Abstract
Genetic disruptions of spindle/centrosome-associated WD40-repeat protein 62 (WDR62) are causative for autosomal recessive primary microcephaly (MCPH) and a broader range of cortical malformations. Since the identification of WDR62 as encoded by the MCPH2 locus in 2010, recent studies that have deleted/depleted WDR62 in various animal models of cortical development have highlighted conserved functions in brain growth. Here, we provide a timely review of our current understanding of WDR62 contributions in the self-renewal, expansion and fate specification of neural stem and progenitor cells that are critical for neocortical development. Recent studies have revealed multiple functions for WDR62 in the regulation of spindle organization, mitotic progression and the duplication and biased inheritance of centrosomes during asymmetric divisions. We also discuss recently elaborated WDR62 interaction partners that include Aurora and c-Jun N-terminal kinases as part of complex signalling mechanisms that may define its neural functions. These studies provide new insights into the molecular and cellular processes that are required for brain formation and implicated in the genesis of primary microcephaly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The WD40-repeat protein 62 (WDR62) gene was identified, relatively recently, as causative for the human condition of autosomal recessive primary microcephaly (MCPH) [1,2,3]. This observation triggered a raft of studies into the role of WDR62 in neural stem and progenitor cell divisions required for normal embryonic brain growth [4,5,6]. WDR62 mutations are additionally implicated in a broader range of cortical malformations [1, 3] indicating pleiotropic functions spanning several stages of neural development. The aim of this review is not to exhaustively discuss the genetic determinants of primary microcephaly. Instead, we defer to recent cogent reviews that have addressed this broader topic in detail [7, 8]. Rather, we will provide a timely summary of our current understanding of the molecular basis of WDR62 structure and function, mechanisms of regulation and physiological importance for cellular processes underpinning neocortex development, which underlies the causative association with MCPH. Although the majority of WDR62 studies have focused on its neuronal functions, WDR62 is ubiquitously expressed and originally identified in a non-neuronal context [9]. Therefore, we will also explore brain-independent functions of the MCPH2 protein.
Pathogenic Human WDR62 Mutations Cause Microcephaly
Microcephaly or ‘small head’ syndrome is a condition of significantly reduced occipital-frontal circumference, defined as greater than 2 s.d. below the mean when matched for age, gender and head shape [10]. Microcephaly vera (or true microcephaly), sometimes also referred to as primary microcephaly, is an inherited autosomal recessive condition with a strict clinical definition. It is categorized by head/brain size that is significantly reduced but structurally normal, mild to moderate cognitive impairment in the absence of other neurological findings and normal height and body weight [11]. Additionally, primary microcephaly is diagnosed during gestation and presents at birth as a consequence of additional, associated developmental abnormalities [10]. This is distinct to secondary microcephaly, a degenerative condition with normal head/brain size at birth but with subsequent cell loss [10]. To date, 17 causative gene loci (MCPH1-17) have been mapped for the condition, including those corresponding to the genes Microcephalin 1 (MCPH1), WDR62 (MCPH2), CDK5RAP2 (MCPH3), CASC5 (MCPH4), ASPM (MCPH5), CENPJ (MCPH6), STIL (MCPH7), CEP135 (MCPH8), CEP152 (MCPH9), ZNF335 (MCPH10), PHC1 (MCPH11), CDK6 (MCPH12), CENPE (MCPH13), SASS6 (MCPH14), MFSD2A (MCPH15), ANKLE2 (MCPH16) and CIT (MCPH17) [12].
The second primary microcephaly locus (MCPH2) was first identified over two decades ago and mapped to chromosome 19q13.1–13.2 in humans [13]. More recently, whole exome sequencing approaches revealed WDR62 as the gene encoded by the MCPH2 locus [1, 3]. Human WDR62 consists of 32 exons, which encode a protein of 1523 amino acid residues and a shorter 1518 amino acid protein generated by an intra-exonic alternative splice acceptor site in exon 27 [2]. WDR62 is not only highly conserved amongst mammalian species, but has functional orthologs in invertebrates, including Drosophila melanogaster [14]. In mice, WDR62 is located on chromosome 7, and 33 coding exons generate a 1524 amino acid protein. Mouse WDR62 displays 75% sequence identity with the human orthologue, with the highest regions of conservation amongst the WD40 domain repeats (Fig. 1). In Drosophila, the WDR62 orthologue (CG7337) is located on the left arm of chromosome 2 and encodes a 2397 amino acid protein. Across the entire coding region, CG7337 is 35% identical to human WDR62, with considerably higher (48.5%) identity in the N-terminal, WD40 repeat-rich region [15] (Fig. 1). This sequence similarity suggested functional conservation for WDR62 in cellular regulation and/or neurodevelopment through evolution, which has been verified through molecular genetic studies (discussed below).
To date, over 30 WDR62 mutations have been genetically linked to primary microcephaly (Table 1). Indeed, WDR62 accounts for ~ 15% of disease cases and thus constitutes the second most frequently mutated MCPH gene [8]. These mutations include deletions, premature terminations, frameshifts and missense mutations, which are not only associated with reduced brain size but also manifest a wide variety of severe cerebral cortical defects previously conceptualised as distinct entities [1,2,3]. WDR62 mutations are recessive which reflects essential developmental functions. A large number of mutations are nonsense, frameshift, base pair duplications or splice-site mutations (Table 1) that result in severe truncations that are likely non-functional or lead to nonsense mediated decay and thus represent null allelles [1,2,3, 22, 24]. In addition to reduced brain growth with simplified gyration, pathogenic WDR62 mutations (Table 1) are linked with different phenotypic variations in cerebral cortical malformations. Individuals harbouring WDR62 mutations most commonly present with neuroradiological features such as pachygyria (incomplete lissencephaly), agenesis or abnormalities of the corpus callosum and a thickened cortex (Table 1). In addition, hippocampal abnormalities, subcortical band heteratopias and schizencephaly (clefts in the cerebral hemispheres) have also been reported, although these are comparatively infrequent in MCPH2 patients (Table 1; [1, 3, 20]). Thus, neurological deficits of MPCH2 patients predominantly impact forebrain development, with mid- and hindbrain abnormalities rarely observed [23].
These clinical studies indicated that the neurological deficits are not easily predicted by the specific mutation type or location of mutation on the WDR62 gene. Indeed, there are several cases of individuals with the same causative WDR62 mutation exhibiting vastly different severity of neurological deficits [1, 20, 24, 29]. Rather, the range of neural deficits associated with WDR62 loss in specific individuals may be the result of modifying environmental (e.g. malnutrition or infection) and additional contributing genotypic factors. Complications during pregnancy such as gestational diabetes were suggested in one study to potentially trigger increased severity of brain malformation during foetal development of a patient with mutated WDR62 [25]. Furthermore, a recent study speculated that tubulin cofactor D (TBCD) mutations and associated defects in microtubule regulation may modify the severity of the WDR62 MCPH phenotype [24]. Regardless, the range of structural and behavioural deficits associated with WDR62 mutations suggests that WDR62 may be a central regulator of multiple neurodevelopmental processes. In support of this notion, Yu et al. reported a post-mortem analysis of a 27-week foetus with an exon 30 frameshift mutation (c.3936_3937insC) that revealed significantly reduced thickness of the cortical plate, streaky heterotopia in the intermediate zone and disorganized clustering of small dividing cells in the subventricular zone [3]. These clinical observations suggest that WDR62 loss can trigger defective neural proliferation, neurogenesis and neuron migration during embryonic development.
