Abstract
Amyloid precursor protein (APP) is cleaved by a set of proteases including α-/β-/γ- and recently identified η-secretases, generating C-terminal fragments (CTFs) of varying lengths and amyloid β (Aβ) peptides, which are considered to play a pivotal role in Alzheimer’s disease (AD) pathogenesis. Cellular cholesterol content/distribution can regulate the production/clearance of APP metabolites and hence modify AD pathology. To determine the functional relation between endosomal-lysosomal (EL) cholesterol sequestration and APP metabolism, we used our recently developed mouse N2a-ANPC cells that overexpress Swedish mutant human APP in the absence of cholesterol-trafficking Niemann-Pick type C1 (Npc1) protein. Here, we report that neither increased levels nor EL cholesterol sequestration altered APP holoprotein levels but caused the intracellular accumulation of APP α-/β-/η-CTFs and Aβ1–40/42 peptides. The levels of APP-cleaved products increased as a function of extracellular serum concentration in N2a-ANPC cells, which are more vulnerable to death than the control cells. Additionally, we show that pH of the lysosomal vesicles in N2a-ANPC cells shifted to a less acidic range with increasing serum concentrations, thus making them less efficient functionally. Interestingly, the addition of cholesterol to the culture media not only increased the levels of cellular cholesterol and APP-cleaved products but also rendered the cells more vulnerable to toxicity. Collectively, our results suggest that extracellular cholesterol concentration in serum under conditions of Npc1 deficiency can influence intracellular cholesterol content/distribution and lysosomal efficacy, triggering the accumulation of toxic APP-cleaved products, eventually leading to cell death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The amyloid precursor protein (APP) plays a central role in the pathogenesis of Alzheimer’s disease (AD), the most common cause of dementia affecting the elderly population worldwide. The importance of APP in AD pathogenesis relates predominantly to its role as the precursor to the potentially cytotoxic amyloid β (Aβ) peptides, one of the molecular hallmarks of AD pathology. Mature APP is usually processed by either non-amyloidogenic α-secretase or amyloidogenic β-secretase pathway [1, 2]. The α-secretase cleavage of APP releases the soluble APPα ectodomain, and the remaining C-terminal fragment (α-CTF) is further processed by γ-secretase to generate Aβ17–40/Aβ17–42 fragments. Alternatively, β-secretase cleavage of APP generates soluble APPβ and an Aβ-containing C-terminal fragment (β-CTF), which is then processed via the same γ-secretase to yield full-length Aβ1–40/Aβ1–42. In addition to this canonical APP processing via α- and β-secretases, APP has recently been reported to be processed by membrane-type 5-matrix metalloproteinases (MT5-MMP), called η-secretase, to generate η-CTF. The η-CTF is subsequently cleaved by a disintegrin and metalloproteinase 10 (ADAM10) or β-site APP cleaving enzyme (BACE1) to generate Aη-α or Aη-β peptides, respectively [3, 4]. Although Aη-α can inhibit long-term potentiation and the η-CTF fragment is found to be enriched in dystrophic neurites in animal models as well as human AD brains [3,4,5], the potential role of CTFs generated by η-secretase in AD pathogenesis remains unclear.
Earlier studies have reported that cholesterol and other lipids can influence the production of APP-cleaved products by regulating α-/β-secretase processing pathways [6,7,8]. This, however, depends not exclusively on the total cellular levels of cholesterol/lipids but also on their subcellular distribution within the cell [9]. Recently, we reported that mouse neuroblastoma cells (N2a-ANPC) that overexpress mutant human APP in the absence of intracellular sterol trafficking protein Niemann-Pick type C1 (Npc1) can lead to cholesterol accumulation within the endosomal-lysosomal (EL) compartments, one of the major sites involved in APP metabolism [10]. These cells exhibit increased levels of APP-CTFs (i.e., α-CTF and β-CTF) as well as Aβ peptides without any alterations in the levels of APP holoprotein. At present, no information is available whether cholesterol sequestration within EL system can influence APP processing via the η-secretase pathway. Additionally, it remains unclear if culture media containing variable concentrations of fetal bovine serum (FBS) that regulates extracellular cholesterol and lipid levels can have an influence on APP processing. Thus, the present study, using cultured N2a-ANPC and the control N2a-APP cells, examined not only the effect of varying concentrations of FBS on APP processing but also the role of cholesterol in the generation of η-CTF to further understand the potential link between EL cholesterol sequestration and APP metabolism.
