Abstract
The extracellular protein tissue inhibitor of metalloproteinase (TIMP)-1 is both a matrix metalloproteinase (MMP) inhibitor and a trophic factor. Mice lacking TIMP-1 exhibit delayed central nervous system myelination during postnatal development and impaired remyelination following immune-mediated injury in adulthood. We have previously determined that the trophic action of TIMP-1 on oligodendrocyte progenitor cells (OPCs) to mature into oligodendrocytes is independent of its MMP inhibitory function. However, the mechanism by which TIMP-1 promotes OPC differentiation is not known. To address this gap in our understanding, herein, we report that TIMP-1 signals via a CD63/β1-integrin receptor complex to activate Akt (protein kinase B) to promote β-catenin signaling in OPCs. The regulation of β-catenin by TIMP-1 to promote OPC differentiation was counteracted, but not abrogated, by canonical signaling evoked by Wnt7a. These data provide a previously uncharacterized trophic action of TIMP-1 to regulate oligodendrocyte maturation via a CD63/β1-integrin/Akt pathway mechanism. These findings contribute to our emerging understanding on the role of TIMP-1 as a growth factor expressed to promote CNS myelination during development and induced in the adult to promote myelin repair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oligodendrocyte progenitor cells (OPCs) are a resident stem cell population responsible for remyelination within the central nervous system (CNS) [1]. Axon demyelination results in white matter lesions that can then become chronically demyelinated due to impaired or arrested OPC maturation [2]. Demyelinated axons have reduced conductive potential leading to disrupted neural network activity [3]. Demyelination can also render axons more susceptible to mechanical injury and inflammation-mediated damage [4, 5]. The impact of chronic demyelination, measured as lesion volume, in diseases like multiple sclerosis (MS), is strongly correlated with disease progression and clinical disability in patients [6, 7]. Thus, improved understanding of the processes that regulate OPC maturation may help identify why remyelination in disease is inadequate or insufficient but is also expected to elucidate mechanisms to promote CNS remyelination [4, 8, 9].
Tissue inhibitors of metalloproteinases (TIMPs) are a family of dual-function extracellular proteins with both matrix metalloproteinase (MMP) inhibitory and growth factor activities [10, 11]. Of the four known mammalian TIMPs, TIMP-1 is robustly expressed by astrocytes in response to myelin injury [12, 13]. In experimental autoimmune encephalomyelitis (EAE), an animal model of immune-mediated demyelination, we had previously shown that TIMP-1-deficient mice (TIMP-1KO) exhibited impaired remyelination following immune-mediated injury [14]. We had also determined that developmental myelination in the spinal cords of TIMP-1KO mice is also delayed compared with wild-type mice [15]. These findings were also corroborated on a cellular level where application of recombinant TIMP-1 protein to primary OPCs in culture increased oligodendrocyte (OL) maturation with expression of myelin basic protein (MBP), while antibodies against TIMP-1 decreased OPC maturation [15]. From these findings, we concluded that TIMP-1 is a dual function MMP inhibitor and growth factor that can promote OPC differentiation and CNS myelination [16]. However, the mechanism by which the trophic function of TIMP-1 stimulates OPC maturation has not been resolved.
TIMP-1 has both MMP-dependent and independent biological functions [10, 11, 16]. Structurally, while the N-terminal portion of the TIMP-1 molecule is critical for inhibiting MMPs, the C-terminal domain of TIMP-1 has been shown to mediate important protein-protein interactions [10]. The tetraspanin protein CD63 has been previously identified as a receptor expressed on transformed cancer cell lines that mediate the actions of TIMP-1 [17]. CD63 is a co-factor for β1-integrins, which together, regulate intracellular signal transduction pathways [17, 18]. Interestingly, previous work has shown that TIMP-1 can regulate β-catenin activity, a process important for OPC migration and differentiation [19]. In this study, we examined the trophic activity of TIMP-1 and define a receptor-mediated action of TIMP-1 that promotes OL differentiation via counter-regulation of β-catenin signaling via activation of Akt.
Materials and Methods
Primary OPC Cultures
OPC cultures were developed from the cortices of neonatal Sprague-Dawley rat pups (P0-2; Harlan), as previously described [15]. Cortices were denuded of meninges and hippocampi and were then mechanically minced and manually homogenized with a flame polished Pasteur pipette into a single cell suspension in culture medium (DMEM/F12, Gibco) containing 10% fetal bovine serum (FBS; Denville Scientific) and 1% penicillin/streptomycin (Fisher Scientific). The cell suspension was spun for 10 min (1800 rpm, RT), and the pellet was resuspended in culture media for plating into flasks coated with poly-l-lysine (PLL 0.3 mg/mL; Sigma-Aldrich). Confluent cell monolayers were then shaken for 1 h (55 rpm, 37 °C); media exchanged with fresh culture media to remove any cellular debris and microglia and then returned to the incubator for 2–4 h (37 °C). Cultures were then shaken overnight (255 rpm) to lift OPCs. Supernatant from shaken flasks was spun down (1800 rpm, 10 min); the pellet resuspended in media, and then incubated (37 °C), for 3–4 h to limit astrocyte contamination. Media was collected, spun down (1800 rpm, 10 min), resuspended in fresh OPC media, and cells plated onto poly-ornithine-coated coverslips. For poly-ornithine coating: glass coverslips were washed in sterile 1 N HCl (30 min, RT), washed with sterile water (three times), and then coated with poly-l-ornithine (0.05 mg/mL; Sigma) for 3 h (37 °C). Once plated onto coverglass, media was exchanged for differentiation media for up to 4 days and then OL maturation was assessed. Differentiation media: neurobasal media (Gibco) with 2% B27 serum-free supplement (Gibco), 2 mM l-glutamine (Gibco), and 10 ng/mL T3 (Sigma). For experimental treatments, OPCs were treated with specific peptides or inhibitors daily for four consecutive days (see below) and their effect on OL differentiation assessed by immunocytochemistry using markers to identify each stage of OL differentiation.
Peptides
All peptides used were purchased from commercial sources. These included full-length recombinant murine (rm)-TIMP-1 (10 ng/mL; R&D Systems), C-terminal domain of TIMP-1 (amino acids 126–184 of TIMP-1, referred to as “TIMP-1(C)”; 100 ng/mL, Anaspec), and Wnt7a (50 ng/mL; R&D Systems). Function blocking antibodies: anti-β1 integrin (5 μM; Ha2/5, BD Pharmingen) and its serotype-matched hamster IgM2 control (Sigma) and anti-CD63 (5 μM; Santa Cruz) and its serotype-matched IgG control (Santa Cruz). Chemical inhibitors used included the broad spectrum MMP inhibitor, GM6001 (12.5 μM; Selleckchem), and the Akt inhibitor, MK-2206 (5 μM; Selleckchem).