Intriguingly, clinical genetic studies have additionally revealed truncating and missense mutations that may result in a partial loss of protein function to provide some insight into WDR62 mechanisms required for neurodevelopment. For example, several groups have reported pathogenic mutations located on exon 30 and 31, leading to premature truncation [2, 3, 22]. Although it is unclear if protein expression is maintained in all cases, at least one frame shift mutation (c.4241dupT) is predicted to generate a stable RNA product [1], and result in C-terminally truncated WDR62. Such a protein will lack C-terminal domains and phosphorylation sites that have been shown through ex vivo studies to be required for protein interactions, quaternary structure and protein regulation and, thus, critical for contributions to neurodevelopmental functions [9, 30, 31]. In addition, patients with non-synonymous missense mutations in WDR62 have been reported which is atypical for MCPH genes [20]. Missense WDR62 mutations are associated with neural deficits that range from reduced brain size with simplified gyri and corpus collosal abnormalities (p.R438H), but can also include pachygyri and cortical thickening (p.D511N, p.W224S) or schizencephaly and polymycrogyria (p.V65M). These missense mutations predominantly alter single amino acids located in the N-terminal half of the protein, amongst the WD40 repeat region (Fig. 2). The majority alter evolutionarily conserved residues that are bioinformatically predicted to be deleterious to protein stability. One exception, however, was a recent report of a MCPH patient with compound heterozygous, biallelic mutations in WDR62 that introduced a premature stop codon on one allele (p.D955Afs*112) and a missense mutation (c.1313G>A) in the second to result in a p.R438H amino acid substitution on the latter [23]. A biochemical analysis of patient-derived lymphocytes indicated residual levels of full-length WDR62 which suggested that the p.R438H WDR62 mutant was expressed but whether the protein was functional was unclear [23]. Although the biochemical basis of MCPH pathogenicity associated with the p.R438H mutation remains unclear, future structural and proteomic studies incorporating these atypical missense mutations may reveal novel WDR62-dependent disease mechanisms and provide insight into roles in normal brain growth and development.
WDR62 in Brain Development: Lessons from In Vivo Model Systems
As outlined above, WDR62 is evolutionarily conserved (Fig. 1) and genetic studies using ablation or attenuation of expression in several model systems have confirmed the requirement for WDR62 in normal brain development. Morpholino-mediated knockdown of zebrafish WDR62 reduced head and eye size [32]. Investigation of retinal neuroepithelial progenitors from morphant embryos revealed reduced cell numbers with an increased mitotic index, which was attributed to a mitotic arrest in prometaphase [32]. Transplantation of WDR62-depleted retinal progenitors into wild-type embryos did not rescue the proliferation defect [32], pointing towards an intrinsic role for WDR62 in mitotic progression and proliferation of retinal progenitors in zebrafish. WDR62 is also required for brain growth as wdr62 fish mutants show a ~ 40% reduction in brain volume [32]. Similarly, global depletion of the WDR62 ortholog in Drosophila (dWDR62) reduced larval brain size [15]. The observation that larval neuroblasts (the fly neuroprogenitor equivalent) were only modestly reduced (~ 5% reduction compared with wild type) was somewhat surprising [15], although a subsequent report suggested reduced brain size can arise at the third instar larval stage following WDR62 RNAi knockdown in neuroblasts and their progeny [33]. Thus, while these studies demonstrate a conserved function for WDR62 in maintenance of neural stem cell populations during normal brain development, an outstanding question was how the modest reduction in neuroblasts could significantly decrease brain growth following global wdr62 depletion.
A rationale emerged from our recent study, where lineage-specific depletion of WDR62 revealed the relative contribution of WDR62 in the neuroblast and surrounding glial lineage to brain growth [34]. The major cellular defect following WDR62 depletion, either globally [15] or specifically in neuroblasts [34], is spindle misorientation, which likely underlies the G2 delay and increased mitotic index. Although neuroblast-specific depletion alone reduced neuroblast number, this was not sufficient to reduce brain size, a likely consequence of the compensatory proliferation of the stem cell daughters [34]. In contrast, WDR62 loss-of-function specifically in the glial lineage profoundly altered brain growth. Thus, although both neuroblasts and glia are depleted following wdr62 loss, WDR62 function is only required in glia for maintaining brain size. Most intriguing, WDR62 depletion in the glia not only impaired brain growth autonomously (i.e. through mitotic defects and associated depletion of glia) but also increased neural stem death and significantly reduced the neuroblast pool. The Drosophila studies demonstrated that the glial lineage provides a supportive environment for neuroblast renewal and differentiation, and highlight the complex lineage-specific WDR62 functions that operate in vivo to determine brain growth.
Thus, WDR62 function in glia might also be integral to microcephaly phenotypes, and examining the lineage-specific contribution of WDR62 using mouse models will be of great interest. In the mammalian brain, radial glia behave as neural stem cells [35] and are supported by an outer radial glial environment, which provides the niche to maintain stem cell renewal [36]. The microglia cells also regulate neural precursor cell behaviour to maintain neuronal cell numbers in the cortex [37]. However, whether MCPH genes such as wdr62 are important for glial cell fate in mammals requires further investigation.
Studies of neural progenitor cell division in the ventricular (VZ) and subventricular (SVZ) zones of embryonic mouse brain are most commonly used as a mammalian model for early human brain development and associated diseases [38,39,40]. In recent years, a number of rodent studies have reinforced the important role for WDR62 in the developing mammalian brain. Although WDR62 is ubiquitously expressed, WDR62 levels are particularly high in the VZ and SVZ of the developing forebrain and corresponded to peak neurogenesis in rodent models [2, 29]. This also corresponds with a similar pattern of enriched VZ expression in developing human brain [1, 33]. Depletion of WDR62 by in utero shRNA electroporation of E14-16.5 embryonic rat brains resulted in shRNA transfected cells residing predominantly in the intermediate zone (IZ) at E17.5-20.5, rather than throughout the VZ, SVZ and cortical plate as expected for actively proliferating and differentiating cells within the developing brain [29]. In addition, Xu et al. reported a loss of radial glial fibers that span the width of the neocortex in transfected cells [29]. Thus, WDR62 is required to maintain neurite projections and facilitate the proper neuron migration essential for cortical lamination (Fig. 3).
