not only involved in depression, but that its activation causes a depressive phenotype. The hippocampi of rats were directly infused with 500 ng of tunicamycin (TM), an agent that initiates the UPR by blocking N-terminal glycosylation. Three to 8 days post-surgery, the rats showed depressive behavior in escape latency, forced swim despair, sucrose preference anhedonia, and also physiological signs of depression like decreased weight. Further, these behavioral changes were associated with enhanced expression of key UPR genes and proteins in the hippocampus. We propose that this model will make an excellent tool for studying depression and for understanding pathways that are affected by the UPR which directly causes depressive behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is a debilitating mental health problem. About 300 million individuals world-wide suffer from depression with a staggering 1.7:1 female to male ratio [1, 2]. This illness has been described as the leading cause of disability according to the World Health Organization [3]. Depression is commonly associated with medical illnesses (e.g., stroke), and medical illnesses have been identified as significant risk factors for both suicidal behavior and suicidal ideation, especially among older adult depressed patients. Specifically, the existence of previous mood disorder, prior history of stroke, and cognitive impairment have been reported to be the most important risk factors for suicidal behavior. [4]. Despite decades of research and a plethora of past and current data on depression and the pathophysiology that comes about as a result of the disease, there is still no consensus on its underlying cause.
The unfolded protein response (UPR) is an evolutionarily conserved series of intracellular cascades which responds to misfolded proteins that accrue in the endoplasmic reticulum (ER) [5]. The primary functions of the UPR are to repair these misfolded proteins; direct them for degradation; or, in extreme cases, initiate and facilitate the apoptotic pathways that lead to cell death [6]. The unfolded protein response can be considered an excellent target for studying the impact of stress on emotionally salient regions of the brain and thus on behavior. Not only is it a direct physiological response that correlates to psychological stress, but it is involved in much of the same physiology that is seen in depression studies. For example, the UPR is involved in inflammation [7, 8] through several means, including direct activation of NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and subsequent inflammatory cytokine production [9, 10] via IκB (inhibitor of kappa B) degradation, through c-Jun N-terminal Kinase (JNK) hyper-activation [11], and through increased toll-like receptor expression and activity [12, 13], which has been studied in human post-mortem tissue with similar expression trends [14, 15].
Interestingly, downstream of UPR activation is regulation and facilitation of apoptosis [5, 6, 16,17,18,19,20,21] which is also implicated in depression [21,22,23,24,25,26,27]. Namely, this occurs via the PERK (eukaryotic translation initiation factor 2-alpha kinase 3, EIF2AK3) pathway by first initiating a global downregulation of protein synthesis through eIF2α (Eukaryotic translation initiation factor 2A) [28]. Under prolonged stress, this pathway leads to the induction of CHOP (C/EBP homologous protein) which induces cell cycle arrest or apoptosis [29]. CHOP regulates the expression of DR5 (death receptor 5) [17] and suppresses the expression of the survival protein BCL2 (B cell lymphoma 2) [30]. Further, this arm, in conjunction with the inflammatory activity of JNK via the IRE1α (Inositol-requiring enzyme 1 α) branch of UPR activation, activates caspases, their cleavage, and their downstream activation and pro-apoptotic signaling which leads to DNA fragmentation and cell death [29]. While not explicitly analyzed in the MRI (magnetic resonance imaging) studies that showed hippocampal atrophy [31,32,33], apoptosis could be inferred as a possible cause of the hippocampal atrophy and thus makes the activation of UPR and its downstream activity of particular interest when discussing underlying pathophysiology in depression.
Previous studies from our lab [12, 21] and others [20, 34] have indicated that the UPR is active in the hippocampus of depressed rats. However, these studies have relied on methods wherein animals were stressed by an outside stimulus and relative UPR activity was studied in hippocampi post-mortem. The hippocampus is a relevant target due to its susceptibility to stress and atrophy in depressed individuals [31,32,33]. In the present paper, we propose that the induction of the UPR in the hippocampus is a critical molecular determinant in developing depression phenotype. We propose that this induction of the UPR causes an acute and severe depressive response which can be utilized for studying severe depression in rodents. Due to previous studies, it is evident that the UPR is involved in the underlying pathophysiology of depression in the hippocampus. Thus, we hypothesize that the induction of the UPR in the hippocampus suffices to cause depressive behavior. Based on the data obtained in this manuscript, we confirm that the activation of the UPR via tunicamycin is sufficient to cause behavioral deficits that are congruent with depressive behavior in rodents.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (300–350 g body weight) were obtained from Envigo (Indianapolis, IN, USA) and housed in similar cages (2 rats/cage) within the same room under standard laboratory conditions (temperature 21 ± 1 °C, humidity 55 ± 5%). Animals were given free access to food and water and adapted to the laboratory environment for 1 week prior to the experiment. All the experiments were carried out according to the National Institutes of Health (NIH) guide for the care and use of laboratory animals and were approved by the Animal Care Committee (IACUC) of the University of Alabama at Birmingham.
Surgical Procedure
Rats were anesthetized with 99.9% isoflurane prior to stereotaxic surgery. The head was placed in a stereotaxic apparatus with the skull exposed. Small burr holes were drilled in the left and right hemispheres for drug administration. A stainless-steel needle (26 gauge) was inserted (coordinates: DV 3.5, AP -4.8, ML ± 2.5) and connected to a Hamilton syringe. A total of 2.5 μL of tunicamycin (TM; 200 ng/μl) in methanol or 150 mM dextrose (control) was infused for ~ 12.5 min; the needle was left in place for 5 additional minutes to allow diffusion of the drug or vehicle. This process was repeated in the second hippocampal location in the opposite hemisphere. After the initial infusion of TM on day 0, rats were assigned to either an “8-day paradigm” or a “12-day paradigm” (named for the day they were sacked).
Behavioral Paradigms
The behavioral paradigm followed in this study has been designed based on a precise timeline which can be viewed in Fig. 1. As mentioned in the previous section, the rats were distributed into two groups (8-day and 12-day paradigm) based on their sacking after TM infusion.