Analysis of OPC Differentiation
The number of mature oligodendrocytes was evaluated by immunocytochemistry using myelin basic protein (MBP) to characterize differentiation and Olig2+ to define cells of oligodendrocyte lineage. The number of cells per field (× 20 magnification) in four fields per coverslip using n = 8 samples per treatment group was used for analysis. Each experiment was performed using triplicate biological replicates from uniquely derived primary cultures.
PCR
Total RNA was isolated using Trireagent (Sigma), and PCR was performed using primers specific for rat CD63: forward primer (5′-CGGTGGAAGGAGGAATGAAG-3′), reverse primer (5′-CTACATTACTTCATAGCCACTTCG-3′), as described previously [20].
Immunocytochemistry
Immunocytochemistry was performed as previously described [15]. Cultures were fixed in 4% paraformaldehyde, washed, and then incubated with primary antisera for MBP (1:500; Millipore, Billerica, MA), Olig2 (1:500; Millipore), CD63 (1:100; Santa Cruz), and β-catenin (1:500; Abcam). Appropriate secondary antibodies were applied for an hour to visualize staining. 4′,6-diamidino-2-phenylindole (DAPI) was applied to identify nuclei. Immunoreactivity was visualized using a fluorescent microscope (Olympus, IX71) with computer-assisted image analysis software (Empix Imaging). All comparative analyses were made using coded samples prepared with the same reagents and processed at the same time.
Co-immunoprecipitation
Recombinant murine TIMP-1 (R&D, Minneapolis, MN, 10 μg) was coupled to Affi-Gel 10 in a mini-column (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. Ethanolamine was added to the column to destroy any unfilled binding areas and then was washed with water. OPC lysates were collected in PBS and some incubated with function blocking anti-CD63 (5 μM; Santa Cruz) for 30 min before lysing. The cells were then lysed in PBS with 1% Tween-20, and a BCA was performed to determine protein content. Forty micrograms of OPC protein was incubated in the column with the coupled Affi-Gel overnight on a ferris wheel at 4 °C to facilitate binding. After overnight mixing, the column was washed with phosphate buffer in 1% Triton X-100, water, then the attached proteins were eluted using an acid buffer (2.5 pH HCl) and then neutralized using 3 M Tris HCl, 8 pH. Three separate subsequent elutions were performed for each OPC lysate sample. Each subsequent elution was kept separate and stored as E1, E2, etc. After elution, the column was washed with water and phosphate buffer in order to run through another OPC lysate sample. The protein content of the separate elutions was determined using a BCA, and 20 μg of eluted protein was separated by electrophoresis and transferred to nitrocellulose membranes. Membranes were blocked in Tris-buffered saline (1% Tween-20) (TBST) + 5% bovine serum albumin (BSA), incubated overnight with anti-rat CD63 primary antibody (Bio-Rad Antibodies, 1:200), washed in TBST, and incubated with the appropriate HRP-conjugated secondary antibody for an hour. Proteins were visualized by chemiluminescence.
Western Blot Analysis
Lysates from OPC cultures grown on poly-l-ornithine were prepared as previously described [15]. Cells were scraped into PBS after treatment, centrifuged (10,000×g), and the pellet resuspended in RIPA buffer (Triton X-100, NaCl, Tris-HCl base, deoxycholic acid, SDS, H2O) after which the pellets were mechanically dissociated using a motorized pellet pestle (Fisher Scientific). Each sample was prepared with 2× loading buffer (Tris-HCl, glycerol, SDS, H2O, BPB, DTT), and 100 μg of total protein was loaded into a graduated 5–15% acrylamide gel (Bio-Rad). After PAGE, protein was transferred onto PVDF membrane (Thermo Scientific). Blots were blocked and then incubated overnight in primary antisera. Primary antisera used were non-phosphorylated “active” β-catenin (Ser33/37/Thr41, Cell Signaling), GSK-3α/β (Cell Signaling), p-GSK-3α/β (Ser21/9, Cell Signaling), pAkt (Ser473, Cell Signaling), pan-Akt (Cell Signaling), and β-actin (Cell Signaling). Secondary antisera against the species of the primary antisera were HRP-conjugated (Vector Labs) for detection using ECL reagent (Bio-Rad). Exposed films were developed and quantified relative to β-actin (Sigma) or GAPDH (Cell Signaling), as indicated, loading for each sample using ImageJ (NIH), as described previously [15].
Statistics
Experiments were performed in triplicate technical replicates for each of three biological replicates per condition. Comparisons between treatments were made using the mean of each biological replicate. Student’s t test or one-way ANOVA, where appropriate, and as indicated. Data are presented as mean ± SEM. The null hypothesis for all experiments was P < 0.05.
Results
TIMP-1 Promotes OPC Differentiation Via Its Trophic Factor Domain
We had previously determined that TIMP-1 is a potent trophic factor for OPC differentiation [15]. In that study, we had not addressed the mode by which TIMP-1 stimulated OPC maturation. Since TIMP-1 is a dual function protein we first determined whether the ability of TIMP-1 to promote OPC maturation was related to either its MMP inhibitory function or mediated by a novel MMP-independent mechanism. To test this, we treated OPCs daily for 4 days and then quantified the number of mature OLs in each experimental condition. OPCs were treated with either full-length recombinant murine (rm)-TIMP-1 or a broad spectrum MMP inhibitor (GM6001). We also tested a recombinant peptide of TIMP-1 (amino acids 126–184), which has previously been defined as the CD63 interacting domain of TIMP-1 [TIMP-1(C)] [17]. When compared with PBS-treated control cultures, we found that GM6001 completely prevented any OPC differentiation (Fig. 1). In contrast, and consistent with our previous study [15], we found that administration of full-length rm-TIMP-1, or the trophic factor domain of TIMP-1 [TIMP-1(C)], significantly increased the number of MBP+/Olig2+ mature OLs in treated cultures (Fig. 1a). There were no significant differences between the proportion of mature OLs in OPC cultures treated with the same concentrations of either rm-TIMP-1 or its trophic domain only peptide, TIMP-1(C) (Fig. 1b). To determine if these treatments to the OPCs affected proliferation or cell death, we counted the number of Olig2+ cells in each field and determined that there were no significant changes in oligodendrocyte number between treatments (Fig. 1c). The differences in the results of these treatments indicated that the trophic function of TIMP-1, and not the MMP-dependent function of TIMP-1, was responsible for promoting OPC differentiation.