Proposed WDR62 role in radial glial cells. WDR62 is required to maintain symmetrical divisions of radial glial cells at the apical membrane for proliferation and expansion of the stem cell pool. WDR62 loss leads to an increase in asymmetric divisions and precocious differentiation to basal progenitors. During late neurogenesis, WDR62 is required for neural migration to the cortical plate. WDR62 loss arrest the movement of immature neurons within the intermediate zone
The depletion of WDR62 reduced neural stem cell proliferation, induced their cell cycle exit and premature differentiation into transit amplifying intermediate progenitors (Tbr2 +ve) [29]. These results were consistent with our previous study that depleted WDR62 in embryonic mouse brains at E14 by in utero electroporation [4]. Specifically, WDR62 siRNA-transfected cells were reduced within the VZ and predominantly present in the IZ, and displayed reduced proliferation and increased cell cycle exit [4]. WDR62 depletion did not substantially increase mitotic index or apoptotic cell death in embryonic rodent brains [4, 29], which suggests the predominant defect following in utero electroporation was one of promiscuous differentiation of apical progenitors and their reduced proliferative capacity (Fig. 3).
In contrast, slightly different findings were reported with WDR62 hypomorphic mice generated through gene-trap mutagenesis [5, 33]. Homozygous mutant mice with gene-trap insertion between exons 14 and 15 of WDR62 exhibited reduced protein expression (~ 25% of wild-type levels) and were reduced in overall body and organ size [5]. Importantly, WDR62 mutant mice had reduced brain size with associated thinning of cortical layers evident at E17.5, consistent with a deficiency in neocortex expansion [5]. However, in contrast to in utero WDR62 depleted brains, premature differentiation of apical progenitors was not observed in WDR62 mutant mice. Rather, increased mitotic index, a delay in mitotic progression, spindle checkpoint activation and apoptosis of apical and basal progenitors during mid-late neurogenesis (E15.5-17.5), was reported [5]. Furthermore, Chen et al. utilized in utero electroporation of WDR62 siRNA and live cell imaging of brain slice cultures to demonstrate delayed cortical neuroprogenitor mitotic progression consistent with their in vivo findings [5].
More recently, Jayaraman et al. reported severely reduced brain size and overall body weight in WDR62 null mice generated by gene-trap insertion between exons 21 and 22 [6]. These mice lacked WDR62 protein and mRNA expression and exhibited thinning of upper cortical layers, reduced numbers of apical progenitors and increased basal progenitor populations [6]. In contrast with hypomorphic mice, WDR62 null mutants did not exhibit substantial cell death at E14.5 supporting precocious differentiation of apical progenitors as underlying brain growth deficits in the absence of WDR62 expression [6]. An independent study of WDR62 mutant mice derived from the same trapped ES cells (Wdr62Gt(CH0428)Wtsi), insertion between exons 21 and 22) also reported microcephaly, with reduced brain weight and size evident during early postnatal stages [33]. Immunohistological studies indicated reduced proliferation, cell cycle exit and increased apoptosis of neuroprogenitors confined to mid-late neurogenesis (E15.5-17.5) [33] and reminiscent of neuroprogenitor defects observed in hypomorphic mice with reduced WDR62 expression [5]. Interestingly, X-gal reporter labelling of embryonic brains revealed expression of truncated WDR62 (aa 1–870) lacking a substantial proportion of the C-terminal [33]. Therefore, the observed neuroprogenitor defects appear to be consistent with a partial loss-of-function mutation and suggest contributions by the C-terminal regions of WDR62 that are specifically required in late-stage neuroprogenitors [5, 33].
These rodent studies collectively reinforce the importance of WDR62 in formation of the mammalian CNS. Although the specific reasons underlying varied observations of neuroprogenitor proliferation, differentiation and survival in gene-trap mice between groups are untested, differences in WDR62 protein expression and partial loss-of-function following gene-trap insertion are likely to contribute to differential impacts on specific neuroprogenitor populations [33]. Our recent studies describing partial WDR62 knockdown in neural progenitors derived from human embryonic stem cells show a significant loss of TBR2 and S100β expression [41], which further highlight complex functions of WDR62 in maintaining the neuroprogenitor pool. However, the precise molecular and subcellular WDR62-dependent mechanisms that underpin neural development remain unclear. The following section of the review will discuss the WDR62-regulated mechanisms and functions that may contribute to brain development.
WDR62 Structural Domains
WDR62 is a relatively large protein (~ 175 kD, aa 1–1513) with multiple protein interaction domains mapped. Prominent features of WDR62 are the 12–15 annotated WD40 protein interaction domains that span aa 109–1298 (Fig. 2) [9]. MAPK-binding protein 1 (MAPKBP1) is another WD40-repeat protein that is a closely related paralog of WDR62 and shares 31% sequence similarity (Fig. 1) [30, 42]. WD40-repeat proteins comprise a class of evolutionarily conserved proteins that form beta propeller structures with multiple interaction interfaces for protein complex assembly [43]. Moreover, WD40 domain-containing proteins can mediate signal transduction by orchestrating the association between regulators, including kinases, and their relevant substrates [43]. The subcellular localizations of WD40 domain-containing proteins may also provide spatial definition to otherwise stochastic interactions.
We have demonstrated that a large proportion of WDR62 N-terminus region (aa 1–841) is both necessary and sufficient for spindle microtubule association [4]. Although the crystal structure of WDR62 has yet to be determined, we speculate an intact N-terminus will form the beta propeller required for microtubule binding. In addition, molecular and biochemical studies have revealed regulatory functions for the C-terminal region (aa 842–1513) of WDR62 that includes a number of phosphorylation sites, interaction motifs required for dimerization and binding of a number of kinases (Fig. 2). The WDR62 C-terminal loop helix motif is essential for homodimerization and heterodimerization with MAPKBP1 [30], which itself can bind JNK. Like WDR62, MAPKBP1 is recruited to the spindle pole during mitosis [44]; however, the extent to which MAPKBP1 is able to functionally compensate for WDR62 loss has not been determined. Homodimerization appears to be crucial for WDR62 recruitment and scaffolding of certain kinases, in particular c-Jun N-terminal kinase (JNK) family members [30]. In addition, several kinase interaction motifs have been identified in WDR62 upstream from the loop helix motif, that enable its interaction with kinases including the JNK isoforms, MKK7β1, MLK3, TAK1 and AURKA (Fig. 2) [5, 29, 31, 45, 46]. Interaction with WDR62 likely facilitates assembly of signalling complexes for the regulation of specific cellular processes including neurogenic divisions.