The paradigm followed in behavioral assessment. a The 8-day paradigm. This figure depicts the 8-day paradigm in which rats’ hippocampi were infused with TM and behavioral studies began on day 3 with EPM. The rats were sacked on the eighth day after the initial infusion. EPM elevated plus maze, ET escape test, SPT# sucrose preference test (day#), FST# forced swim test (day#). b The 12-day paradigm. This figure depicts the 12-day paradigm in which rats’ hippocampi were infused with TM and behavioral studies began on day 7 with EPM. The rats were sacked on the 12th day after the initial infusion. EPM elevated plus maze, ET escape test, SPT# sucrose preference test (day#), FST# forced swim test (day#). Data presented are the mean ± SEM
Eight-Day Paradigm
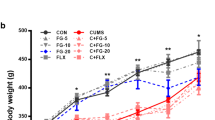
A timeline of TM injections and subsequent behavioral tests are depicted in Fig. 1a. In this paradigm, the rats were subjected to a series of behavioral tests beginning 3 days after the initial injection. Body weights of each rat were taken on day 0 and days 4 and 8 post-injection (see Fig. 2). Rats were sacked on day 8 post-injection. This paradigm was utilized to assess depressive behavior as quickly as possible post-surgery.
Change in weight by paradigm and group. This figure depicts the contrast in the weights from both the 8-day (n = 10/group) and the 12-day (n = 10/group) paradigms. The shades of red show the changes present in the TM-infused groups while the shades of blue depict the control groups. The 8-day paradigm had three weigh-in points while the 12-day had 5 points. The most significant differences between groups were observed on day 4 (8-day; p = 0.43) and day 6 (12-day; p = 0.54). This indicates that TM had a small window for maximum performance. The level of significance is indicated with *p ≤ 0.05. Data presented are the mean ± SEM
Twelve-Day Paradigm
A timeline of TM injections and subsequent behavioral tests are depicted in Fig. 1b. In this timeline, rats were injected, and the normal regimen of behaviors was not executed until 7 days post-injection. Rats’ weights were taken every 3 days instead of 3× over the 8-day period of the first study (see Fig. 2). Rats were sacked on day 12 post-injection. This was used to assess how long the TM would impact behavior.
Behavioral Analyses
Sucrose Preference Test
This is a 5-day procedure that assesses anhedonia in the rodents. During the week prior to and after surgery, rats underwent a sucrose preference test. Following is the procedure which has been adapted from a previously published report [35]. Briefly, on day 1, both standard hydro-packs were replaced with 500 mL of 1% sucrose in bottles; this allowed for acclimation to the new bottles. Day 2—one of the two bottles was replaced with a 500 mL bottle of regular tap water. This allowed the rats to become acclimated to the concept of choice between sucrose and regular tap water. Day 3—All water and food was removed for 24 h. Day 4—The rats were moved to individual cages; food was added and two pre-measured bottles with 500 mL of 1% sucrose for one bottle and regular tap water was supplied. The rats were left for an overnight sucrose preference test. Day 5—The sucrose and water consumption were then removed and measured. Finally, the rats were returned to their original cage with standard hydro-packs and food that they had access to ad libidum.
Percent sucrose consumption was calculated by dividing the volume of 1% sucrose solution consumed by the total volume of all liquid consumed (1% sucrose + tap water). Further, the sucrose preference percentage was compared between a before-surgery total to an after-surgery total (sucrose preference testing completed during the behavioral portion of the study). The percentage from before surgery was subtracted from the percentage after to give a within-subjects change in preference as a result of the surgery. Finally, the sucrose preference percentage changes in the control group were compared to the changes in the TM-injected rats, thus yielding a within-group and between-groups result.
Elevated Plus Maize
Rats were acclimated to the testing room for 15 min. After acclimation, the rat was placed in the center of the maze and the recording was started. After a 5-min testing period, the rat was then removed from the testing apparatus and returned to its cage. The maze was wiped down with ethanol in preparation for the next rat. The next rat was then placed in the center of the maze and the test was started again. The previously described methods were used for each successive rat [36].
Shuttle Escape Test
To determine if the rats were exhibiting helpless/depressed behavior, they were subjected to an escape test (via electric shock to the foot; 0.6 mA on a VI schedule) in an escape shuttle (Model no. ENV-413, Med Associates, Lafayette, IN, USA) as described earlier [21, 37]. The rats were brought to the testing room with the escape test apparatuses and acclimated for 15 min. Rats were placed individually in the shuttle escape apparatuses. Once inside the chamber, the program started the timed sequence for FR-1. FR-1 is programmed such that, after 5 min of acclimation, the rat will have electrical shock applied to the feet (0.6 mA). In order to deactivate the current, the rat must pass under an archway and successfully move to the other side of the chamber; this takes place five times. Next, FR-2 is initiated. FR-2 requires the rat to escape from the initial shock. The current is continued on the opposite side, and the rat must return to the original position to escape the shock. This shock is delivered 25 times. After completion, the rat was returned to its cage, and the shuttle boxes were cleaned.
The learned helpless (LH) behavior was assessed based on the average amount of time it took the rats to escape the shock. The cut-off time for exposure to constant shock was 30 s. Any rat that took 25–30 s to escape was considered severely depressed (or severe LH), while 20–24 s was considered moderately depressed. For this study, the TM-infused rats ranged from 24.7 s escape latency to the full 30-s time while the control rats ranged from 1.93 to 6.5 s escape latency.
Forced Swim Test
The forced swim test protocol followed here has been adopted from previous literature [38] and was broadly broken down into 2 days; before beginning the test each day, the rats were brought to the room to acclimate for 15 min prior to testing. On day 1, after acclimation to the room, a rat was placed in a large cylinder containing water filled to an appropriately marked level (so the rat could not stand on the bottom) and was unable to escape for 15 min. Afterward, the rat was retrieved, dried, and returned to its cage. The cylinder was cleaned with chlorhexidine and warm water. On day 2, after acclimation to the room, the rat was placed in a large cylinder of water. A video recorder was placed in front of the cylinder, and the rat was recorded for a total time of 6 min. After that time, the rat was retrieved, dried off, and returned to its cage. The cylinder was cleaned with chlorhexidine and warm water. The cylinder was refilled for the next rat.