TIMP-1 induces OPC differentiation through an MMP-independent mechanism. Analysis of the trophic actions of recombinant TIMP-1 determined that the MMP inhibitory function of TIMP-1 is dispensable for its ability to promote OPC maturation to MBP+/Olig2+ mature OLs. a Representative images of immunofluorescent staining for Olig2 (left column), MBP (middle column), and merged image with DAPI nuclear counterstain (blue; right column) of oligodendrocytes in culture following 4 days of differentiation media and treatment with either vehicle (PBS), recombinant murine (rm)-TIMP-1 (10 ng/mL), a broad spectrum MMP inhibitor (GM6001; 12.5 μM), or a recombinant peptide of the trophic domain of TIMP-1, “TIMP-1(C)” (100 ng/mL). Scale bar = 100 μm. b Quantitative analysis of the percent of mature MBP+/Olig2+ OLs in each treatment group. Data are presented as the percent mean ± SEM compared to PBS vehicle control set to 100% differentiation (one-way ANOVA, Dunnett’s multiple comparisons test: PBS vs. rm-TIMP-1 [*P < 0.05], PBS vs. GM6001 [****P < 0.0001], PBS vs. TIMP-1(C) [**P < 0.005]). PBS used as vehicle control for compounds. c Analysis of the number of Olig2+ cells/field confirmed that the treatments did not increase proliferation or cell death as the number of OPC lineage cells/field was consistent across all treatment groups (one-way ANOVA, P = 0.04788, n.s. not significant)
The Trophic Function of TIMP-1 on OPCs Is Receptor-Mediated
The tetraspanin family protein, CD63, has been previously described as a cell surface receptor for TIMP-1 on breast cancer cells [17], although it has not been previously determined whether CD63 is also a receptor for TIMP-1 on any CNS cell type. To test this possibility, we first performed immunocytochemistry for CD63 on primary Olig2+ OPCs in culture and identified CD63 expression (Fig. 2a). We next collected mRNA from primary rat OPC cultures at each stage of maturation to assay for CD63 gene expression by PCR. We found that CD63 mRNA was expressed by OPCs and maintained at all stages of OL maturation (Fig. 2b). To validate the interaction between TIMP-1 and CD63 on OPCs, we performed co-immunoprecipitation using rm-TIMP-1 as the bait to screen OPC protein lysate. Using the full-length recombinant TIMP-1, we found that CD63, from OPC lysate, was attached to the recombinant TIMP-1 in the column (Fig. 2c). The same column, with rm-TIMP-1 attached, was subsequently eluted three times with an acid wash. The first elution (OPC E1) caused the detachment of all CD63 protein from the column, while the 2nd (OPC E2) and 3rd (OPC E3) elutions from the same column contained no CD63 protein. OPC lysates treated with the CD63 function blocking antibody (αCD63) and then incubated in the column did not elute CD63, showing that blocking the CD63 receptor inhibits binding with TIMP-1 (Fig. 2c). Running three separate elutions and probing for CD63 in the OPC lysates treated with αCD63 showed no CD63 protein in any of the elutions. These data substantiate a protein-protein interaction between TIMP-1 and CD63 on OPCs.
CD63 and β1 integrin mediate the trophic function of TIMP-1 on primary OPC cultures. a, b Expression of the tetraspanin CD63 in OL-lineage cells and its sustained expression throughout maturation. Analysis of CD63 mRNA expression in primary OPC cultures identified CD63 expression in early OPC, late OPC (lOPC), early OL (eOL), and mature OL cells (mOL). (RT indicates negative control of reverse transcriptase reaction without cDNA substrate). c Western blot probed for CD63 (50 kD) of OPC lysates, OPC elutions from the co-immunoprecipitation column, and OPCs treated with αCD63, lysed, and then eluted from the column (E = elution). The first elution from the rm-TIMP-1 column (OPC E1) detached all CD63 protein bound to the column as no CD63 was detected in the 2nd subsequent elution (OPC E2) or the 3rd subsequent elution (OPC E3) from the same column. d–e Representative images of Olig2+ (green) and MBP+ (red) OLs to determine the effect of blocking CD63 on rm-TIMP-1-mediated tropism on OPC maturation. Function blocking antisera against CD63 (αCD63, 5 μM), or isotype matched IgG control (5 μM), was applied with rm-TIMP-1 (10 ng/mL) to primary OPCs in culture. OPCs were treated for 4 consecutive days in the presence of differentiation media. Scale bar in “C” = 100 μm for d, e, g, and h. f Quantitative analysis of the percent of mature MBP+/Olig2+ OLs in each treatment group. Data are presented as the percent mean ± SEM compared to IgG control set to 100% differentiation (one-way ANOVA, Dunnett’s multiple comparisons test: IgG vs. αCD63 [**P < 0.01], IgG vs. rm-TIMP-1 [*P < 0.05], IgG + rm-TIMP-1 vs. αCD63 + rm-TIMP-1 [**P < 0.005]). g, h Representative images of differentiated OLs following treatment with rm-TIMP-1 (10 ng/mL) and either Ha2/5 (5 μM) the, β1-integrin function blocking antisera, or the hamster (h) IgG serotype-matched control antisera (5 μM). OPCs were treated for 4 consecutive days in the presence of differentiation media. i Quantitative analysis of the percent of mature MBP+/Olig2+ OLs in each treatment group. Data are presented as the percent mean ± SEM compared to hIgG control set to 100% differentiation (one-way ANOVA, Dunnett’s multiple comparisons test: hIgG vs. Ha2/5 [***P < 0.001], hIgG vs. rm-TIMP-1 [****P < 0.0001], hIgG + rm-TIMP-1 vs. Ha2/5 + rm-TIMP-1 [**P < 0.01]). j, k Quantitative analysis of number of Olig2+ cells counted per field from f and i presented as mean ± SEM (one-way ANOVA, P = 0.0.3737 for j and P = 0.0866 for k, n.s. not significant)
To address whether CD63 was responsible for mediating the trophic effects of TIMP-1 on OPCs, we tested several commercially available antibodies against CD63 and identified one that exerted a function-blocking effect (Fig. 2d–f). When administered to primary OPC cultures with rm-TIMP-1 this αCD63 antisera significantly diminished the number of MBP+/Olig2+ mature OLs, when compared with rm-TIMP-1 alone, or when applied with a serotype-matched IgG control antisera (Fig. 2f). Even without the addition of rm-TIMP-1, αCD63 alone was able to reduce the amount of mature OLs (Fig. 2f). These results indicated that rm-TIMP-1 may require the CD63 signaling complex to initiate its trophic actions on OPCs in cultures.