Subcellular Spatiotemporal Distribution of WDR62
Defining the subcellular localization of WDR62 has provided clues to cellular functions. Immunofluorescent staining of immortalized human cell lines (U2OS, HeLa and HEK), with verification by RNA interference, revealed WDR62 localization within the cytoplasm, particularly in the golgi apparatus, centrosomes and mitotic spindles [2,3,4, 29, 47, 48]. The diverse subcellular distributions reported in separate studies, revealed through immunofluorescence staining, appear to be a result of antibodies raised to distinct regions of WDR62. For example, polyclonal antibodies raised against sequences corresponding to aa 900–950 detect centrosome-localized WDR62 [47], while antibodies raised against aa 700–750 predominantly detect WDR62 in the cytoplasmic golgi compartment [2]. This may reflect context-dependent and/or distinct functional sub-classes of WDR62. Notably, the majority of WDR62 antibodies detect spindle pole microtubule localization in all mitotic cells regardless of cell type or developmental context [2, 4, 23]. Interestingly, ectopically expressed WDR62 fusion proteins (e.g. GFP-tagged) are localized to the cytoplasm in interphase and to the spindle poles in mitosis [2, 4]. This may be due to the factors required for trafficking WDR62-GFP to golgi and centrosomal compartments becoming limiting as a consequence of overexpression. One study also reported nuclear WDR62 localization in immortalized keratinocytes (HaCat), hepatocytes (HepG2) and breast epithelial (A549) cells; however, this has not been observed in HeLa or HEK cell lines [20]. Moreover, WDR62 nuclear localization may require verification using RNAi depletion to confirm signal specificity.
The localization of WDR62 in neural stem and progenitor cells may also provide insight into its cellular functions required for neurodevelopment. However, the poor cross-reactivity of commercially available human WDR62 antibodies for the mouse protein hinders detection in murine cells/tissue (our unpublished observations). As a consequence, the subcellular localization of WDR62 during murine cortical development has remained somewhat enigmatic. Nevertheless, several groups have consistently reported WDR62 expression in apical and basal progenitors located in the VZ and SVZ layers and in cortical plate neurons in rodent brain sections [1, 2, 5, 29]. In agreement, immunofluorescence staining of human brain sections, from CS22 gestational stage embryos, show WDR62 expression in the cytoplasm of apical progenitors in proximity to centrosomes and nuclear localization in newborn neurons at the outermost layers [2]. Interestingly, using a custom-generated antibody to mouse WDR62, Chen et al. (2014) observed cytoplasmic, but not nuclear staining of cortical plate neurons. Although there may be species or developmental context-dependent differences, it remains unclear whether WDR62 is a bona fide nuclear protein.
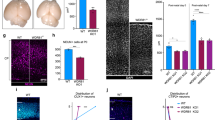
In addition, despite the distinctive spindle pole localization of WDR62 in immortalized human cell lines, studies have yet to reveal in vivo spindle-associated WDR62 in brain sections. This may be due to the loss or depolymerisation of microtubules during fixation and/or processing of tissue sections [2]. Although further studies are required, we predict WDR62 will be required on microtubules in neural stem and progenitor cells as we observe polar localization in mitotic Pax6 +ve neuroprogenitors from human ES cell-derived cultured neurospheres that is consistent with spindle pole localization (Fig. 4). Similarly, the Drosophila homolog of WDR62 is spindle-associated in asymmetrically dividing neuroblasts [15]. Functional evidence also suggests spindle pole localization is essential for neural development as WDR62 mutant proteins, recapitulating MCPH-associated missense mutations, fail to localize to the spindle in mitotic HeLa cells [2, 49]. Similarly, defective spindle pole localization of mutant WDR62 was reported in immortalized lymphocytes and fibroblasts derived from MCPH patients [23, 33]. This reinforces the notion that appropriate mitotic spindle localization of WDR62 may be required for normal brain development.
WDR62 localization in mitotic human neural progenitors. In vitro cultures of neurospheres containing neural progenitors were derived from pluripotent human embryonic stem cells. Fixed sections of neurosphere rosettes were immunostained for Pax6 as a marker of neuroprogenitors and human WDR62. DAPI staining revealed condensed chromosomes aligned at the metaphase plate indicative of mitotic phase
WDR62 Interactions with Aurora Kinases
The distinctive spindle pole localization of WDR62 in cultured human cells is highly dependent on cell cycle stage, particularly the phase of mitosis, and reflects precise regulation by mitotic signalling mechanisms. WDR62 association with spindle microtubules in the vicinity of separated centrosomes increases rapidly following mitotic entry [4, 47]. WDR62 levels at the spindle pole peak at prometaphase and decline with anaphase transition [50]. A proportion of WDR62 may remain at the poles, and additionally localize to the central spindle during late mitosis, although this has not been universally reported and appears dependent on the cell line investigated [20, 23, 50]. Regardless, the dynamic subcellular distribution of WDR62 associated with mitotic transitions are reminiscent of other microtubule-binding spindle pole proteins such as p150Glued and CEP170 [51, 52], with the later a known binding partner of WDR62 identified in proteomic studies [51, 52]. Although WDR62-MCPH mutants display aberrant mitotic localization, whether the genesis of human neurogenic deficiencies arises from defects in interphase and/or mitotic WDR62 functions is currently debatable. Delineating the mechanisms that define WDR62’s spatiotemporal distribution will be essential to dissect these cell cycle stage-specific functions required for normal development and which are likely dysregulated in disease.
The increase in WDR62 spindle association corresponds with mitotic entry and, thus, increased activity of mitotic kinases such as Aurora A (AURKA) [49]. Chen et al. (2014), co-immunoprecipitation screening of candidate spindle assembly factors, reported the interaction between WDR62 and AURKA [5]. LC-MS/MS-based studies, using WDR62 as bait for affinity isolation from mitotic cell lysates, independently identified AURKA-WDR62 complexes and further validated WDR62 and AURKA as direct binding partners [49]. Domain mapping studies for WDR62 defined the AURKA interaction region between aa 621 and 1138 [5]; however, further characterization is required to define the precise AURKA/WDR62 binding domain to determine the effects of specifically disrupting the WDR62-AURKA interactions without disrupting interactions with other protein partners.
WDR62 is mitotically phosphorylated and a direct phosphorylation target of AURKA [48, 49]. Moreover, phosphoproteomic analyses have revealed that multiple serine/threonine residues (S32, S33, S49, T50 and S52) at the N-terminus of WDR62 are targeted by AURKA (Fig. 2) [48, 53]. AURKA phosphorylation of WDR62 directs the association of the latter with microtubule filaments [48, 49]. Indeed, ectopic expression of AURKA is sufficient to promote WDR62 microtubule binding, irrespective of cell cycle stage [48]. Thus, AURKA, which is localized specifically to the centrosome and spindle pole [54], can modulate the distribution of WDR62 on microtubule filaments. Mutagenesis studies have also confirmed that AURKA-phosphorylated N-terminal WDR62 residues are required for normal protein localization and function during mitosis [48, 49]. AURKA-WDR62 signalling interactions, therefore, represent a significant mechanism for delineating mitotic WDR62 localization and function.