Method
Reagents
NuPAGE 4–12% Bis-Tris gels, Alexa Fluor 488-conjugated secondary antibody, LysoSensor Yellow/Blue DND-160, ProLong Gold anti-fade reagent, and ELISA kits for the detection of human Aβ1–40 and Aβ1–42, were purchased from Life Technologies (Burlington, ON, Canada). The bicinchoninic acid protein assay (BCA) kit, Amplex red cholesterol assay kit, and enhanced chemiluminescence kit were from ThermoFisher Scientific Inc. (Nepean, ON, Canada). Anti-APP C-terminal antibody (clone Y188) was purchased from Abcam while the anti-β-actin antibody, chloroquine, water soluble cholesterol, and filipin were obtained from Sigma-Aldrich, Co. (Oakville, ON, Canada). LAMP1 (clone ID4B) antibody was obtained from Developmental studies Hybridoma Bank, University of Iowa, USA, and the polyclonal NTG449 antibody was generated in our lab against a fusion protein corresponding to the ectodomain residues 306–600 of human APP 695 isoform. Horseradish peroxidase-conjugated secondary antibodies and Npc1/scramble shRNA lentiviral particles were from Santa Cruz Biotechnology (Paso Robles, CA, USA). All other chemicals were from Sigma-Aldrich, ThermoFisher Scientific, or Life Technologies, Corp. (Burlington, ON, Canada).
Cell Culture
Mouse N2a cells stably overexpressing the human Swedish mutant (K670N, M671L) APP (N2aAPPsw, clone Swe.10) were maintained in N2a growth medium [11]. The amount of Npc1 protein was reduced in N2aAPPsw cells (referred as N2a-ANPC) by mRNA silencing with lentiviruses expressing Npc1 shRNA and subjected to puromycin selection according to the manufacturer’s instructions (Santa Cruz Biotechnology) [10]. The controls, N2aAPPsw cells, were transduced with lentiviruses shRNA encoding a scrambled sequence (referred as N2a-APP). Clones stably expressing the shRNAs were maintained in N2a growth medium containing puromycin (5 μg/ml). The cells were grown to 70% confluency in complete medium, rinsed in phosphate-buffered saline (PBS), and then incubated for 24 h under the following three different medium conditions prior to harvesting: (i) Opti-MEM® I without any serum supplementation, (ii) Opti-MEM® I containing 5% FBS, and (iii) Opti-MEM® I with 10% FBS. In a separate series of experiments, cultured cells after maintenance in complete medium were exposed to 0% FBS in the presence or absence of different concentrations (25 and 50 μM) of cholesterol as described recently [12]. After 36 h incubation, cultured N2a-APP and N2a-ANPC cells were harvested for further processing.
Filipin Staining and Fluorescence Microscopy
N2a-APP and N2a-ANPC cells grown on coverslips were fixed with 4% paraformaldehyde and processed for immunocytochemistry as described earlier [10]. Briefly, fixed cells on coverslips were incubated overnight at 4 °C with anti-LAMP1 (1:500) antibody and subsequently labeled with Alexa Fluor 488-conjugated secondary antibody for 2 h at room temperature. For visualization of unesterified cholesterol, immunolabeled cells were incubated with 25 μg/ml of filipin in PBS for 30 min in the dark under agitation. Coverslips were finally washed with PBS and mounted in ProLong Gold anti-fade reagent. Filipin-stained cells were examined at UV excitation around 360 nm and emission around 480 nm and imaged using a Zeiss multiphoton confocal laser scanning microscope (LSM510, Carl Zeiss, Inc.) equipped with a × 63 Plan-apochromatic oil-immersion lens.
Cholesterol Assay
N2a-APP and N2a-ANPC cells cultured under different FBS conditions, as mentioned above, were homogenized in PBS and centrifuged at 10,000×g for 10 min. Supernatants were collected and protein concentrations were estimated using BCA method. Total cholesterol levels were determined using an Amplex red cholesterol assay kit according to the manufacturer’s instructions. Fluorescence was measured using a SpectraMax M5 plate reader (Sunnyvale, CA, USA) at excitation/emission wavelengths of 560/590 nm. In parallel, N2a-APP and N2a-ANPC cells cultured with 0 and 10% FBS or following supplementation with extracellular cholesterol for 36 h were homogenized and mass of cholesterol was determined using gas-liquid chromatography as described earlier [13, 14]. All samples were assayed in duplicate and each experiment was repeated three times.