β1-Integrin, together with CD63, forms a TIMP-1 receptor complex [17]. To fully evaluate the role of this CD63 receptor complex for TIMP-1 trophic actions on OPCs, we next tested the requirement of β1-integrin, which has been previously shown to be expressed on OPCs [21]. To test whether rm-TIMP-1 also requires β1-integrin we administered a function blocking antibody against β1-integrin (Ha2/5) to OPC cultures treated with rm-TIMP-1. Blocking β1-integrin using Ha2/5 was found to significantly reduce the number of MBP+/Olig2+ mature OLs in response to adding rm-TIMP-1 to OPCs, when compared with a serotype-matched antibody control (Fig. 2g–i). Without rm-TIMP-1 added to the OPCs, the addition of Ha2/5 decreased the amount of OPC differentiation when compared to the hIgG control (Fig. 2i). To assay for proliferation or cell death with these varying treatments, we counted the number of Olig2+ cells and found no significant differences in either experiments (Fig. 2j, k). Together, these data suggest a CD63/β1-integrin receptor complex mediated the trophic signaling function of TIMP-1 on OPCs.
TIMP-1 Activates Akt to Promote OPC Differentiation
We next sought to determine the required signaling elements downstream of the CD63/β1-integrin receptor that were activated in OPCs by rm-TIMP-1. Protein kinase B (PKB), or Akt, is a kinase known to be downstream of the CD63/β1-integrin receptor complex in other cell types [22]. Akt has also been shown to have importance for promoting OPC differentiation and CNS myelination [23]. To determine whether rm-TIMP-1 activated Akt signaling, and to determine whether Akt was required for its trophic action, we applied to primary OPCs in culture the Akt-specific inhibitor, MK2206, coincidently with rm-TIMP-1. To test whether rm-TIMP-1 activated Akt in primary OPCs in culture, we used western blotting to analyze phosphorylated Akt (pAkt) as an indicator of its level of activation and compared it to overall levels of pan-Akt. In response to rm-TIMP-1, we found elevated pAkt levels after 2 h of treatment when compared with control (PBS-treated) levels (Fig. 3a). Importantly, this increase in pAkt in response to rm-TIMP-1 was completely blocked by co-administration of MK2206 (Fig. 3a, b). In addition, we probed for phosphorylated GSK-3α/β (Ser21/9), an Akt substrate, in the same treatment conditions and found a decrease in p-GSK-3α/β in MK2206 treated OPCs after 1 h (Fig. 3c, d).
Akt mediates TIMP-1 trophic function on OPCs in primary culture. a Western blot of phosphorylated (Ser473) Akt and total Akt expression in primary OPC cultures vehicle treated (PBS), treated with rm-TIMP-1 (10 ng/mL), Akt inhibitor: MK2206 alone (5 μΜ), or rm-TIMP-1 with MK2206 for 2 h in differentiation media. Relative changes in Akt activation (pAkt) in each treatment conditioned was quantified relative to Akt levels (lower panel) from the same blot. Each lane represents an independent measurement. b Quantification of Akt activation in OPCs in response to treatments (one-way ANOVA, P < 0.0001; *P < 0.05 PBS vs rm-TIMP-1; **P < 0.01, PBS vs MK2006 or MK2206 + rm-TIMP-1). c Western blot of total GSK-3α/β, phosphorylated GSK-3α/β (Ser21/9), and GAPDH in OPC cultures treated with PBS, rm-TIMP-1 (10 ng/mL), Akt inhibitor: MK2206 alone (5 μΜ), or rm-TIMP-1 with MK2206 for 1 h in differentiation media. α at 51 kDa and β at 47 kDa. Each lane represents an independent measurement. d Quantification of p-GSK activation in OPCs in response to treatments (one-way ANOVA, P < 0.005; *P < 0.05 PBS vs rm-TIMP-1). e–g Representative images of mature OLs in primary culture (MBP+/Olig2+) following treatment with either rm-TIMP-1, MK2206, or rm-TIMP-1 with MK2206. h Quantification of MK2206 effect on MBP+/Olig2+ mature OLs in response to rm-TIMP-1 and effect of blocking Akt using MK2206. Data are presented as the percent mean ± SEM compared to PBS vehicle control set to 100% differentiation (one-way ANOVA, Dunnett’s multiple comparisons test: PBS vs. MK2006 [****P < 0.0001], PBS vs. MK2206 + rm-TIMP-1 [****P < 0.0001]). i Quantitative analysis of number of Olig2+ cells counted per field presented as mean ± SEM (one-way ANOVA, P = 0.1425, n.s. not significant)
We next determined whether inhibition of Akt by MK2206 affected the re-TIMP-1 trophic action to promote OPC maturation. The number of mature oligodendrocytes in culture (MBP+/Olig2+), which was enhanced by rm-TIMP-1, was blocked by co-application of the Akt inhibitor MK2206 (Fig. 3e–h). Since blocking Akt signaling may also affect cell survival, we confirmed that the reduced OL maturation with MK2206 treatment was not a result of cell death by assessing total number of Olig2+ cells per field in each treatment condition. No differences in the numbers of Olig2+ cells were observed between any treatment group (Fig. 3i). Thus, inhibiting Akt by MK2206 did not reduce cell viability but did block the trophic action of re-TIMP-1. Moreover, these data indicate that Akt signaling, and not GSK, is required as a downstream effector, or at the very least, a regulator of the trophic action of TIMP-1 on OPCs.
TIMP-1 Activates β-Catenin in OPCs
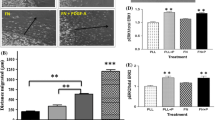
We considered the signaling downstream of Akt related to OPC maturation and identified β-catenin as a transcriptional regulator that is controlled by Akt activity [9, 24]. β-Catenin is constitutively held in the cytoplasm where it is targeted for degradation by the proteasome until activation of Akt releases β-catenin, which then translocates to the nucleus where it promotes the transcription of target genes. β-catenin signaling has been implicated as an important signal controlling OPC maturation and CNS myelination [25, 26]. Since we had determined that Akt was activated by rm-TIMP-1, we next sought to determine whether this activation had any impact on the regulation of β-catenin within OPCs. We first determined whether rm-TIMP-1 was sufficient to promote the activation of β-catenin in primary OPCs by examining the phosphorylation state of β-catenin and its sub-cellular localization following application of rm-TIMP-1 (Fig. 4a). Using western blotting, we also found that with rm-TIMP-1 treatment, active β-catenin was increased but then decreased with application of Wnt7a, a direct canonical activator of Akt (Fig. 4b, c). These data suggested that β-catenin in OPCs was activated by application of rm-TIMP-1.