The videos of the rats were watched by other members of the lab to ensure no biases; the rater was blind to the animal grouping (vehicle or TM infused). The video analysts recorded how much time was spent by the rat making overt attempts to escape the container (noted by exasperated motion, rapid kicking, and use of front paws in motion. Non-exasperated motion was considered equilibrating or attempting to keep the rat afloat). The total amount of time swimming was then compared to the total amount of time the rats were in the containers. The initial 2 min was analyzed separated from the full 6 min as this showed the rats’ initial response to the water and any effect was not dampened by exhaustion shown in the latter 4 min. The percentage of time swimming was then compared across both groups.
The Test of Mobility: SHIRPA
The SHIRPA (SmithKline Beecham, Harwell, Imperial College, Royal London Hospital, Phenotype Assessment) method was used to assess the mobility and motor coordination of the animals. This procedure is typically used for assessing the extent of cerebellar ataxia [39]. Kyphosis, ledge balance, gain, and grasp were assessed to determine motor ability in the rats. The scoring system is based on observation of the aforementioned areas and is ranked from 0 (no problem in this task) to 3 (severely debilitated) [40].
Tissue Collection
Rats were sacrificed on day 5 of the sucrose preference test paradigm in all three timelines. After anesthetizing the rats, blood was collected via exsanguination of the heart followed by decapitation. Brains were removed and hippocampi were dissected and flash frozen in liquid nitrogen. Tissues were stored at − 80 °C until they were analyzed.
RNA Isolation and qPCR-Based Gene Expression Assay
RNA was isolated using TRIzol® (Life Technologies, USA), as described earlier [21]. RNA purity (260/280 nucleic acid quantification; cut-off ≥ 1.8) was determined with NanoDrop spectrophotometer (ThermoScientific, Waltham, MA, USA). The RNA quality was further assessed using denaturing agarose gel electrophoresis and evaluating the 28S and 18S rRNA band integrity. Finally, only those samples were selected for analyses which showed 260/280 ≥ 1.8 and 28S:18S rRNA = 2:1.
M-MLV-based reverse transcription of hippocampal RNA was performed following oligo (dT) priming method. One microgram (1 μg) total RNA was reverse transcribed using M-MLV Reverse Transcriptase (Invitrogen, Grandsland, NY, USA) and oligo (dT)18 primer (Invitrogen, Grandsland, NY, USA). The oligo (dT)18 primer annealing step was carried out at 5 μM concentration in presence of 1 mM dNTPs (Invitrogen, Grandsland, NY, USA) by incubating the reaction at 65 °C for 5 min. The reaction was quenched by holding at 4 °C for 2 min. The reaction was mixed with 1× first-strand synthesis buffer (Invitrogen, Grandsland, NY, USA), 0.01 mM DTT (Invitrogen, Grandsland, NY, USA), 2 U of RNaseOut (Invitrogen, Grandsland, NY, USA), and 200 U of M-MLV Reverse Transcriptase and incubated at 37 °C for 50 min. Finally, the reaction was inactivated at 70 °C.
The relative abundance of transcripts was measured with a quantitative real-time PCR machine (AriaMx Real-Time PCR System; Agilent Technologies, USA) using 1× EvaGreen qPCR Mastermix (Applied Biological Material Inc., Canada) in combination with 0.8 μM each of gene-specific forward and reverse primers. A list of primers is given in Table 1. Forty-fold diluted raw cDNA was used as template for qPCR amplification using a thermal parameter of initial denaturation at 95 °C for 10 min followed by repeating 40 cycles of denaturation at 95 °C for 10 s, primer annealing at 60 °C for 15 s, and an extension of amplicon at 72 °C for 20 s. The possibility of primer dimer formation and secondary product amplification was ruled out by running a single cycle of EvaGreen-specific dissociation curve analysis program with an initial denaturation at 95 °C for 1 min followed by annealing at 55 °C for 30 s and repeat denaturation 95 °C for 30 s. The relative gene expression level was quantified after normalization with Gapdh as a reference gene, and fold change value was determined following Livak’s ∆∆Ct calculation method. Data are presented as fold change.
Western Blot-Based Protein Quantification
Protein lysates prepared in RIPA buffer (Tris–HCl (pH 8.0) 50 mM, NaCl 150 mM, NP-40 1%, sodium deoxycholate 1%, SDS 0.1%, supplemented with 1× protease inhibitors, 1 mM phenylmethylsulfonyl fluoride, and 25 μm proteasome and calpain inhibitor MG-132) were subjected to immunoblot analysis using denatured discontinuous SDS–polyacrylamide gel electrophoresis (SDS-PAGE). Probing with primary antibody was performed after titrating the optimum dilution point for the detection of a specific protein on blot. All antibodies (Table 2) were diluted in 2% non-fat milk in TBST (1 L of 1× Tris-buffered saline and 1 mL of Tween 20 [polysorbate 20]). GAPDH was used to normalize the protein expression. Image J (v.1.51; https://imagej.nih.gov/ij/download. html)-based densitometry quantification was performed by averaging at least three independent experiments. The fresh lysate was taken from the same tissue for the second batch of experiments which confirmed the findings of our previous observation with the first batch.
Data Collection and Statistical Analysis
Statistical Package for the Social Sciences (SPSS) was used for all the data analysis. The data are represented as mean ± SEM. Control and restraint groups were compared using two-tailed, independent sample t test. Significance level was set at p ≤ 0.05.
Results
Body Weights
In the two timelines, rats were weighed on days 0, 4, and 8 (8-day paradigm, n = 10) and on days 0, 3, 6, 9, and 12 (12-day paradigm, n = 11) depicted in Fig. 2. Within the control rats of the 8-day group, there was no significance between the three measures of weight when analyzed by one-way ANOVA [F (2, 27) = 1.3425, p = 0.27]. Within the TM-infused rats of the same group, there was a significant difference among the three measurements [F F(2, 27) = 6.7125, p < 0.005]. Applying two-way ANOVA with replication helped us to determine the difference between control and TM rats which showed F value (5.27) higher than the F critical value (4.01), whereas the level of significance was lower (p < 0.025) than the specified alpha value (α = 0.05) in the statistical analysis. When comparing the average weights (via two-tailed t tests) by day between control and TM groups, there was no significant difference on day 0 and 8 (p = 0.99; p = 0.10), but a significant difference was found on day 4 (p < 0.05). However, adjusting the p values with one-tail t test, we were unable to show statistical significance for day 0 (p = 0.49) and day 8 (p = 0.054).