During development, AURKA and WDR62 co-operate to promote brain growth in vivo. Double heterozygous WDR62 +/−, AURKA +/− mice have significantly decreased brain size compared with WDR62 heterozygotes alone, reinforcing the role of AURKA-WDR62 interaction in neurodevelopment [5]. Drosophila genetic studies suggest conservation of the WDR62-AURKA signalling axis, but the effects on brain growth are lineage-dependent [34]. Neuroblast-specific AURKA knockdown significantly increased the stem cell population and drove brain overgrowth, as reported for global AURKA loss-of-function [55, 56]; WDR62 co-knockdown reduced neuroblast number and brought brain size to within the wild-type range. The brain overgrowth associated with AURKA loss-of-function therefore requires endogenous WDR62. In contrast, AURKA knockdown in glia significantly decreased glial number and brain volume, and co-depletion of WDR62 enhanced glial cell death to further impair brain growth [34]. That co-knockdown of WDR62 in both contexts reduced brain size suggests AURKA likely acts to promote WDR62-dependent glial proliferation, but antagonises WDR62 function in the neuroblast lineage in the context of normal brain development. Moreover, the function of the WDR62-AURKA axis in glial is essential for non-autonomous maintenance of the neural stem cell population and brain growth. Thus, lineage-specific signalling of AURKA-WDR62 orchestrates larval brain growth and development.
In mammalian systems, apical neuroprogenitors express glial markers and represent the first glial cells that undergo a switch from neurogenesis to gliogenesis during mid-late gestation. It would be of interest to determine the contribution of WDR62 in more committed glial subtypes (e.g. astrocytes) with significant emerging functions in mammalian neurodevelopment [57].
An outstanding question concerns the precise AURKA-WDR62 signalling mechanism required for neurodevelopment. Interestingly, AURKA protein levels are downregulated in WDR62 hypomorphic mice [5]. In addition, the overexpression of WDR62, in combination with Tpx2, an AURKA co-activator, modestly increased AURKA autophosphorylation and expression [5] which suggests that WDR62 regulates AURKA expression and activity. However, the knockdown or deletion of WDR62 in cultured cells did not substantially perturb AURKA abundance, nor did it disrupt mitotic localization [48]. Thus, WDR62 may also be involved in complex feedback mechanisms enabling regulation of AURKA activity in vivo. We have shown that WDR62 is a direct phosphorylation target of AURKA and this is required for mitotic regulation in cultured cells [48, 49]. Functional rescue studies in WDR62 null mice with phosphorylation deficient WDR62 mutants will be required to define the contribution of AURKA signalling to WDR62 in mammalian neurodevelopment.
Emerging evidence also indicates that a proportion of WDR62 may co-localize and interact with Aurora B (AURKB) at the centromeres [33]. WDR62 loss in patient fibroblasts resulted in decreased AURKB levels at kinetochores and may account for mitotic progression delay [33]. This raises the question of WDR62-regulated AURKB contributions in neocortical progenitor self-renewal and proliferation. Sgourdou et al. (2017) further revealed genetic interactions between WDR62 and AURKB in Drosophila larval neuroblast growth [33]. Thus, WDR62 interactions with multiple Aurora kinases and functions in several mitotic processes may be significant for brain development.
WDR62 Interactions with JNK Signalling Cascade
As outlined above, WDR62 interacts with the JNK mitogen-activated protein kinase (MAPK) signalling pathways family, implicated in a plethora of intracellular responses in response to a broad range of extracellular stimuli [50]. The WDR62 motif required for JNK interaction spans aa 1294–1301 and is also known as the JNK binding domain (JBD) (Fig. 2) [9]. This interaction not only occurs during basal cellular homeostasis but also provides a mechanism for modulating JNK signal specificity in response to cellular stress and during mitosis [9, 50]. In addition to JNK, WDR62 also binds upstream kinases in the JNK cascade, MLK3 and MKK7β1 [30]. Although canonically MKK7β1 is required for JNK activation, the MKK7β1/WDR62 interaction results in recruitment of an unidentified phosphatase that prevents aberrant JNK phosphorylation of WDR62 [30]. Therefore, under non-stress conditions, WDR62 scaffolding provides spatial-temporal regulation of MLK3/MKK7β1/JNK1 signalling [45].
Although the JNK/WDR62 interaction occurs under basal cellular conditions, their interaction is important for spatial determination of JNK signalling under cellular stress conditions [9]. In response to arsenite-induced cellular stress, JNK and WDR62 are redistributed from the cytoplasm into stress granules, transient sites enabling stress-specific translational regulation, through accumulation of stalled initiation complexes for house-keeping mRNAs together with increased translation of stress-induced transcripts [58]. Furthermore, WDR62 protein expression increases upon JNK activation, which suggests feedback regulation of WDR62 turnover. Interestingly, WDR62 is also a substrate of JNK itself and is phosphorylated at several serine/threonine residues in the C-terminus upon arsenite stimulation [9]. The role of stress-stimulated WDR62-mediated JNK phosphorylation and its localization to stress granules remains unclear, but suggests a possible role for WDR62 in mRNA homeostasis.
The important functions of neural JNK isoforms are well recognized [59, 60]. The generation of JNK1/2 double-knockout mice first revealed the critical requirements for JNK signalling in neural tube formation [61]. In particular, JNK1 is highly expressed and active in the developing neocortex and regulates neurogenesis [62]. WDR62 interactions with JNK have been also implicated in neurogenesis [29, 50]. Xu et al. (2014) demonstrated that WDR62-JNK signalling is required for neural proliferation and migration. More recently, in utero electroporation studies revealed JNK1 isoforms mirrored WDR62 functions, being specifically required to maintain self-renewal of apical progenitors [29]. Moreover, overexpression of constitutively active JNK1 or expression of WDR62 with an intact JBD was sufficient to functionally rescue WDR62 depletion defects [29]. The sum of these findings suggests that WDR62-JNK1 interactions are required for optimal JNK1 activity in the neocortex and embryonic brain growth.
In contrast, WDR62 scaffolding of JNK2 signalling appears to be significant for migratory behaviour of newly generated neurons. JNK signalling has a long-established role in cell migration and implicated in negative regulation of radial migration of multipolar cells in the SVZ [62]. In contrast, in utero electroporation and depletion of WDR62 arrested cells in the intermediate zone of the developing cortex, consistent with a migration defect. Zhang et al. (2016) demonstrated that WDR62, in the murine neocortex, was likely required for activation of the JNK2 isoform in complex cross-talk signalling with the TGF-β pathway [46]. Moreover, in partnership with another scaffold protein POSH, WDR62 brought TAK1, a downstream effector kinase of TGF-β receptor, into complex with MKK4/7 and JNK2 [46]. Thus, WDR62-scaffolded JNK2 signalling constitutes a non-canonical effector of TGF-β pathway signalling required for neuronal migration during brain development [46].
Taken together, these studies suggest that MCPH associated with WDR62 loss may stem, at least in part, from disrupted or misdirected JNK signalling during brain development. However, it remains unclear whether JNK signalling is altered in MCPH patients. An assessment of the localization and/or activation of JNK isoforms in clinical samples would serve to resolve this knowledge gap. Alternatively, investigation of JNK signalling in in vivo or in vitro organoid culture models of MCPH2 may provide additional insights.