Activation of β-catenin by TIMP-1 and Antagonism by Wnt7a controls OPC maturation. a Immunocytochemistry of β-catenin in primary OPCs showing basal pattern of cytoplasmic β-catenin staining and rapid nuclear translocation in response to application of rm-TIMP-1 (10 ng/mL) for 2 h. Graph: quantification of percent OPCs with nuclear β-catenin basally and following rmTIMP-1 treatment (t test, *P < 0.02). b Western blot of unphosphorylated (active) β-catenin and β-actin in primary OPC cultures following 2 h of treatment with rm-TIMP-1 (10 ng/mL), Wnt7a (50 ng/mL), and rm-TIMP-1 and Wnt7a. PBS control runs on one blot; rm-TIMP-1, Wnt7a, and rm-TIMP-1 + Wnt7a run on another blot. Blots were exposed to identical reagents and exposed on same film. Each lane represents an individual measurement. c Western blot analysis of β-catenin activation in OPCs in response to rmTIMP-1 (10 ng/mL), Wnt7a (50 ng/mL), or a combination of rmTIMP-1 with Wnt7a. Data represented as average fold change ± SEM. Equivalent protein loading was verified using β-actin, and quantification of β-catenin activation was performed (one-way ANOVA, Dunnett’s multiple comparisons test: PBS vs. rm-TIMP-1 [****P < 0.0001], PBS vs. Wnt7a [**P < 0.01], rm-TIMP-1 vs. rm-TIMP-1 + Wnt7a [**P < 0.01]). d–f Representative images of MBP+/Olig2+ mature OLs in response to either rm-TIMP-1 (10 ng/mL), Wnt7a (50 ng/mL), or rm-TIMP-1 with Wnt7a over four consecutive days in differentiation media. g Analysis of OL maturation in response to increasing concentrations of Wnt7a when co-administered in the presence of rm-TIMP-1 (10 ng/mL). Data are presented as the percent mean ± SEM compared to vehicle control (PBS) + rm-TIMP-1 set to 100% differentiation (one-way ANOVA, Dunnett’s multiple comparisons test: vehicle + rm-TIMP-1 vs. 50 ng/mL Wnt7a + 10 ng/mL rm-TIMP-1 [*P < 0.05]). h Quantitative analysis of number of Olig2+ cells counted per field in panel G presented as mean ± SEM (one-way ANOVA, P = 0.1937, n.s. not significant). i Analysis of OL maturation (MBP+/Olig2+) in response to increasing concentrations of rm-TIMP-1 when Wnt7a was co-administered at 50 ng/mL. Data are presented as the percent mean ± SEM compared to vehicle control (PBS) + 50 ng/mL Wnt7a set to 100% differentiation (one-way ANOVA, Dunnett’s multiple comparisons test). j Quantitative analysis of number of Olig2+ cells counted per field in i presented as mean ± SEM (one-way ANOVA, P = 0.0939, n.s. not significant)
TIMP-1 Trophic Actions to Promote OPC Differentiation Are Antagonized by Wnt7a
We next determined what role TIMP-1 regulation of β-catenin might play in regulating the differentiation of OPCs. Elevated expression of Wnt/β-catenin regulated genes has been reported in white matter (WM) lesions in MS, which has led to the suggestion that aberrant over-activation of β-catenin signaling by Wnt may be associated with remyelination failure in MS [27, 28]. Since rm-TIMP-1 activated β-catenin, we next considered whether TIMP-1 and canonical Wnt signaling, using Wnt7a, interacted to influence OPC maturation. Consistent with previous work [29], we found that increasing concentrations of Wnt7a (beginning at 0.5 ng/mL up to 50 ng/mL) resulted in significantly fewer mature (MBP+) OLs after 96 h of treatment, even in the presence of rm-TIMP-1 (Fig. 4d–g). We hypothesized that the activation of β-catenin by TIMP-1, which promotes OPC maturation, may therefore compete with Wnt7a-induced signaling, which prevents OPC maturation. We tested whether increasing concentrations of rm-TIMP-1 (ranging from 1 to 50 ng/mL) could induce OPC maturation in the presence of the highest concentration of Wnt7a (50 ng/mL) (Fig. 4i). Analysis of MBP+/Olig2+ OLs determined that the concentration range of rm-TIMP-1 tested did not increase OL maturation in the presence of this highest concentration of Wnt7a (50 ng/mL) (Fig. 4i). The combination of these treatments, both increasing concentrations of rm-TIMP-1 in the presence of Wnt7a did not alter cell death or proliferation of OPCs (Fig. 4j). Secondly, we tested whether increasing the concentrations of Wnt7a in the presence of rm-TIMP-1 (10 ng/mL) would affect OPC maturation. Even in the presence of low concentrations of Wnt7a, adding rm-TIMP-1 did not increase differentiation. Although, at the highest concentration of Wnt7a tested (50 ng/mL) OL maturation was significantly reduced (Fig. 4h; *P < 0.05 rm-TIMP-1 + vehicle vs rm-TIMP-1 + Wnt7a), we found that this amount of OL maturation was significantly higher in the presence of mTIMP-1 than with Wnt7a treatment alone (Fig. 4g; *P < 0.05, vehicle + Wnt7a vs 10 ng/mL rm-TIMP-1 + Wnt7a). Overall, there were no changes seen in proliferation or cell death with these treatments (Fig. 4h, j). These data indicated that over a range of concentrations there exist a physiological antagonism between TIMP-1 and Wnt signaling that can result in counteracting influences on β-catenin signaling affecting OPC maturation.