Western Blotting
Cells cultured under various FBS concentrations as well as in the presence or absence of extracellular cholesterol were homogenized in ice-cold radioimmunoprecipitation (RIPA) lysis buffer. Proteins (15 μg) were separated on NuPAGE 4–12% Bis-Tris gels, transferred to polyvinylidene difluoride membranes, then incubated overnight at 4 °C with anti-APP C-terminal antibody (clone Y188, dilution 1:5000) or anti-NTG449 antibody (1:1000). The membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies (dilution 1:5000) and immunoreactive proteins were visualized using enhanced chemiluminescence. All blots were re-probed with anti-β-actin antibody (1:5000) to monitor equal protein loading and quantified using a MCID image analyzer (Imaging Research, Inc., St Catherines, ON, Canada) as described earlier [14].
ELISA for Aβ1–40 and Aβ1–42
Aβ from the cultured N2a-APP and N2a-ANPC cells from various experimental conditions were extracted by solubilization in 5 M guanidine-HCl and 50 mM Tris-HCl (pH 8.0) for 4 h followed by centrifugation at 16,000×g for 20 min [15]. The levels of extracted human Aβ1–40 or Aβ1–42 were measured using commercial ELISA kits following the manufacturer’s instructions. The OD value was converted to picograms per milliliter according to a standard curve. All samples were assayed in duplicate and each experiment was repeated three times.
Flow Cytometry
N2a-APP and N2a-ANPC cells following different experimental paradigms were incubated with LysoSensor Yellow/Blue DND-160 (1 μM) for 30 min, washed in PBS, and analyzed using LSR-Fortessa Flow Cytometer (BD Biosciences). The two detectors had 450/50 nm and 530/30 nm filters with excitation of 355 nm (20-mW UV laser). Data were analyzed using FCS Express 4 Flow Cytometry software (De Novo Software, Los Angeles, CA, USA).
Cell Viability
Viability of N2a-APP and N2a-ANPC cells cultured under various FBS concentrations as well as in the presence or absence of extracellular cholesterol supplement was determined using the colorimetric MTT [3-(4,5-dimethylthiozolyl)-2,5-diphenyl-tetrazolium bromide] assay as described earlier [10]. Briefly, cells grown in different FBS conditions/cholesterol supplement were treated with or without 100 μM of chloroquine for 5 h. Subsequently, they were incubated with 0.25% MTT in fresh media for 3 h at 37 °C. The reaction product was solubilized in dimethylsulfoxide and measured spectrophotometrically at 570 nm. Each experiment was performed in quadruplet and repeated three times.
Statistical Analysis
Data are expressed as means ± S.E.M. Statistical significance was determined by one-way ANOVA followed by Newman-Keuls post hoc analysis for multiple comparisons or unpaired two-tailed Student’s t test for single comparison with a significance threshold of p < 0.05. All analyses were performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA).
Results
The Influence of FBS Concentrations on APP Processing in N2a-ANPC Cells
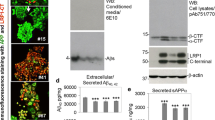
As reported earlier [10], reducing Npc1 protein expression sequestered filipin-labeled unesterified cholesterol in LAMP1-positive lysosomes in N2a-ANPC cells compared to the control N2a-APP cells (Fig. 1a). To determine the influence of extracellular serum concentration on APP metabolism in Npc1-deficient cells, N2a-APP and N2a-ANPC cells were cultured in N2a growth medium containing 5% FBS and then treated with three different FBS concentrations (0, 5, and 10%) in Opti-MEM for 24 h. Our results clearly showed that cellular cholesterol levels, as detected by Amplex Red cholesterol assay kit, were not altered in N2a-ANPC cells compared to N2a-APP cells at 0% FBS but were significantly increased at 5 and 10% FBS conditions (Fig. 1b). APP holoprotein levels were also not affected either in N2a-APP or N2a-ANPC cells following incubation with variable concentrations of FBS (Fig. 2a, b). The levels of α-CTF and β-CTF were, however, significantly higher in N2a-ANPC cells than N2a-APP cells at 5 and 10% FBS conditions. Interestingly, cells maintained in Opti-MEM without any FBS supplementation (0% FBS) for 24 h showed no significant difference in the levels of α-CTF or β-CTF in N2a-ANPC cells compared to N2a-APP cells (Fig. 2a, c, d). Similar to α-CTF and β-CTF, the steady state levels of η-CTF, detected by Y188 antibody, markedly increased following intracellular cholesterol sequestration in N2a-ANPC cells compared to N2a-APP cells cultured in 5 and 10% FBS conditions but not in 0% FBS (Fig. 2a, e). The identity of η-CTF is substantiated with our NTG-449 antibody that is raised against a fusion protein corresponding to the ectodomain residues 306–600 of human APP695. Additionally, the intracellular levels of human Aβ1–40 and Aβ1–42 were also significantly enhanced in N2a-ANPC as compared with N2a-APP cells when the cells were incubated with 5 and 10% FBS, but not in serum-free Opti-MEM. Moreover, intracellular Aβ1–40 and Aβ1–42 levels were found to increase as a function of increasing serum concentration between 0 and 10% FBS in N2a-ANPC cells (Fig. 2f, g). Taken together, these results suggest that intracellular accumulation of APP-cleaved products in Npc1-deficient cells could possibly be a function of extracellular serum supplementation.