WDR62 in Mitotic Spindle Regulation
A significant question remains as to the specific cellular defects, arising from WDR62 loss, underlying the development of MCPH in humans. The first reports of WDR62 depletion in cultured cells described bipolar spindles with disrupted spindle pole organization post spindle assembly [50]. WDR62 depletion resulted in reduced spindle-centrosome attachment reminiscent of the disruption cdk5rap2, a centrosome and MCPH protein [63]. This suggests that WDR62 was required for spindle pole maintenance (Fig. 5). As a consequence, spindle orientation and mitotic progression are disrupted with WDR62 loss [50].
Cell cycle specific functions of WDR62. Several distinct functions of WDR62 during different stages of the cell cycle have been reported. This includes stress granule formation, cilia regulation and centriole biogenesis during interphase and spindle regulation and spindle-centrosome attachment during mitosis. WDR62 is also required for timely cell cycle progression
Bipolar spindle polarity and subsequent division orientation in neuroprogenitors are thought to play key roles in determining the balance of proliferative (symmetric) versus neurogenic (asymmetric) divisions, with the later triggering neuroprogenitor self-renewal [64, 65]. Conserved plasma membrane protein complexes, comprising key factors such as Pins/LGN and Mud/NUMA, recruit dynein motors to capture astral microtubules, connecting the cell cortex with the spindle pole for spindle positioning [64]. In Drosophila neuroblasts, there is a strict requirement for spindle alignment along an apico-basal axis for asymmetric divisions [39]. However, the significance of spindle orientation in apical progenitor division in mammals is less clear [39]. This may be related to the relative difficulty in experimentally measuring spindle orientation in the mouse brain, compared to Drosophila neuroblasts, or the interplay between more complex interactions amongst several distinct neuroprogenitors subsets in defining cell fate in the mammalian neocortex [66].
Nevertheless, WDR62 expression is required to maintain apico-basal spindle polarity and timely mitosis in Drosophila neuroblasts highlighting conserved functions in spindle regulation [15, 34]. In utero WDR62 depletion in murine embryos disrupts spindle pole structure [29], while MCPH patient lymphocytes with WDR62 mutations manifest fragmented centrosomes, disorganized spindles and associated mitotic delay in agreement with WDR62 knockdown in HeLa cells [23]. WDR62 depletion also increases spindle length, frequency of multipolar spindles and misaligned chromosomes at the metaphase plate [50]. MEFs isolated from WDR62 hypomorphic mice displayed similar defects, but the mitotic delay was also associated with spindle checkpoint activation and apoptotic cell death [5]. However, the brain size reductions in WDR62 hypomorphic or WDR62 knockout mice were not accompanied by equivalent changes in division orientation of apical progenitors [5, 6]. Therefore, disrupted spindle organization as a consequence of WDR62 loss has been most apparent in 2D cell culture and Drosophila neuroblast studies. As a consequence of these contradictory findings, there is a lack of consensus on the role for WDR62-regulated spindle orientation and mitotic progression in defining mammalian neural stem cell fate in vivo. A detailed study targeting WDR62 in 3D neurosphere or brain organoid cultures or conditional targeting of WDR62 in the brain may yet provide more definitive conclusions.
WDR62 interactions with JNK also contribute to mitotic regulation [50]. WDR62 protein lacking the JBD motif fails to rescue mitotic spindle defects resulting from WDR62 depletion [50], highlighting the importance of WDR62-JNK interactions for spindle and mitotic regulation in cultured cells. As WDR62-JNK1 interactions are required to maintain neural proliferation [29], it is tempting to speculate that this may be mediated through mitotic spindle regulation and division orientation. The identification and characterization of mitotic JNK substrates involved in spindle control may provide further insights into the contribution of mitosis-specific WDR62-JNK signalling in neurogenesis.
WDR62 in Centrosome Regulation
WDR62 has been reported to localize to centrosomes where it may contribute to centriole duplication and partitioning in dividing cells [6, 15, 47]. Centrosomal WDR62 is localized to the proximal ends of centriole barrels where it promotes centriole duplication (Fig. 5) in concert with other MCPH proteins cdk5rap2 (MCPH3), CEP152 (MCPH4) and CEP63 (MCPH12) [6, 47]. WDR62 is recruited to centrosomes, by the centriole satellite protein MOONRAKER, in a hierarchical manner (CDK5RAP→CEP152→WDR62→CEP63) forming a complex necessary to localize cyclin-dependent kinase 2 (CDK2) to the centriole to promote duplication [47]. In addition, WDR62 interacts with ASPM to recruit CEP63 and subsequently CPAP/CENPJ, a component of the cartwheel assembly, for the elongation of daughter centrioles [6]. The loss of WDR62 in vitro or in vivo resulted in defective centriole duplication leading to S phase cells with abnormal centriole numbers and acentriolar cells [6]. Thus, WDR62 joins the growing list of MCPH proteins with functions in centriole biogenesis, a common defect underlying genetic microcephaly.
Compelling evidence supports centriole biogenesis defects as underlying microcephaly in WDR62 null mice [6]. The severity of centriole loss following gene dose-dependent WDR62 and ASPM loss correlated with the degree of brain size reduction, cortical thinning and precocious neuroprogenitor differentiation in the mouse embryo [6]. In addition, brain growth deficits and the delamination of apical progenitors in WDR62 null mice mirror that of CEP63 or SAS-4 deficiency which similarly results in centriole loss [67, 68]. However, the reduction in centrosomes associated with CEP63 and SAS-4 deficiency did not impair neuroprogenitor proliferation per se but rather triggered p53-mediated apoptosis leading to neuronal loss [67, 68]. Microcephaly defects in CEP63 and SAS4 mice are rescued by p53 deletion for example [67, 68]. Interestingly, increased apoptosis was not reported in WDR62 null mice with centriole deficiencies which suggest that centriole loss in this context may not be as severe or directly comparable to mice lacking core components essential for centriole duplication [6, 67]. Abnormal centrosome numbers in mitosis may also impact spindle and division orientation of apical progenitors, but this was similarly unaffected in the absence of WDR62 [6]. This raises the question of the precise link between centriole loss and microcephaly in WDR62 null mice.
Jayaraman et al. revealed that WDR62 was required to maintain the organization of apical complex proteins (Pals, aPKC, Par3/6) that couple progenitor cell fate and cortical development [69]. Precisely how WDR62 functions in centriole and apical complex regulation are mechanistically linked was undefined. Mature centrioles are also responsible for organizing the primary cilium which regulate radial glial cell morphology and cell fate as they extend into the ventricular space to respond to various signalling factors present in cerebrospinal fluid [70, 71]. In addition, incomplete cilia disassembly and asymmetric inheritance of centriole-associated ciliary membrane remnants may be a mechanism to expedite ciliogenesis and self-renewal of daughter cells [72]. Consistent with this mechanism, WDR62 loss triggered a reduction in primary cilia within neurogenic zones and the dissociation of ciliary membranes from centrosomes which could conceivably alter apical progenitor cell fate [6]. Thus, as centriole loss potentially impacts several cellular processes throughout cell cycle stages, precisely how WDR62 loss triggers microcephaly remains unclear and may be multi-factorial.