Discussion
Our data demonstrate a novel role for TIMP-1 signaling via its C-terminal domain through an interaction with the CD63 receptor, which we have found to be expressed OPCs, to promote differentiation. This study establishes TIMP-1 as an extracellular signaling protein that can activate β1-integrin in conjunction with CD63 which together acts as a receptor complex to phosphorylate Akt. Activation of Akt results in phosphorylation of GSK-3β, which promotes β-catenin, thereby inducing OPC differentiation. On the other hand, activation of β-catenin via canonical Wnt7a signaling inhibits OPC differentiation, demonstrating dual pathway regulation of β-catenin by Wnt and TIMP-1 (Fig. 5). Previously, the trophic action of TIMP-1 and the downstream effectors, which lead to OPC maturation, were unknown. Uncovering this mechanism by which TIMP-1 can directly promote OPC differentiation provides a new understanding of this complex interplay between processes regulating OPC fate.
a TIMP-1 regulation of intracellular signaling via Akt/GSK can stimulate beta-catenin translocation to the nucleus to promote OPC differentiation. b In the presence of Wnt7a, counter-regulation of beta-catenin signaling which, based on published studies, would promote OPC migration over differentiation. Physiologically, during development, the opposing influences of TIMP-1 and Wnt may together coordinate the timing and location of myelination by OPCs. In the context of CNS remyelination in diseases, such as MS, a deficiency of TIMP-1 would be hypothesized to result in impaired myelination by OPCs
Receptor-mediated signaling of TIMP proteins has garnered attention as the actions of TIMP family proteins are independent of their expected MMP inhibitor functions, and these receptor-mediated actions play important physiological roles [10, 11]. Our data indicate that a primary action of TIMP-1 on OPCs is MMP-independent. This finding is consistent with previous work by others that reported an important role for MMPs as mediators of OPC maturation [30]. These findings are also consistent with other reports which have demonstrated important signaling functions of TIMP-1 in non-neuronal systems. We found that the trophic action of TIMP-1 to promote OPC differentiation was mediated by a cell surface protein complex requiring CD63 and β1-integrin. Using function-blocking antisera against either CD63 or β1-integrin, we found that by blocking either of these factors the tropic action of TIMP-1 to promote OPC maturation was attenuated. Interestingly, even without the presence of TIMP-1 blocking, either CD63 or β1-integrin decreased the percent of OPC differentiation. The OPCs had been treated with differentiation media, containing thyroid hormone (T3), known to promote differentiation, along with the function-blocking antisera. β1-Integrin has been found to have an integral role in OPC differentiation [31]; therefore, blocking it, even without TIMP-1, inhibited differentiation. Blocking CD63 while OPCs were under differentiating conditions also inhibited OPC differentiation, indicating that CD63 is an important mediator of TIMP-1 signaling on OPCs. Further studies will be needed to determine the precise role of CD63 signaling on OPCs, exclusive of its function described herein as a receptor for TIMP-1. Indeed, other tetraspanin molecules, including CD82 and CD9, have been previously implicated in promoting OL maturation [32, 33]. To our knowledge, this is the first characterization of the expression and function of the tetraspanin CD63 on oligodendrocyte maturation. Interestingly, CD82, has also been identified as a putative TIMP-1 receptor, offering the possibility of additional TIMP-1 signaling pathways and cell-specific signaling by TIMP-1 [34]. Thus, while our data indicate an important functional contribution for CD63 as a mediator of the effect of TIMP-1 on OPC maturation, CD63 may not be the only TIMP-1 receptor to mediate signaling functions of TIMP-1 in the CNS.
Our data also provide a new connection between β1-integrin, in association with CD63, as a receptor complex for the trophic actions of TIMP-1. β1-Integrin has been previously reported to be important for OPC migration [21] and is involved as a co-factor linking extracellular signals to OPC differentiation [33, 35]. Developmental myelination of the CNS in β1-integrin KO mice has been reported to have deficient compact myelination resulting from a defect in oligodendrocyte process outgrowth [31]. This defect in the β1-integrin KOs was found to be related to a failure in the activation of Akt [31]. This data prompted us to examine whether TIMP-1 signals through a β1-integrin complex to also activate Akt. Indeed, we found that the trophic actions of TIMP-1 stimulated and required Akt. Activation of Akt is now a recognized activator of CNS myelination as overexpression of active Akt stimulates prolific CNS myelination [23]. The downstream signaling from Akt includes glycogen synthase kinase-3β (GSK-3β), a kinase substrate inhibited by Akt-mediated phosphorylation. GSK-3β controls β-catenin function as GSK-3β-mediated phosphorylation of β-catenin in the cytoplasm targets β-catenin for degradation by the proteasome [36, 37]. Activation of GSK-3β has been associated with impaired CNS myelination [38], while inhibition of GSK-3β has also been shown to promote CNS myelination [25]. Thus, our findings that TIMP-1 can activate Akt which influences GSK-3β phosphorylation support TIMP-1 as an activator of the Akt/GSK-3β pathway in OPCs to promote differentiation.
As mentioned above, downstream of Akt/GSK-3β our data also provide evidence that TIMP-1 can modulate β-catenin activity in oligodendrocytes. The impact of this finding was equivocal until we evaluated whether TIMP-1 could influence Wnt7a-mediated inhibition of OPC differentiation, since Wnt7a is an established regulator of the β-catenin pathway. Our data suggest that whether β-catenin promotes or blocks OPC maturation may depend upon which factor(s) and pathways are concurrently activated. The precise mechanism by which β-catenin controls OPC fate is complex and intricate. A possible mechanism by which divergent roles may emerge from the convergence of TIMP-1 and Wnt on β-catenin is that there are manifold intermediary factors that regulate β-catenin signaling. For instance, insulin-growth factor (IGF)-1 which also promotes β-catenin activity does so by stimulating cyclin D1, a co-factor involved in regulating cyclin-dependent kinase activities, to induce OPC proliferation and enhance OPC survival in vitro [24]. Similarly, Wnt signaling requires bone morphogenic proteins (BMPs) as intermediaries to inhibit OPC maturation [39], and this can be repressed by expression of the Wnt effector transcription factor 7-like 2 (TF7L2) to promote OPC differentiation [40]. Wnt signaling is a potent process involved in developmental patterning and CNS myelination. In this study, we selected Wnt7a for study as it is an astrocyte-produced gene that, through its cell surface receptor frizzled, is known to induce β-catenin signaling and impair OPC differentiation [27]. It is also important to note that in terms of development, where the influence of Wnt factors on β-catenin transcriptional activity are best characterized, are also time-sensitive. For example, the transcription factor c-myc, a β-catenin transcriptional target, has been shown to function as a “switch” for the transition from proliferating OPCs to mature OLs [41]. Future studies comparing TIMP-1 and Wnt transcriptional signatures may serve to refine our understanding on functional roles of specific transcriptional targets of β-catenin controlled by these opposing signals [42].