A one-way ANOVA was used to analyze the average weights within the groups for the 12-day paradigm. Within the control rats, there was a significant difference in the weights collected across the five time points [F(4, 50) = 5.212, p < 0.005]. In contrast, the TM group did not show a significant difference in the five time points of weight collection [F(4, 50) = 1.040, p = 0.39]. When comparing the average weights (via two-tailed t tests) by day between control and TM groups, there were no significant differences on days 0, 3, 9, and 12 (p = 0.74, p = 0.74, p = 0.16, p = 0.38), but there was a near significant difference on day 6 (p = 0.054) when two-tailed t test was applied but adjusting the value for one-tailed t test resulted in a highly significant difference on day 6 (p = 0.027).
Behavior
Escape Tests
In the 8-day paradigm (n = 12 control, n = 12 TM; this included rats from a preliminary study, hence the larger n), the average escape latency for the control group was 3.5 s while the TM rats had an average of 28.4 s (p < 0.005). In the 12-day paradigm (n = 11 control, n = 11 TM), the average escape latency for the control group was 7.5 s while the TM rats had an average of 7.9 s (p = 0.88) (Fig. 3).
Animal behavior by paradigm and group. This figure contrasts the behavioral measures between the 8-day paradigm (panel a) and the 12-day paradigm (panel b). Overall, the TM-infused rats showed a significant change in their behavioral battery compared to the controls in panel a while there was no significant change in panel b. These data are indicative of depressive behavior if the delay from infusion to testing is kept brief (i.e., the 8-day paradigm shows significant changes) which, again, reiterates the impact of TM being short-lived. In panel a, the number of subjects is as follows by behavior: ET: n = 12/group (preliminary rodents included); FST: n = 10/group; SPT: n = 10/group. In panel b, all experiments were conducted in 10 rats/group. Data presented are the mean ± SEM
Forced Swim Test
The forced swim test was analyzed in 1-min increments and totaled for the amount of time swimming vs time floating. The first 2 min was studied for the sake of significant differences in groups, though all time points were analyzed. In the 8-day paradigm rats (n = 10 control, n = 10 TM), the control rats spent 56.9% (average of 68.3 s) of the first 2 min actively swimming/attempting to escape while the TM group spent 33.8% (average of 40.6 s) of the time swimming/attempting to escape (p < 0.005). In the 12-day paradigm (n = 11 control, n = 11 TM), the control rats spent an average of 36% (average of 43.1 s) of the first 2 min actively swimming/attempting to escape while the TM group spent 37.3% (average of 44.7 s) of the first 2 min actively swimming/attempting to escape (p = 0.93) (Fig. 3).
Sucrose Preference Test
The sucrose preference test (SPT) was administered before and after surgery to establish baseline sucrose preference. Sucrose preference is described as the amount of sucrose water consumed divided by the amount of sucrose water and tap water consumed. After the second SPT data was collected, the amount of change from baseline to post-surgery was recorded (decrease in SPT pre/post in Fig. 3). For the rats who underwent the 8-day paradigm, the control rats (n = 10) had a 2.6% overall decrease in sucrose preference between baseline and post-surgery while the TM-infused group (n = 10) had an overall decreased preference in sucrose of 15.6%.
Figure 3 shows the total sucrose preference differences in control and TM rats after surgery. In the 8-day paradigm, the control rats had an average of 83.8% preference for sucrose while the TM rats had an average of 71.8% (p < 0.05). In the 12-day paradigm, the control rats had an average of 92.7% preference for sucrose while the TM rats had an average of 87.8% (p = 0.20).
Molecular Analyses
RNA Level Expression Analysis of Unfolded Protein Response Genes
In a random sample of the behaviorally tested rats of the 8-day paradigm (6 control and 6 TM rats), qPCR analysis revealed that TM rats expressed a significant increase in all tested genes that are of particular importance in UPR activity. Grp78 (78 kDa glucose-regulated protein) showed a 2.7-fold increased expression in TM-infused rats when compared to control rats (p < 0.005), Grp94 (94 KDa glucose-regulated protein) showed a 2.2-fold increased expression in TM rats compared to control (p < 0.001), Xbp-1 (X-box binding protein 1) showed a 1.5-fold increased expression in TM rats compared to control rats (p < 0.05), Xbp-1s (previously designed and described, see Timberlake M. et al. [12]) showed a 5.7-fold increased expression in TM rats compared to control rats (p < 0.001); Atf6 (Activating transcription factor 6) showed a 1.3-fold increased expression in TM rats compared to control rats (p = 0.05); Atf4 (Activating transcription factor 4) showed a 1.9-fold increased expression in TM rats compared to control rats (p < 0.005); and Chop showed a 2.4-fold increased expression in TM rats compared to control rats (p < 0.001) (see Fig. 4).
Expression of UPR-associated genes in hippocampus of TM-infused rats. In this figure, key genes of all three branches of UPR activation are compared in the TM-infused rats to the control vehicle rats; these genes are Grp78, Grp94, Xbp-1, Xbp-1s, Atf6, Atf4, and Chop. Between the two paradigms, the greatest fold change and significance was observed in the 8-day paradigm rats. A trend was seen in the 12-day rats; however, there was no significant change between the expressions in the TM-infused and the control rats. The data is derived from a sample of n = 6/group for the 8-day paradigm and n = 5/group for the 12-day paradigm. Data presented are the mean ± SEM
In the 12-day paradigm (Fig. 4), a random sample of behaviorally tested rats (n = 5 control and 5 TM) was analyzed. While the trend persists, there were no significantly elevated genes expressed in this paradigm. Grp78 showed a 1.3-fold increased expression in TM-infused rats when compared to control rats (p = 0.18), Grp94 showed a 1.3-fold increased expression in TM rats compared to control (p = 0.18), Xbp-1 showed a 1.6-fold increased expression in TM rats compared to control (p = 0.24), Xbp-1s showed a 1.8-fold increased expression in TM rats compared to control (p = 0.12), Atf6 showed a 1.1-fold increased expression in TM rats compared to control (p = 0.35), Atf4 showed a 1.2-fold increased expression in TM rats compared to control (p = 0.29), and Chop showed a 1.1-fold increased expression in TM rats compared to control (p = 0.41).