Recent studies have also revealed that loss or truncation of WDR62 leads to abnormal centrosome inheritance in neuroprogenitors [15, 33]. The asymmetric partitioning of duplicated centrosomes has been shown to correlate with self-renewal [73]. In Drosophila, the older mother centriole is specifically partitioned into fate committed ganglion mother cells during type I neuroblast asymmetric divisions [74]. Nair et al. (2016) demonstrated a requirement for Drosophila WDR62 in maintaining centrosome asymmetry, with mutant neuroblasts incorrectly missegregating mother and daughter centrosomes during asymmetric divisions [15]. However, centrosomal defects were uncoupled from reduced brain size and neuroblast numbers, which suggests that asymmetric partitioning of centrosomes may not be a primary determinant of Drosophila neuroblast fate in self-renewing asymmetric divisions [66]. In the mammalian neocortex, centrosomes containing the older mother centriole are specifically inherited by self-renewed apical progenitors at the VZ [75]. The missegregation of centrosomes in WDR62 mutant mice is associated with defects in the migration of newborn neurons, decreased neural proliferation and brain size [33]. Although the underlying mechanisms are not fully defined, WDR62 association and regulation of microtubule organization appears to be necessary. Drosophila WDR62 stabilizes interphase microtubules which are required to position the centrosome with younger mother centriole at the apical membrane for specific inheritance by the self-renewed neuroblast [15]. In mammalian cells, WDR62 mutants fail to bind spindle microtubules leading to disrupted spindle and centrosome positioning [23, 33, 48]. Thus, parallel mechanisms may determine WDR62 contribution to proper centrosome segregation during asymmetric divisions of mammalian neuroprogenitors.
Non-neuronal Functions of WDR62
Although WDR62 mutations manifest primarily as neurodevelopmental deficits, WDR62 is ubiquitously expressed and likely has non-neuronal functions. It is unclear why WDR62 mutations in humans impact the brain specifically although one could suggest that functional redundancy from WDR62 paralogs (e.g. MAPKBP1) may partially compensate for WDR62 loss in non-neuronal cells. An analysis of WDR62 expression in a human tissue panel indicated elevated mRNA levels in the adult heart, skeletal muscle and testes that exceeded that observed in foetal brain [9]. The role of WDR62 in non-neuronal tissues and whether they parallel spindle and centrosome regulatory functions defined in neural precursors and immortalized human cell lines have not been reported to date although some studies suggest significant contributions to fertility and tumour progression [5, 6, 76].
The reduced fertility of WDR62 hypomorphic mice was indicated in one study but was not further elaborated [5]. However, this suggests that WDR62 expression may be involved in germ cell development and/or gametogenesis which would be consistent with previously defined requirements for MPCH proteins in normal reproduction [68, 77,78,79]. The loss or mutation MCPH proteins ASPM and Cdk5rap2 result in reduced germ cell production, decreased gonad size and infertility in mice [78, 79]. CEP63 deficiency specifically impairs tested development and spermatogenesis due primarily to male meiotic recombination defects [68]. As previously mentioned, ASPM and CEP63 are complexed with WDR62 [6, 47]. Therefore, it would be interesting to determine if WDR62 is similarly required for germ cell specification/maintenance or meiotic spindle organization and recombination events during spermatogenesis.
Recent clinical studies have additionally linked WDR62 with non-neuronal pathologies. The overexpression of WDR62 has also been linked to poor prognosis and chemoresistance in a range of human cancers [76, 80, 81]. WDR62 overexpression was associated with amplified centrosomes in lung and ovarian cancer [76, 80] and poor differentiation of gastric tumours in humans [81]. Interestingly, the suppression of WDR62 using siRNA suppressed gastric tumour growth, indicating that WDR62 maintained proliferation or survival of gastric cancer cells [81]. WDR62 inhibition arrested gastric cancer cells in G2/M and induced apoptosis reminiscent of neuroprogenitors from WDR62 hypomorphic mice [5, 81]. Thus, WDR62 overexpression or gain-of-function may be detrimental in the context of adult tissues. However, the molecular and cellular consequences of overexpressed WDR62 remain undefined.
Conclusions and Future Directions
Following identification of WDR62 as encoded by the MCPH2 locus, recent WDR62 studies have provided valuable insights into processes that underpin cortical development. WDR62 has been implicated in centrosome duplication, spindle regulation, turnover of the primary cilium and cell cycle progression (Fig. 5), reinforcing the critical contribution of these processes towards carefully co-ordinated neural stem cell proliferation, self-renewal and differentiation in the expanding neocortex. The importance of DNA damage and repair response in neurogenesis has also been implicated amongst other MCPH proteins [8]. A similar requirement for WDR62 in maintaining genome stability has not been reported to date. In addition, WDR62 neural functions extend to the maintenance of neuronal migration [46] and apical membrane integrity [6] reflective of the diverse range of cortical malformations observed in MCPH2 patients [3]. It remains to be seen whether the range of neural and cellular deficits are recapitulated with specific WDR62 MCPH missense mutations that retain protein expression [23]. A comparison of phenotypes of mutant WDR62 proteins may further delineate mechanisms directly linked to specific neural stem processes. Deciphering WDR62 protein interactions has helped elaborate the scaffolding mechanisms that define compartmentalized activity of specific JNK isoforms involved in neurodevelopment [29, 46, 50] and the hierarchy of centriolar complexes required for duplication [47]. However, the function of WDR62 interactions with further centrosomal and mitotic proteins (e.g. CEP170) remains uncharacterized [2, 49]. Clearly, WDR62 has pleiotropic functions in the brain and the challenge remains to define the specific defects that are causative of MCPH. Lastly, future studies of WDR62 in non-neuronal systems would define its broader functions and determine whether these are consistent with cytoskeletal and signalling functions reported in the brain.