FBS and cholesterol: a Fluorescence photomicrographs showing the sequestration of filipin-labeled unesterified cholesterol in LAMP1-positive lysosomes in Npc1-deficient N2a-ANPC cells (lower panel) cultured in 5% FBS. N2a-APP cells (upper panel) expressing normal levels of Npc1 protein show only a diffuse labeling with filipin. b Histograms depicting cellular levels of cholesterol, as detected by Amplex red cholesterol assay kit, in N2a-APP (gray) and N2a-ANPC (black) cells in the presence of increasing FBS concentrations. Values represent means ± SEM from three independent experiments, each performed in duplicate. *p < 0.05
FBS and APP processing. a Representative immunoblots depicting amounts of APP holoprotein and C-terminal fragments (α-CTF, β-CTF, and η-CTF) in N2a-APP and N2a-ANPC cells cultured in N2a growth medium for 48 h and then treated with three different concentrations of FBS (0, 5, and 10%) in Opti-MEM medium for 24 h. b–e Quantitation of the amounts of APP holoprotein (b) and APP-CTFs (c–e) in N2a-APP (gray) and N2a-ANPC (black) cells cultured in 0, 5, and 10% FBS conditions for 24 h. All blots were re-probed with a β-actin antibody to monitor equal protein loading. Values represent means ± SEM of three independent experiments, *p < 0.05. f and g Intracellular levels of human Aβ1–40 (f) and Aβ1–42 (g) as detected by ELISA in N2a-APP (gray) and N2a-ANPC (black) cells cultured with increasing FBS concentrations. Values represent means ± SEM of three independent experiments, each performed in duplicate. *p < 0.05, ***p < 0.001
Influence of FBS Supplementation on Cell Metabolic Functions
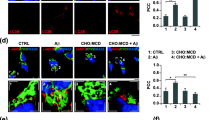
Multiple lines of experimental evidence suggest that EL system plays a critical role in APP metabolism [1, 2]. The acidic environment of the EL system is suitable for various enzymes regulating production/clearance of Aβ-related peptides [1, 16, 17]. Any alteration in its pH may influence the activity of the enzymes regulating APP metabolism. To determine if variable FBS concentrations in the media can have a role in regulating lysosomal pH in Npc1-deficient cells, we stained N2a-APP and N2a-ANPC cells with the ratiometric probe LysoSensor Yellow/Blue DND-160, which produces yellow fluorescence in acidic organelles and blue fluorescence in less acidic organelles. Since LysoSensor Yellow/Blue DND-160 exhibit fluorescence in both yellow and blue range, we measured the pH of the vesicles of N2a-APP and N2a-ANPC cells by dividing the intensity of yellow by that of the blue channel obtained using a LSR-Fortessa Flow Cytometer. Our ratiometric analyses of the two cell lines cultured in different FBS concentrations (Fig. 3a–g) clearly showed that the intracellular acidic milieu is less evident in N2a-ANPC cells than in N2a-APP cells at both 5 and 10% FBS concentrations despite an overall increase in both yellow and blue fluorescence intensities (Fig. 3b–g). However, serum starvation for 24 h did not show any significant difference in yellow and blue fluorescence intensities between the cell lines (Fig. 3a, b, g). Additionally, to establish if the cellular cholesterol level/sequestration following extracellular serum supplementation can affect cell viability, we treated N2a-APP and N2a-ANPC cells with chloroquine, a lysosomotropic agent that induces cell death by neutralizing lysosomal pH. Interestingly, consistent with the accumulation of CTFs and Aβ peptides, N2a-ANPC cells were found to be significantly more vulnerable to chloroquine-induced toxicity at 5 and 10% FBS, but not at 0% FBS, as compared with N2a-APP cells (Fig. 3h).