In the same sample of the behaviorally tested rats of the 8-day paradigm (6 control and 6 TM rats), qPCR analysis revealed that TM rats expressed a significant increase in several tested genes that are of particular importance in activity downstream of UPR activation by TM infusion. Figure 5 depicts genes that are highly expressed in depression physiology and are related to inflammation. Nf-κb showed a 1.644-fold increased expression in TM-infused rats when compared to controls (p < 0.001), Tnf-α (tumor necrosis factor-alpha) showed a 3.23-fold increased expression in TM-infused rats when compared to controls (p < 0.01), Il-6 (interleukin-6) showed a 10-fold increased expression in TM-infused rats when compared to controls (p < 0.001), Il-1β (interleukin-1 beta) showed a 1.87-fold increased expression in TM-infused rats when compared to controls (p < 0.05), Tlr2 (Toll-like receptor 2) showed a 5-fold increased expression in TM-infused rats when compared to controls (p < 0.01), Tlr4 (Toll-like receptor 4) showed a 3.8-fold increased expression in TM-infused rats when compared to controls (p < 0.01), Tlr7 (Toll-like receptor 7) showed a 3.7-fold increased expression in TM-infused rats when compared to controls (p < 0.01), and Tlr9 (Toll-like receptor 9) showed a 2.6-fold increased expression in TM-infused rats when compared to controls (p < 0.01).
Expression of key inflammatory genes in hippocampus of TM-infused rats. In this figure, key genes shown to have significant contribution to inflammation or a pro-inflammatory environment are compared in the TM-infused rats to the control vehicle rats; these genes are Tnf-α, Il-6, Il-1b, Tlr2, Tlr4, Tlr7, Tlr9, and Nf-κβ. The data is derived from a sample of n = 6/group from rats who underwent the 8-day TM-infusion paradigm. Data presented are the mean ± SEM
Protein Level Expression Analysis of Unfolded Protein Response Genes
In a sample of the behaviorally tested rats of the 8-day paradigm, Western blot results revealed that TM rats had significantly increased expression in all tested proteins when compared to the control group. GRP94 showed a 0.71-fold increased expression in TM rats (p < 0.05), CHOP showed a 1.2-fold increased expression in TM rats (p < 0.005), and the sensor protein PERK showed a 9.3-fold increased expression in TM rats (p < 0.05) compared to the control group (Fig. 6).
Expression of UPR proteins in hippocampus of TM-infused rats. In this figure, the proteins GRP94, CHOP, and PERK were analyzed using western blot in the 8-day paradigm TM rat experiment. All three proteins showed significant fold increase in the TM-infused rats when compared to the control; all proteins were normalized to GAPDH. The data is derived from a sample of n = 4/group for GRP94, CHOP and n = 6/group for PERK. Data presented are the mean ± SEM
Discussion
From our previous studies, it is evident that the UPR is active in the hippocampus of depressed rats. In the present study, we hypothesize that the induction of the UPR in the hippocampus is sufficient to cause depressive behavior. Based on the data reported in this manuscript, we confirm that the activation of the UPR via tunicamycin is sufficient to cause behavioral deficits that are congruent with depressive behavior in rodents. In light of our previous data, we designed a novel model that utilized the activation of the UPR in the hippocampus of rats. The present study highlights the results of this new model. Not only did we show an acute, severe depressive response in behavior, but we also showed significant increases in expression at both the gene and the protein level of relevant components of the UPR. Most important to this finding are the significant changes in rodent behavior that correlate with severe depressive behavior. We also found that this effect, when the UPR is directly induced via TM, is time-sensitive; rats who had an infusion of TM but were left for 1 week before beginning behavioral (12-day paradigm) testing did not show the depressive behavior that the TM-infused rats who were injected and began behavioral testing within 3–4 days showed (the 8-day paradigm). The magnitude of these changes in behavior is indicative of an acute, severe, depressive episode.
In the 8-day paradigm, rats infused with TM showed significant changes in depressive behavior when compared to controls that also underwent the same procedure but with an infusion of 150 mM dextrose instead of TM. The first significant finding was that of the escape test which has previously been used for validating depression in rats [37]. Unlike the traditional learned helplessness model of depression, this model does not utilize a round of inescapable shock. Instead, the rats are placed directly in the shuttle escape chamber 4 days post-injection to assess learned helpless behavior. As evident from the data in the control group, rats who were placed in this environment escaped the aversive shocks rapidly. On average, the control rats escaped the shock within 3.5 s. In contrast, the TM rats had an average escape latency of 28.5 s. This suggests that activated UPR localized to the hippocampus is sufficient to cause learned helpless behavior in rats. In our previous findings [21], we showed that rats that exhibited the learned helpless behavior had a correlated heightened expression of UPR genes, namely Grp94, Grp78, Xbp-1, Atf6, Atf4, and Chop. In contrast to these data, rats that underwent the 12-day paradigm did not show significant changes between groups in their escape latency. The control group had an average escape latency of 7.5 s while the TM group had an average of 7.9 s. This reinforces the idea that TM-induced UPR activation and subsequent depressive behavior is acute in nature.
The next behavior analyzed was forced swim test (FST). In the 8-day paradigm, the control rats spent an average of 68.3 s in the first 2 min attempting to escape. The TM-infused group spent only 40.6 s on average in the first 2 min. This significant decrease in attempted escape time is indicative of behavioral despair [41, 42]. By contrast, rats that underwent the 12-day paradigm did not show a significant difference in their escape time; control rats spent an average of 40.9 s attempting to escape while the TM rats spent an average of 45.9 s.