Wnt7a was of particular interest because it is expressed at high levels in white matter lesions in MS suggesting a potential involvement of developmental signaling with pathological processes associated with impaired remyelination in the adult CNS. The complexity of Wnt regulation of OPC fate cannot be understated; there are many Wnt proteins and while canonical Wnt activity and constitutive β-catenin activity have been reported to inhibit oligodendrocyte differentiation and delay myelination [43], other Wnt factors have been suggested to point to stage-specific effects on OPC maturation and CNS myelination [26]. Thus, while β-catenin signaling may represent an important pathway activated by TIMP-1 to promote OL maturation, our data indicate that this is counter-regulated by Wnt7a. Whether this counteracting effect of TIMP-1 on Wnts is also observed with other Wnt proteins remains to be tested. However, on the basis of the different effects that TIMP-1 and Wnt7a have on OPC differentiation, there may exist a role for TIMP-1 during CNS development also related to Wnt signaling. We have previously reported that in mice lacking TIMP-1, CNS myelination is delayed [15]. Recent work by Fancy and colleagues have demonstrated an important function for Wnt7a during an earlier critical period of prenatal development, wherein Wnt7a promotes OPC migration while preventing differentiation [44]. Future studies may consider whether, during CNS development, TIMP-1 and Wnt7a cooperate to coordinate the migration and timing of OPCs to establish CNS myelination. Given that these factors both seemingly converge on β-catenin, it would be similarly important to delve into how this targeted pathway is differentially regulated by these two factors to ultimately control myelination in vivo.
In the context of applying our findings to understand remyelination failure in diseases like multiple sclerosis, it is important to point out that TIMP-1 has very low constitutive expression in healthy adult CNS but is widely reported to be highly inducible in response to infection, inflammation, or injury where it is most often described to be expressed by astrocytes at or around the site of damage [10, 12, 16]. Our previous work using TIMP-1-deficient (KO) mice has shown that mice lacking TIMP-1 have delayed developmental myelination [15] and exhibit poor remyelination as adults following experimental autoimmune encephalomyelitis (EAE), a model of immune-mediated CNS demyelination [14]. In contrast, mice that overexpress TIMP-1 from astrocytes (GFAP-TIMP-1) are more resistant to EAE-induced myelin injury [45]. These animal studies data present a striking correlation with human studies which have shown a positive correlation between elevated CNS expression of TIMP-1 remyelination: in acute demyelinating encephalomyelitis (ADEM), a temporary condition with complete remyelination, TIMP-1 expression is dramatically elevated [46], whereas TIMP-1 expression is not increased in patients with MS, which is marked by minimal remyelination [47]. These data initially led us to consider how loss of TIMP-1 expression, and its role as a trophic factor, may contribute to chronic demyelination in diseases like MS [16] and now indicate that future studies exploring how TIMP-1 may interact with expression of Wnts could also provide insight into the etiology of myelin pathophysiology in disease (Fig. 5). Studies on the neuropathology of white matter lesions in MS have indicated that OPCs are recruited to demyelinated lesions yet fail to differentiate and provide remyelination [48, 49]. It has been suggested that this is evidence that OPCs fail to differentiate because of a deficit in the environment [50]. We suggest that absence of TIMP-1 from the lesion environment may be a contributing factor to myelin regeneration failure in MS lesions.
References
Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD (2000) NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci 20(17):6404–6412
Crawford AH, Tripathi RB, Foerster S, McKenzie I, Kougioumtzidou E, Grist M, Richardson WD, Franklin RJM (2016) Pre-existing mature oligodendrocytes do not contribute to remyelination following toxin-induced spinal cord demyelination. Am J Pathol 186:511–516
Sbardella E, Tona F, Petsas N, Upadhyay N, Piattella MC, Filippini N, Prosperini L, Pozzilli C et al (2015) Functional connectivity changes and their relationship with clinical disability and white matter integrity in patients with relapsing-remitting multiple sclerosis. Mult Scler 21(13):1681–1692
Franklin RJ (2002) Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci 3(9):705–714
Mei F, Lehmann-Horn K, Shen YAA, Rankin KA, Stebbins KJ, Lorrain DS, Pekarek K, A Sagan S et al (2016) Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. Elife 5
De Stefano N et al (1998) Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain 121(Pt 8):1469–1477
Mammi S, Filippi M, Martinelli V, Campi A, Colombo B, Scotti G, Canal N, Comi G (1996) Correlation between brain MRI lesion volume and disability in patients with multiple sclerosis. Acta Neurol Scand 94(2):93–96
Fancy SP et al (2011) Myelin regeneration: a recapitulation of development? Annu Rev Neurosci 34:21–43
Gaesser JM, Fyffe-Maricich SL (2016) Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp Neurol 283(Pt B):501–511
Crocker SJ, Pagenstecher A, Campbell IL (2004) The TIMPs tango with MMPs and more in the central nervous system. J Neurosci Res 75(1):1–11
Stetler-Stevenson WG (2008) Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal 1(27):re6
Pagenstecher A, Stalder AK, Kincaid CL, Volk B, Campbell IL (2000) Regulation of matrix metalloproteinases and their inhibitor genes in lipopolysaccharide-induced endotoxemia in mice. Am J Pathol 157(1):197–210
Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32(18):6391–6410
Crocker SJ, Whitmire JK, Frausto RF, Chertboonmuang P, Soloway PD, Whitton JL, Campbell IL (2006) Persistent macrophage/microglial activation and myelin disruption after experimental autoimmune encephalomyelitis in tissue inhibitor of metalloproteinase-1-deficient mice. Am J Pathol 169(6):2104–2116
Moore CS, Milner R, Nishiyama A, Frausto RF, Serwanski DR, Pagarigan RR, Whitton JL, Miller RH et al (2011) Astrocytic tissue inhibitor of metalloproteinase-1 (TIMP-1) promotes oligodendrocyte differentiation and enhances CNS myelination. J Neurosci 31(16):6247–6254
Moore CS, Crocker SJ (2012) An alternate perspective on the roles of TIMPs and MMPs in pathology. Am J Pathol 180(1):12–16