FBS and cell metabolic activity. a–d Flow cytometric analysis of N2a-APP and N2a-ANPC cells stained with the ratiometric probe LysoSensor Yellow/Blue DND-160. Note the overall increase in acidic yellow (b, d) and less acidic blue (a, c) organelles in N2a-ANPC compared to N2a-APP cells treated with 10% FBS (c, d) but not with 0% FBS (a, b) for 24 h. e, f Representative photomicrographs of N2a-APP and N2a-ANPC cells stained with the ratiometric probe LysoSensor Yellow/Blue DND-160. g A ratio of yellow-to-blue intensities in cells labeled with LysoSensor Yellow/Blue DND160 indicating a reduction in the intracellular acidic milieu following EL cholesterol accumulation in N2a-ANPC compared to N2a-APP cells cultured in 5 and 10% FBS, but not in 0% FBS, for 24 h. h Histogram depicting cell metabolic activity as detected by MTT assay in N2a-APP and N2a-ANPC cells maintained in 0, 5, and 10% of FBS for 24 h and subsequently treated with 100 μM chloroquine for 5 h. Data are expressed as a percentage of no chloroquine treatment for the respective FBS concentration and cell line. Values represent means ± SEM from three independent experiments, each assayed in quadruplet. *p < 0.05, **p < 0.01, and ***p < 0.001
Effects of Cholesterol Supplementation on APP Metabolism and Cell Viability
FBS contains numerous lipids including cholesterol, which has previously been linked to APP processing [7, 18, 19]. A recent study reported that increasing concentrations of cholesterol in the culture media can enhance the levels of APP and its cleaved products in astrocytes [12]. In order to evaluate whether the cholesterol component of the serum is partly responsible for the effect on APP metabolism, we treated N2a-APP and N2a-ANPC cells cultured with 0% FBS with either 25 or 50 μM cholesterol for 36 h. Our results clearly showed that levels of cellular cholesterol, APP, APP-CTFs (i.e., α-/β-/η-CTFs), and Aβ1–40 peptides (Fig. 4a–h) in cultured N2a-APP and N2a-ANPC cells were enhanced following cholesterol supplement compared to those observed in 0% FBS. Additionally, ratiometric analysis of two cell lines revealed that intracellular acidic milieu is somewhat less evident in N2a-ANPC cells than in N2a-APP cells especially at 25 μM cholesterol paradigm and their vulnerability is reduced following exposure to chloroquine (Fig. 5a, b). These results indicate that extracellular cholesterol present in the FBS may have a role not only in enhancing the levels of APP and its cleaved products but also in altering the intracellular acidic milieu as well as the vulnerability of cells to toxicity.
Cholesterol and APP processing. a Histograms depicting cellular levels of cholesterol, as determined by gas-liquid chromatography, in N2a-APP and N2a-ANPC cells cultured with 0 and 10% FBS or following exposure to 25 and 50 μM cholesterol in the medium. Values represent percentage of respective control (i.e., 0% FBS) from three independent experiments. b Representative immunoblots depicting amounts of APP holoprotein and C-terminal fragments (α-CTF, β-CTF, and η-CTF), as detected by Y188 antibody, in N2a-APP and N2a-ANPC cells cultured with 0 and 10% FBS or following exposure to 25 and 50 μM cholesterol in the media. c–f Histograms representing quantitation of the amounts of APP holoprotein (c) and APP-CTFs (d–f) in N2a-APP and N2a-ANPC cells. All blots were re-probed with a β-actin antibody to monitor equal protein loading. Values represent means ± SEM of three independent experiments. g Representative immunoblot and quantification depicting amounts of η-CTF, as detected by NTG449 antibody, in N2a-APP and N2a-ANPC cells cultured with 0 and 10% FBS or following exposure to 25 and 50 μM cholesterol in the media. The η-CTF blot was re-probed with a β-actin antibody to monitor equal protein loading. Values represent means ± SEM of three independent experiments. h Intracellular levels of human Aβ1–40 as detected by ELISA in N2a-APP and N2a-ANPC cells cultured with 0 and 10% FBS or following exposure to 25 and 50 μM cholesterol in the conditioned media. Values represent means ± SEM of three independent experiments, each performed in duplicate. *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001