The final behavior analyzed was that of sucrose preference (SPT) [42, 43] which is a measure of anhedonia. For this, there were both within/between-subjects’ analyses as well as between-subject analysis for the within/between-subjects test; rats were tested for a baseline sucrose preference before and after surgery. The total changes in preference were then analyzed within the groups and controlled between the two groups. For this study, sucrose preference is defined as a total amount of 1% sucrose water consumed divided by the total consumption of tap water + 1% sucrose. In the 8-day paradigm, there was a noted decrease in sucrose preference in the TM rats by an average of 16% compared to the 2.6% decrease in sucrose preference in the control group. Further, in analyzing only the post-surgery SPT differences, control rats showed an average 83.8% preference of sucrose to tap water while the TM group showed a 71.8% preference. For the rats that underwent the 12-day paradigm, there was no decrease in sucrose preference in the within/between-group analysis. There was a small difference in sucrose preference in the between-subjects-only analysis, but it was not significant. Control rats had an average of 92.7% sucrose preference compared to the TM-infused rats which had an 87.7% preference.
Finally, we studied expression levels of genes relevant to UPR activity in the hippocampus which have previously been shown to be significantly upregulated in stress-induced-depressed rats [12, 21]. In the 8-day paradigm, the genes Grp78, Grp94, Xbp-1, Xbp-1s, Atf6, Atf4, and Chop were all shown to be significantly upregulated in the TM-infused group compared to their control counterparts. This is relevant as we have shown in both LH and restraint-stress rats that these same genes were significantly increased in hippocampus [12, 13, 21]. As mentioned in “Introduction,” aberrant changes in the expression of CHOP and the sensory protein PERK may lead to the activation of caspases with functional consequences linked to cellular apoptosis. Earlier studies have suggested a significant loss of hippocampal functions due to apoptosis in dentate gyrus and CA3 regions of hippocampus. For example, a systematic review on this topic has been published by Lucassen et al. where they discussed the importance of hippocampal apoptosis in relation to stress and depression [44]. In addition, apoptosis has been reported in entorhinal cortex, subiculum, dentate gyrus, and CA4 brain areas of depressed patients [45]. Interestingly, in a recent report, the ontological enrichment of genes with the apoptotic function was detected from a pool of 38 depressed patients when compared to control subjects. Even pathway enrichment and network analysis on differentially expressed genes identified a higher degree of involvement in apoptotic and inflammatory pathways in these patients [46]. Further, the proteins of GRP94, CHOP, and the sensor protein PERK were significantly upregulated in the TM rats. Not only is this indicative that the UPR is an important feature of depressive behavior but also that the TM is, in fact, affecting the hippocampus and activating the UPR. The rats that underwent the 12-day paradigm showed a similar trend in the expression of the aforementioned genes; however, there was no significant change between the groups suggesting that the TM-induced UPR may have some residual and lasting effect, but it does not impact the behavior after a certain amount of time.
In the same group, we also analyzed several genes relevant to inflammation which reinforce our previous claim that the UPR acts on a pro-inflammatory environment in the hippocampus of depressed subjects [12, 13]. The increased expression of Tlr2,4,7, and 9 in TM rats show that pro-inflammatory environment is directly related to UPR activity. This is also reinforced by other findings which suggest that TLR-mediated inflammation is a key component in the underlying pathophysiology of depression [12,13,14,15, 27, 47,48,49,50,51,52,53,54]. Further, a direct and increased measure of the cytokines Tnf-α, Il-6, and Il-1b as well as the transcription factor Nf-kβ, which is a known regulator of cytokine production, indicated active inflammation in the hippocampus. This also correlates with many studies that have shown the relevance of inflammation in the depressed brain [7, 9, 21, 55,56,57,58,59,60,61]. Moreover, earlier studies have suggested the role of tunicamycin in inducing NF-κB through the catalytic activity of the IRE1α kinase [62]. Studies have also documented the involvement of tunicamycin in regulating several inflammatory genes with their roles in immune function. Many of them include various cytokines and their receptors (e.g., IL23a, IL1a, IL6, IFNAR1, IL17ra, IL6ra), chemokines (CCL2, CCL9), adhesion molecules (ICAM1), and prostaglandin synthetases (PTGS2, COX-2). These findings further validate the replication of this model in mimicking known depression physiology associated with neuroinflammation [63].
This new model allows the study of an acute, severe depressive episode by activation of the UPR directly in the hippocampus via stereotaxic injection. A benefit of this model is that its delivery and implementation are very specific and the TM dose is not lethal (2.5 μL at 200 ng/μL). Further, the rats, though exhibiting depressive behavior, are not locomotor compromised. Using a modified version of the SHIRPA method to assess locomotor activity, all rats, control, and TM displayed a score of “0” in each of the four categories observed (kyphosis, ledge, clasp, and gait) [39]. This suggests that there is no locomotor abnormality associated with TM injection.
We emphasize that the proposed model is a method for studying an acute, severe depressive episode in rats. Further, we propose that this model is most relevant as a method to circumvent inducing depression by means of a behavioral stressor like inescapable shock or chronic restraint stress and directly induces depression via pharmacological activation of the UPR. We also propose that this solidifies our prior findings that correlated the activation of the UPR with depressive behavior in rats, and it may have broader implications for expanding the study of the UPR in the context of depression. Collectively, our findings from the current study suggest that pharmacological activation of UPR system by tunicamycin may lead to depression phenotype with increased susceptibility of the hippocampus to acute stress, causing dysfunction in programmed cell death. This may hold clinical significance, considering the fact that the failure of the UPR system in the susceptible regions of depressed brain may perhaps be critical in immunomodulation.
Future studies can be conducted in this model where a pharmacological agent, like a lentiviral construct targeting a specific gene, could be introduced before the infusion of TM (via cannulation) and observation of behavior. Further, studies with antidepressants could also be used if they are administered for the recommended week before behavioral testing. The primary disadvantage of this new model is that the duration of the effect is acute, despite the severity of depression. We have tested the use of cannulas with this model; however, a second infusion is not sufficient to prolong the effect of TM. The rats that underwent a second infusion of TM via cannulation did not show significant changes in behavior that mirrored the 8-day paradigm rats (data not shown). Instead, they mimicked the results of the 12-day group wherein there was an apparent recovery or resistance to the initial effects of TM. This could be due to resistance to the drug, or possibly due to a type of over-compensation which ameliorates the pharmacological effect of TM. This is speculative in nature, but presently, our data suggests that a second dose of TM at the same concentration is not sufficient to alter behavior; further, higher doses have not been tested beyond the 500 ng/hippocampus.