Jung KK, Liu XW, Chirco R, Fridman R, Kim HRC (2006) Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. EMBO J 25(17):3934–3942
Berditchevski F (2001) Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci 114(Pt 23):4143–4151
Egea V, Zahler S, Rieth N, Neth P, Popp T, Kehe K, Jochum M, Ries C (2012) Tissue inhibitor of metalloproteinase-1 (TIMP-1) regulates mesenchymal stem cells through let-7f microRNA and Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A 109(6):E309–E316
Schroder J, Lullmann-Rauch R, Himmerkus N, Pleines I, Nieswandt B, Orinska Z, Koch-Nolte F, Schroder B et al (2009) Deficiency of the tetraspanin CD63 associated with kidney pathology but normal lysosomal function. Mol Cell Biol 29(4):1083–1094
Milner R, Edwards G, Streuli C, ffrench-Constant C (1996) A role in migration for the alpha V beta 1 integrin expressed on oligodendrocyte precursors. J Neurosci 16(22):7240–7252
Chirco R, Liu XW, Jung KK, Kim HRC (2006) Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev 25(1):99–113
Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA et al (2008) Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci 28(28):7174–7183
Ye P, Hu Q, Liu H, Yan Y, D’ercole AJ (2010) Beta-catenin mediates insulin-like growth factor-I actions to promote cyclin D1 mRNA expression, cell proliferation and survival in oligodendroglial cultures. Glia 58(9):1031–1041
Azim K, Butt AM (2011) GSK3beta negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia 59(4):540–553
Dai ZM, Sun S, Wang C, Huang H, Hu X, Zhang Z, Lu QR, Qiu M (2014) Stage-specific regulation of oligodendrocyte development by Wnt/beta-catenin signaling. J Neurosci 34(25):8467–8473
Fancy SP et al (2009) Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev 23(13):1571–1585
Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S et al (2002) Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 8(5):500–508
Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SPJ, Zahed H, Maltepe E et al (2014) Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 158(2):383–396
Larsen PH, Yong VW (2004) The expression of matrix metalloproteinase-12 by oligodendrocytes regulates their maturation and morphological differentiation. J Neurosci 24(35):7597–7603
Barros CS, Nguyen T, Spencer KSR, Nishiyama A, Colognato H, Muller U (2009) Beta1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth. Development 136(16):2717–2724
Mela A, Goldman JE (2009) The tetraspanin KAI1/CD82 is expressed by late-lineage oligodendrocyte precursors and may function to restrict precursor migration and promote oligodendrocyte differentiation and myelination. J Neurosci 29(36):11172–11181
Terada N, Baracskay K, Kinter M, Melrose S, Brophy PJ, Boucheix C, Bjartmar C, Kidd G et al (2002) The tetraspanin protein, CD9, is expressed by progenitor cells committed to oligodendrogenesis and is linked to beta1 integrin, CD81, and Tspan-2. Glia 40(3):350–359
Zhang J, Wu T, Zhan S, Qiao N, Zhang X, Zhu Y, Yang N, Sun Y et al (2017) TIMP-1 and CD82, a promising combined evaluation marker for PDAC. Oncotarget 8(4):6496–6512
Siskova Z et al (2006) Fibronectin impedes “myelin” sheet-directed flow in oligodendrocytes: a role for a beta 1 integrin-mediated PKC signaling pathway in vesicular trafficking. Mol Cell Neurosci 33(2):150–159
Aberle H, Bauer A, Stappert J, Kispert A, Kemler R (1997) Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16(13):3797–3804
Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P (1996) Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science 272(5264):1023–1026
Luo F, Burke K, Kantor C, Miller RH, Yang Y (2014) Cyclin-dependent kinase 5 mediates adult OPC maturation and myelin repair through modulation of Akt and GsK-3beta signaling. J Neurosci 34(31):10415–10429
Feigenson K, Reid M, See J, Crenshaw IIIEB, Grinspan JB (2011) Canonical Wnt signalling requires the BMP pathway to inhibit oligodendrocyte maturation. ASN Neuro 3(3):e00061
Hammond E, Lang J, Maeda Y, Pleasure D, Angus-Hill M, Xu J, Horiuchi M, Deng W et al (2015) The Wnt effector transcription factor 7-like 2 positively regulates oligodendrocyte differentiation in a manner independent of Wnt/beta-catenin signaling. J Neurosci 35(12):5007–5022
Magri L, Gacias M, Wu M, Swiss VA, Janssen WG, Casaccia P (2014) c-Myc-dependent transcriptional regulation of cell cycle and nucleosomal histones during oligodendrocyte differentiation. Neuroscience 276:72–86
Guo F, Lang J, Sohn J, Hammond E, Chang M, Pleasure D (2015) Canonical Wnt signaling in the oligodendroglial lineage—puzzles remain. Glia 63(10):1671–1693
Fancy SP et al (2014) Parallel states of pathological Wnt signaling in neonatal brain injury and colon cancer. Nat Neurosci 17(4):506–512
Tsai HH, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien AC, Kuo CJ et al (2016) Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 351(6271):379–384
Althoff GE et al (2010) Long-term expression of tissue-inhibitor of matrix metalloproteinase-1 in the murine central nervous system does not alter the morphological and behavioral phenotype but alleviates the course of experimental allergic encephalomyelitis. Am J Pathol 177(2):840–853
Ichiyama T et al (2006) Serum levels of matrix metalloproteinase-9 and its tissue inhibitor (TIMP-1) in acute disseminated encephalomyelitis. J Neuroimmunol 172(1–2):182–186
Avolio C, Ruggieri M, Giuliani F, Liuzzi GM, Leante R, Riccio P, Livrea P, Trojano M (2003) Serum MMP-2 and MMP-9 are elevated in different multiple sclerosis subtypes. J Neuroimmunol 136(1–2):46–53
Chang A, Tourtellotte WW, Rudick R, Trapp BD (2002) Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med 346(3):165–173
Wolswijk G (1998) Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci 18(2):601–609
Franklin RJ, Kotter MR (2008) The biology of CNS remyelination: the key to therapeutic advances. J Neurol 255(Suppl 1):19–25
Acknowledgements
We thank Xiufang Liu, Sangita Karki, and Dr. Anthony W. Giampetruzzi for technical expertise and assistance, and we gratefully acknowledge Dr. Elisa Barbarese for helpful suggestions and Dr. David Martinelli for assistance with the Co-IP and feedback on this manuscript.
Funding
This work was supported, in part, by funding from the National Institutes of Health (5R21NS087578-02) and National Multiple Sclerosis Society (RG5001-A-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nicaise, A.M., Johnson, K.M., Willis, C.M. et al. TIMP-1 Promotes Oligodendrocyte Differentiation Through Receptor-Mediated Signaling. Mol Neurobiol 56, 3380–3392 (2019). https://doi.org/10.1007/s12035-018-1310-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1310-7