Cholesterol and cell metabolic activity. a A ratio of yellow-to-blue intensities in N2a-ANPC (black) compared to N2a-APP (gray) cells labeled with LysoSensor Yellow/Blue DND-160 indicating a reduction in the intracellular acidic milieu following exposure to cholesterol in the conditioned medium. b Histogram depicting cell metabolic activity as detected by MTT assay in N2a-APP (gray) and N2a-ANPC (black) cells with 0 and 10% FBS or following exposure to 25 and 50 μM cholesterol in the medium and treated with 100 μM chloroquine for 5 h. Data are expressed as a percentage of no chloroquine treatment for the respective condition and cell line. Values represent means ± SEM from three independent experiments, each assayed in quadruplet. *p ≤ 0.05 and **p ≤ 0.01
Discussion
Using mouse neuroblastoma cells stably overexpressing mutant human APP in the absence of Npc1 protein, we demonstrate that increased cellular cholesterol level/sequestration within EL system can enhance the intracellular levels of η-CTF along with those of α-/β-CTFs and Aβ1–40/42 peptides. Interestingly, the increased accumulation of APP-cleaved products in Npc1-deficient cells occurs with increased concentrations of extracellular FBS. Additionally, varying the extracellular serum concentrations in Npc1-deficient cells can affect the pH of endosomal/lysosomal vesicles, which serve as a major site of APP metabolism and are known to be markedly altered in “at-risk” neurons in the brains of patients with AD [6, 20, 21]. We further showed that EL cholesterol sequestration with extracellular serum supplementation can render the cells vulnerable to cell death. Interestingly, direct treatment of the Npc1-deficient cells with cholesterol in the culture media not only enhance APP metabolism and alter pH of the intracellular vesicles but also render cells less viable to toxicity—highlighting the role of cholesterol in AD-associated neurodegenerative diseases.
Increasing evidence suggests that altered lipid metabolism, modulation of cellular phospholipid content, or lipid asymmetry in the plasma membrane plays a pivotal role in AD pathogenesis [7, 22, 23]. The critical factor by which cholesterol influences AD might not be limited to total cellular cholesterol content but also to its distribution among different cellular compartments [9]. Under normal conditions, cholesterol derived from astrocytes is taken up by neurons via receptor-mediated endocytosis and is delivered first to the EL system, from where it is distributed to the other cellular compartments involving Npc1/Npc2-dependent trafficking mechanisms [24]. Deficiency of Npc1 protein in neurons does not affect the total cellular content of cholesterol but inhibits its exit from the late-endosomes/lysosomes and hence reduces its availability to the other cellular compartments such as the plasma membrane and ER [25, 26]. In this study, we show that cholesterol sequestration within the EL system subsequent to Npc1 deficiency can increase the intracellular levels of APP-CTFs and Aβ peptides in the presence of serum in the media and can be depleted by 24 h serum starvation or increased following 36 h cholesterol supplementation of the culture media. Interestingly, our results also show that accumulation of Aβ peptides in Npc1-deficient cells increases with enhancing serum levels in the extracellular milieu and following cholesterol supplementation. This is consistent with a recent study which showed that increasing concentrations of cholesterol in the culture media can not only trigger cholesterol accumulation but also elevate the levels of APP and its metabolites in astrocytes [12].
Recently, it has been reported that a subset of APP undergoes alternative processing by a novel η-secretase pathway generating 25-kD CTF fragments called the η-CTFs [3, 4]. These η-CTFs, under normal conditions, are rapidly degraded via the lysosomal machinery, specifically by cathepsin L, but may be altered during AD pathogenesis or other neurodegenerative conditions characterized by lysosomal malfunction. This is consistent with our observation that η-CTF levels were increased in our N2a-ANPC cells compared to the control N2a-APP cells as a function of serum concentrations. Additionally, increasing concentrations of cholesterol were found to increase the steady-state levels of η-CTF as revealed by two distinct antibodies (Y1-88 and NTG449), thus indicating a critical role for cellular cholesterol in modulating η-CTF metabolism. It is of interest to note that increased levels of η-CTFs were also observed in our recently developed ANPC mouse model [10, 14], specifically in brain regions severely affected by cholesterol accumulation (unpublished data).