References
Belmaker RH, Agam G (2008) Major depressive disorder. N Engl J Med 358(1):55–68. https://doi.org/10.1056/NEJMra073096
Albert PR (2015) Why is depression more prevalent in women. J Psychiatry Neurosci 40(4):219–221
Organization WH (2018) Depression.
Pompili M, Venturini P, Lamis DA, Giordano G, Serafini G, Belvederi Murri M, Amore M, Girardi P (2015) Suicide in stroke survivors: epidemiology and prevention. Drugs Aging 32(1):21–29. https://doi.org/10.1007/s40266-014-0233-x
Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110(10):1389–1398. https://doi.org/10.1172/jci16886
Ron D (2002) Translational control in the endoplasmic reticulum stress response. J Clin Invest 110(10):1383–1388. https://doi.org/10.1172/jci16784
Garg AD, Kaczmarek A, Krysko O, Vandenabeele P, Krysko DV, Agostinis aP (2012) ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol Med 18(10):589–598
Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23(21):7448–7459
Hotamisligil GS (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140(6):900–917. https://doi.org/10.1016/j.cell.2010.02.034
Yamazaki H, Hiramatsu N, Hayakawa K, Tagawa Y, Okamura M, Ogata R, Huang T, Nakajima S et al (2009) Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J Immunol (Baltimore, Md : 1950) 183(2):1480–1487. https://doi.org/10.4049/jimmunol.0900017
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C et al (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science (New York, NY) 306(5695):457–461. https://doi.org/10.1126/science.1103160
Timberlake MI, Prall K, Roy B, Dwivedi Y (2018) Unfolded protein response and associated alterations in toll-like receptor expression and interaction in the hippocampus of restraint rats. Psychoneuroendocrinology 89:185–193. https://doi.org/10.1016/j.psyneuen.2018.01.017
Timberlake M II, Dwivedi Y (2018) Linking unfolded protein response to inflammation and depression: potential pathologic and therapeutic implications. Mol Psychiatry 24:987–994. https://doi.org/10.1038/s41380-018-0241-z
Hung Y-Y, Kang H-Y, Huang K-W, Huang T-L (2014) Association between toll-like receptors expression and major depressive disorder. Psychiatry Res 220:283–286
Pandey GN, HS R XR, Dwivedi Y (2014) Toll-like receptors in the depressed and suicide brain. J Psychiatr Res 53:62–68
Szegezdi E, Fitzgerald U, Samali A (2003) Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann NY Acad Sci:186–194
Yamaguchi H, Wang HG (2004) CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem 279(44):45495–45502. https://doi.org/10.1074/jbc.M406933200
Endo M, Mori M, Akira S, Gotoh T (2006) C/EBP homologous protein (CHOP) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation. J Immunol (Baltimore, Md : 1950) 176(10):6245–6253
Meares GP, Mines MA, Beurel E, Eom TY, Song L, Zmijewska AA, Jope RS (2011) Glycogen synthase kinase-3 regulates endoplasmic reticulum (ER) stress-induced CHOP expression in neuronal cells. Exp Cell Res 317(11):1621–1628. https://doi.org/10.1016/j.yexcr.2011.02.012
Zhang Y, Liu W, Zhou Y, Ma C, Li S, Cong B (2014) Endoplasmic reticulum stress is involved in restraint stress-induced hippocampal apoptosis and cognitive impairments in rats. Physiol Behav 131:41–48. https://doi.org/10.1016/j.physbeh.2014.04.014
Timberlake M, II, , Dwivedi Y (2015) Altered expression of endoplasmic reticulum stress associated genes in Hippocampus of learned helpless rats: relevance to depression pathophysiology. Front Pharmacol 6:319. doi:https://doi.org/10.3389/fphar.2015.00319
Eilat E, Mendlovic S, Doron A, Zakuth V, Spirer Z (1999) Increased apoptosis in patients with major depression: a preliminary study. J Immunol 163(1):533–534
Fossati P, Radtchenko A, Boyer P (2004) Neuroplasticity: from MRI to depressive symptoms. Eur Neuropsychopharmacol 14(Suppl 5):S503–S510. https://doi.org/10.1016/j.euroneuro.2004.09.001
Dwivedi Y (2009) Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr Dis Treat 5:433–449
McKernan DP, TGD JFC (2009) “Killing the blues”: a role for cellular suicide (apoptosis) in depression and the antidepressant response? Prog Neurobiol 88(4):246–263
Gold PW, Licinio J, Pavlatou MG (2013) Pathological parainflammation and endoplasmic reticulum stress in depression: potential translational targets through the CNS insulin, klotho and PPAR-gamma systems. Mol Psychiatry 18(2):154–165. https://doi.org/10.1038/mp.2012.167
Yanxiao Xiang HY, Zhou J, Zhang Q, Hanley G, Caudle Y, LeSage G, Zhang X, Yin D (2015) The role of toll-like receptor 9 in chronic stress-induced apoptosis in macrophage. PLoS One 10(4):1–14
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C et al (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11(3):619–633
Fribley A, Zhang K, Kaufman RJ (2009) Regulation of apoptosis by the unfolded protein response. Methods Mol Biol (Clifton, NJ) 559:191–204. https://doi.org/10.1007/978-1-60327-017-5_14
McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21(4):1249–1259. https://doi.org/10.1128/mcb.21.4.1249-1259.2001
McEwen BS (2000) Effects of adverse experiences for brain structure and function. Biol Psychiatry 48(8):721–731
Sala M, Perez J, Soloff P, Ucelli di Nemi S, Caverzasi E, Soares JC, Brambilla P (2004) Stress and hippocampal abnormalities in psychiatric disorders. Eur Neuropsychopharmacol 14(5):393–405. https://doi.org/10.1016/j.euroneuro.2003.12.005
Frodl T, Schaub A, Banac S, Charypar M, Jager M, Kummler P, Bottlender R, Zetzsche T et al (2006) Reduced hippocampal volume correlates with executive dysfunctioning in major depression. J Psychiatry Neurosci 31(5):316–323
Nevell L, Zhang K, Aiello AE, Koenen K, Galea S, Soliven R, Zhang C, Wildman DE et al (2014) Elevated systemic expression of ER stress related genes is associated with stress-related mental disorders in the Detroit neighborhood health study. Psychoneuroendocrinology 43:62–70. https://doi.org/10.1016/j.psyneuen.2014.01.013