Several lines of evidence have suggested a role of lysosomal dysfunction in AD and NPC pathogenesis [21, 27]. Autophagic vacuoles, an early component of the autophagic-lysosome degradation pathway, are known to accumulate extensively in AD brains [20, 28]. These vesicles accrue Aβ peptides along with β-CTF and γ-secretase components, suggesting a role in AD pathogenesis. Moreover, there is evidence that fusion of late endosomes with lysosomes is impaired in cholesterol-accumulating Npc1-deficient cells [29]. Collectively, these observations suggest that lysosomal dysfunction plays a major role in both AD and NPC pathogenesis. In line with this, the present study reveals that EL cholesterol sequestration markedly alters the pH of the lysosomal vesicles from an acidic to a less acidic range which might interfere with the activity of the lysosomal enzymes, thereby regulating the clearance of cellular wastes. Our results further show that the shift towards basic pH upon intracellular cholesterol sequestration in Npc1-deficient cells can be modulated by extracellular serum levels. These findings make it rather intriguing to speculate that EL cholesterol sequestration can directly interfere with the lysosomal degradative efficiency leading to intracellular accumulation of α-, β-, and η-CTFs and Aβ peptides, which can subsequently influence neurodegeneration in AD-related pathology. Consistent with this notion, our results show that N2a-ANPC cells accumulating APP-CTFs and Aβ peptides are more vulnerable to cell death upon exposure to chloroquine. This is further reinforced by the evidence that N2a-ANPC cells cultured with increasing concentrations of cholesterol in the culture media not only enhance APP metabolism but also render the cells less acidic and vulnerable to chloroquine-induced toxicity.
In summary, using N2a-ANPC and N2a-APP cells as model systems, we show that increased level/sequestration of cholesterol within the EL vesicles does not affect APP holoprotein levels but triggers an accumulation of APP α-, β-, and η-CTFs as well as Aβ1–40/42 peptides. These results also suggest a role for EL cholesterol sequestration on η-CTF accumulation. Additionally, we show that extracellular serum concentration, possibly by altering the functioning of the lysosomal clearance machinery, can influence the accumulation of APP metabolites in the cells and render them more vulnerable to cell death.
References
Haass C, Kaether C, Thinakaran G, Sisodia S (2012) Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med 2(5):a006270. https://doi.org/10.1101/cshperspect.a006270
Thinakaran G, Koo EH (2008) Amyloid precursor protein trafficking, processing, and function. J Biol Chem 283(44):29615–29619. https://doi.org/10.1074/jbc.R800019200
Wang H, Sang N, Zhang C, Raghupathi R, Tanzi RE, Saunders A (2015) Cathepsin L mediates the degradation of novel APP C-terminal fragments. Biochemistry 54(18):2806–2816. https://doi.org/10.1021/acs.biochem.5b00329
Willem M, Tahirovic S, Busche MA, Ovsepian SV, Chafai M, Kootar S, Hornburg D, Evans LD et al (2015) η-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature 526(7573):443–447. https://doi.org/10.1038/nature14864
Baranger K, Khrestchatisky M, Rivera S (2016) MT5-MMP, just a new APP processing proteinase in Alzheimer’s disease? J Neuroinflammation 13(1):167. https://doi.org/10.1186/s12974-016-0633-4
Maulik M, Westaway D, Jhamandas JH, Kar S (2013) Role of cholesterol in APP metabolism and its significance in Alzheimer’s disease pathogenesis. Mol Neurobiol 47(1):37–63. https://doi.org/10.1007/s12035-012-8337-y
Di Paolo G, Kim TW (2011) Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci 12(5):284–296. https://doi.org/10.1038/nrn3012
Morgado I, Garvey M (2015) Lipids in amyloid-β processing, aggregation, and toxicity. Adv Exp Med Biol 855:67–94. https://doi.org/10.1007/978-3-319-17344-3_3
Puglielli L, Konopka G, Pack-Chung E, Ingano LA, Berezovska O, Hyman BT, Chang TY, Tanzi RE et al (2001) Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nat Cell Biol 3(10):905–912. https://doi.org/10.1038/ncb1001-905
Maulik M, Peake K, Chung J, Wang Y, Vance JE, Kar S (2015) APP overexpression in the absence of NPC1 exacerbates metabolism of amyloidogenic proteins of Alzheimer’s disease. Hum Mol Genet 24(24):7132–7150. https://doi.org/10.1093/hmg/ddv413
Thinakaran G, Teplow DB, Siman R, Greenberg B, Sisodia SS (1996) Metabolism of the “Swedish” amyloid precursor protein variant in neuro2a (N2a) cells. Evidence that cleavage at the “beta-secretase” site occurs in the golgi apparatus. J Biol Chem 271(16):9390–9397
Avila-Munoz E, Arias C (2015) Cholesterol-induced astrocyte activation is associated with increased amyloid precursor protein expression and processing. Glia. https://doi.org/10.1002/glia.22874
Amritraj A, Peake K, Kodam A, Salio C, Merighi A, Vance JE, Kar S (2009) Increased activity and altered subcellular distribution of lysosomal enzymes determine neuronal vulnerability in Niemann-Pick type C1-deficient mice. Am J Pathol 175(6):2540–2556. https://doi.org/10.2353/ajpath.2009.081096
Maulik M, Ghoshal B, Kim J, Wang Y, Yang J, Westaway D, Kar S (2012) Mutant human APP exacerbates pathology in a mouse model of NPC and its reversal by a β-cyclodextrin. Hum Mol Genet 21(22):4857–4875. https://doi.org/10.1093/hmg/dds322
Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N et al (2001) Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem 276(24):21562–21570. https://doi.org/10.1074/jbc.M100710200
Haque A, Banik NL, Ray SK (2008) New insights into the roles of endolysosomal cathepsins in the pathogenesis of Alzheimer’s disease: cathepsin inhibitors as potential therapeutics. CNS Neurol Disord Drug Targets 7(3):270–277
Torres M, Jimenez S, Sanchez-Varo R, Navarro V, Trujillo-Estrada L, Sanchez-Mejias E, Carmona I, Davila JC et al (2012) Defective lysosomal proteolysis and axonal transport are early pathogenic events that worsen with age leading to increased APP metabolism and synaptic Abeta in transgenic APP/PS1 hippocampus. Mol Neurodegener 7:59. https://doi.org/10.1186/1750-1326-7-59
Ariga T, McDonald MP, RK Y (2008) Role of ganglioside metabolism in the pathogenesis of Alzheimer’s disease—a review. J Lipid Res 49(6):1157–1175. https://doi.org/10.1194/jlr.R800007-JLR200
Haughey NJ, Bandaru VV, Bae M, Mattson MP (2010) Roles for dysfunctional sphingolipid metabolism in Alzheimer’s disease neuropathogenesis. Biochim Biophys Acta 1801(8):878–886. https://doi.org/10.1016/j.bbalip.2010.05.003
Nixon RA, Yang DS (2011) Autophagy failure in Alzheimer’s disease—locating the primary defect. Neurobiol Dis 43(1):38–45. https://doi.org/10.1016/j.nbd.2011.01.021
Orr ME, Oddo S (2013) Autophagic/lysosomal dysfunction in Alzheimer’s disease. Alzheimers Res Ther 5(5):53. https://doi.org/10.1186/alzrt217
Kosicek M, Hecimovic S (2013) Phospholipids and Alzheimer’s disease: alterations, mechanisms and potential biomarkers. Int J Mol Sci 14(1):1310–1322. https://doi.org/10.3390/ijms14011310
Lemkul JA, Bevan DR (2011) Lipid composition influences the release of Alzheimer’s amyloid beta-peptide from membranes. Protein Sci Publ Protein Soc 20(9):1530–1545. https://doi.org/10.1002/pro.678
Vanier MT (2015) Complex lipid trafficking in Niemann-Pick disease type C. J Inherit Metab Dis 38(1):187–199. https://doi.org/10.1007/s10545-014-9794-4
Karten B, Vance DE, Campenot RB, Vance JE (2002) Cholesterol accumulates in cell bodies, but is decreased in distal axons, of Niemann-Pick C1-deficient neurons. J Neurochem 83(5):1154–1163
Peake KB, Vance JE (2010) Defective cholesterol trafficking in Niemann-Pick C-deficient cells. FEBS Lett 584(13):2731–2739. https://doi.org/10.1016/j.febslet.2010.04.047
Liao G, Yao Y, Liu J, Yu Z, Cheung S, Xie A, Liang X, Bi X (2007) Cholesterol accumulation is associated with lysosomal dysfunction and autophagic stress in Npc1−/− mouse brain. Am J Pathol 171(3):962–975. https://doi.org/10.2353/ajpath.2007.070052
Nixon RA, Wegiel J, Kumar A, WH Y, Peterhoff C, Cataldo A, Cuervo AM (2005) Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol 64(2):113–122
Goldman SD, Krise JP (2010) Niemann-pick C1 functions independently of Niemann-Pick C2 in the initial stage of retrograde transport of membrane-impermeable lysosomal cargo. J Biol Chem 285(7):4983–4994. https://doi.org/10.1074/jbc.M109.037622
Funding
This work was supported by grants from Canadian Institutes of Health Research (MOP-123258 to SK), the University of Alberta Hospital Foundation (RES0029064 to SK). MM is a recipient of President’s International Doctoral Award from the University of Alberta and a studentship award from Alberta Innovates Health Solutions (AIHS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Maulik, M., Vergote, D., Phukan, G. et al. The Effects of Extracellular Serum Concentration on APP Processing in Npc1-Deficient APP-Overexpressing N2a Cells. Mol Neurobiol 55, 5757–5766 (2018). https://doi.org/10.1007/s12035-017-0799-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0799-5