Mao QQ, Huang Z, Ip SP, Xian YF, Che CT (2012) Peony glycosides reverse the effects of corticosterone on behavior and brain BDNF expression in rats. Behav Brain Res 227(1):305–309. https://doi.org/10.1016/j.bbr.2011.11.016
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14(3):149–167
Dwivedi Y, Mondal AC, Shukla PK, Rizavi HS, Lyons J (2004) Altered protein kinase a in brain of learned helpless rats: effects of acute and repeated stress. Biol Psychiatry 56(1):30–40. https://doi.org/10.1016/j.biopsych.2004.03.018
O'Donovan S, Kennedy M, Guinan B, O'Mara S, McLoughlin DM (2012) A comparison of brief pulse and ultrabrief pulse electroconvulsive stimulation on rodent brain and behaviour. Prog Neuro-Psychopharmacol Biol Psychiatry 37(1):147–152. https://doi.org/10.1016/j.pnpbp.2011.12.012
Hatcher JP, Jones DN, Rogers DC, Hatcher PD, Reavill C, Hagan JJ, Hunter AJ (2001) Development of SHIRPA to characterise the phenotype of gene-targeted mice. Behav Brain Res 125(1–2):43–47
Rogers DC, Fisher EMC, Brown SDM, Peters J, Hunter AJ, Martin JE (1997) Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome 8(10):711–713. https://doi.org/10.1007/s003359900551
Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229(2):327–336
Wang Q, Timberlake M II, Prall K, Dwivedi Y (2017) The recent progress in animal models of depression. Prog Neuro-Psychopharmacol Biol Psychiatry 77:99–109. https://doi.org/10.1016/j.pnpbp.2017.04.008
Klein DF (1974) Endogenomorphic depression. A conceptual and terminological revision. Arch Gen Psychiatry 31(4):447–454
Lucassen PJ, Heine VM, Muller MB, van der Beek EM, Wiegant VM, De Kloet ER, Joels M, Fuchs E et al (2006) Stress, depression and hippocampal apoptosis. CNS Neurol Disord Drug Targets 5(5):531–546
Lucassen PJ, Muller MB, Holsboer F, Bauer J, Holtrop A, Wouda J, Hoogendijk WJ, De Kloet ER et al (2001) Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol 158(2):453–468. https://doi.org/10.1016/S0002-9440(10)63988-0
Woo HI, Lim SW, Myung W, Kim DK, Lee SY (2018) Differentially expressed genes related to major depressive disorder and antidepressant response: genome-wide gene expression analysis. Exp Mol Med 50(8):92. https://doi.org/10.1038/s12276-018-0123-0
Hemmi H, Kaisho T, Takeda K, Akira S (2003) The roles of toll-like receptor 9, MyD88, and DNA-dependent protein kinase catalytic subunit in the effects of two distinct CpG DNAs on dendritic cell subsets. J Immunol 170(6):3059–3064
Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A (2003) Toll-like receptor 9–mediated recognition of herpes simplex Virus-2 by plasmacytoid dendritic cells. J Exp Med 198(3):513–520
Kai Zacharowski PAZ, Koch A, Baban A, Tran N, Berkels R, Papewalis C, Schulze-Osthoff K, Knuefermann P et al (2006) Toll-like receptor 4 plays a crucial role in the immune–adrenal response to systemic inflammatory response syndrome. PNAS 103(16):6392–6397
Sher GTA (2007) Cooperation of toll-like receptor signals in innate immune defence. Nat Rev Immunol 7:179–190
Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrancois L, Li Z (2007) Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity 26(2):215–226. https://doi.org/10.1016/j.immuni.2006.12.005
Ungaro RFM, Hsu D, Hernandez Y, Breglio K, Chen A, Xu R, Sotolongo J, Espana C et al (2009) A novel toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am J Physiol Gastrointest Liver Physiol 296:1167–1179
Kawasaki T, Kawai T (2014) Toll-like receptor signaling pathway. Front Immunol 5(461):1–8
Ming-Kung Wu T-LH, Huang K-W, Huang Y-L, Hung Y-Y (2015) Association between toll-like receptor 4 expression and symptoms of major depressive disorder. Neuropsychiatr Dis Treat 11:1853–1857
Charles L, Raison LC, Miller AH (2006) Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27(1):24–31
AH M, V M CLR (2009) Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65(9):732–741
Lotrich F (2012) Inflammatory cytokines, growth factors, and depression. Curr Pharm Des 18(36):5920–5935
Felger JC, Lotrich FE (2013) Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 246:199–229. https://doi.org/10.1016/j.neuroscience.2013.04.060
Joep Grootjans AK, Kaufman RJ, Blumberg RS (2016) The unfolded protein response in immunity and inflammation. Nat Rev Immunol 16:469–484
Raison AHMCL (2016) The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16:22–34
Setiawan E, Attwells S, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Xu C, Sharma S et al (2018) Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. Lancet Psychiatry 5(4):339–347. https://doi.org/10.1016/s2215-0366(18)30048-8
Kaneko M, Niinuma Y, Nomura Y (2003) Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull 26(7):931–935
Kim SY, Hwang JS, Han IO (2013) Tunicamycin inhibits Toll-like receptor-activated inflammation in RAW264.7 cells by suppression of NF-kappaB and c-Jun activity via a mechanism that is independent of ER-stress and N-glycosylation. Eur J Pharmacol 721(1–3):294–300. https://doi.org/10.1016/j.ejphar.2013.09.022
Acknowledgments
We would like to acknowledge Kevin Prall for technical help.
Funding
The research was partly supported by grants from National Institute of Mental Health (R01MH082802; 1R01MH101890; R01MH100616; 1R01MH107183) to Dr. Dwivedi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the experiments were carried out according to the National Institutes of Health (NIH) guide for the care and use of laboratory animals and were approved by the Animal Care Committee (IACUC) of the University of Alabama at Birmingham.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Timberlake II, M., Roy, B. & Dwivedi, Y. A Novel Animal Model for Studying Depression Featuring the Induction of the Unfolded Protein Response in Hippocampus. Mol Neurobiol 56, 8524–8536 (2019). https://doi.org/10.1007/s12035-019-01687-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01687-6