References
Bilguvar K, Ozturk AK, Louvi A, Kwan KY, Choi M, Tatli B, Yalnizoglu D, Tuysuz B et al (2010) Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Nature 467(7312):207–210. https://doi.org/10.1038/nature09327
Nicholas AK, Khurshid M, Desir J, Carvalho OP, Cox JJ, Thornton G, Kausar R, Ansar M et al (2010) WDR62 is associated with the spindle pole and is mutated in human microcephaly. Nat Genet 42(11):1010–1014. https://doi.org/10.1038/ng.682
Yu TW, Mochida GH, Tischfield DJ, Sgaier SK, Flores-Sarnat L, Sergi CM, Topcu M, McDonald MT et al (2010) Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nat Genet 42(11):1015–1020. https://doi.org/10.1038/ng.683
Bogoyevitch MA, Yeap YY, Qu Z, Ngoei KR, Yip YY, Zhao TT, Heng JI, Ng DC (2012) WD40-repeat protein 62 is a JNK-phosphorylated spindle pole protein required for spindle maintenance and timely mitotic progression. J Cell Sci 125(Pt 21):5096–5109. https://doi.org/10.1242/jcs.107326
Chen JF, Zhang Y, Wilde J, Hansen KC, Lai F, Niswander L (2014) Microcephaly disease gene Wdr62 regulates mitotic progression of embryonic neural stem cells and brain size. Nat Commun 5:3885. https://doi.org/10.1038/ncomms4885
Jayaraman D, Kodani A, Gonzalez DM, Mancias JD, Mochida GH, Vagnoni C, Johnson J, Krogan N et al (2016) Microcephaly proteins Wdr62 and Aspm define a mother centriole complex regulating centriole biogenesis, apical complex, and cell fate. Neuron 92(4):813–828. https://doi.org/10.1016/j.neuron.2016.09.056
Gilmore EC, Walsh CA (2013) Genetic causes of microcephaly and lessons for neuronal development. WIREs Dev Biol 2(4):461–478. https://doi.org/10.1002/wdev.89
Morris-Rosendahl DJ, Kaindl AM (2015) What next-generation sequencing (NGS) technology has enabled us to learn about primary autosomal recessive microcephaly (MCPH). Mol Cell Probes 29(5):271–281. https://doi.org/10.1016/j.mcp.2015.05.015
Wasserman T, Katsenelson K, Daniliuc S, Hasin T, Choder M, Aronheim A (2010) A novel c-Jun N-terminal kinase (JNK)-binding protein WDR62 is recruited to stress granules and mediates a nonclassical JNK activation. Mol Biol Cell 21(1):117–130. https://doi.org/10.1091/mbc.E09-06-0512
Woods CG, Parker A (2013) Investigating microcephaly. Arch Dis Child 98(9):707–713. https://doi.org/10.1136/archdischild-2012-302882
Woods CG, Bond J, Enard W (2005) Autosomal recessive primary microcephaly (MCPH): a review of clinical, molecular, and evolutionary findings. Am J Hum Genet 76(5):717–728. https://doi.org/10.1086/429930
Zaqout S, Morris-Rosendahl D, Kaindl AM (2017) Autosomal recessive primary microcephaly (MCPH): an update. Neuropediatrics 48(3):135–142. https://doi.org/10.1055/s-0037-1601448
Roberts E, Jackson AP, Carradice AC, Deeble VJ, Mannan J, Rashid Y, Jafri H, McHale DP et al (1999) The second locus for autosomal recessive primary microcephaly (MCPH2) maps to chromosome 19q13.1-13.2. Eur J Hum Genet 7(7):815–820. https://doi.org/10.1038/sj.ejhg.5200385
Pervaiz N, Abbasi AA (2016) Molecular evolution of WDR62, a gene that regulates neocorticogenesis. Meta Gene 9:1–9. https://doi.org/10.1016/j.mgene.2016.02.005
Ramdas Nair A, Singh P, Salvador Garcia D, Rodriguez-Crespo D, Egger B, Cabernard C (2016) The microcephaly-associated protein Wdr62/CG7337 is required to maintain centrosome asymmetry in drosophila neuroblasts. Cell Rep 14(5):1100–1113. https://doi.org/10.1016/j.celrep.2015.12.097
Banerjee S, Chen H, Huang H, Wu J, Yang Z, Deng W, Chen D, Deng J et al (2016) Novel mutations c.28G>T (p.Ala10Ser) and c.189G>T (p.Glu63Asp) in WDR62 associated with early onset acanthosis and hyperkeratosis in a patient with autosomal recessive microcephaly type 2. Oncotarget 7(48):78363–78371. 10.18632/oncotarget.13279
Ahmad I, Baig SM, Abdulkareem AR, Hussain MS, Sur I, Toliat MR, Nurnberg G, Dalibor N et al (2016) Genetic heterogeneity in Pakistani microcephaly families revisited. Clin Genet 92(1):62–68. https://doi.org/10.1111/cge.12955
Sajid Hussain M, Marriam Bakhtiar S, Farooq M, Anjum I, Janzen E, Reza Toliat M, Eiberg H, Kjaer KW et al (2013) Genetic heterogeneity in Pakistani microcephaly families. Clin Genet 83(5):446–451. https://doi.org/10.1111/j.1399-0004.2012.01932.x
Bastaki F, Mohamed M, Nair P, Saif F, Tawfiq N, Aithala G, El-Halik M, Al-Ali M et al (2016) Novel splice-site mutation in WDR62 revealed by whole-exome sequencing in a Sudanese family with primary microcephaly. Congenit Anom 56(3):135–137. https://doi.org/10.1111/cga.12144
Bhat V, Girimaji SC, Mohan G, Arvinda HR, Singhmar P, Duvvari MR, Kumar A (2011) Mutations in WDR62, encoding a centrosomal and nuclear protein, in Indian primary microcephaly families with cortical malformations. Clin Genet 80(6):532–540. https://doi.org/10.1111/j.1399-0004.2011.01686.x
Memon MM, Raza SI, Basit S, Kousar R, Ahmad W, Ansar M (2013) A novel WDR62 mutation causes primary microcephaly in a Pakistani family. Mol Biol Rep 40(1):591–595. https://doi.org/10.1007/s11033-012-2097-7
Kousar R, Hassan MJ, Khan B, Basit S, Mahmood S, Mir A, Ahmad W, Ansar M (2011) Mutations in WDR62 gene in Pakistani families with autosomal recessive primary microcephaly. BMC Neurol 11:119. https://doi.org/10.1186/1471-2377-11-119
Farag HG, Froehler S, Oexle K, Ravindran E, Schindler D, Staab T, Huebner A, Kraemer N et al (2013) Abnormal centrosome and spindle morphology in a patient with autosomal recessive primary microcephaly type 2 due to compound heterozygous WDR62 gene mutation. Orphanet J Rare Dis 8:178. https://doi.org/10.1186/1750-1172-8-178
Poulton CJ, Schot R, Seufert K, Lequin MH, Accogli A, Annunzio GD, Villard L, Philip N et al (2014) Severe presentation of WDR62 mutation: is there a role for modifying genetic factors? Am J Med Genet A 164A(9):2161–2171. https://doi.org/10.1002/ajmg.a.36611
Murdock DR, Clark GD, Bainbridge MN, Newsham I, Wu YQ, Muzny DM, Cheung SW, Gibbs RA et al (2011) Whole-exome sequencing identifies compound heterozygous mutations in WDR62 in siblings with recurrent polymicrogyria. Am J Med Genet A 155A(9):2071–2077. https://doi.org/10.1002/ajmg.a.34165
Najmabadi H, Hu H, Garshasbi M, Zemojtel T, Abedini SS, Chen W, Hosseini M, Behjati F et al (2011) Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature 478(7367):57–63. https://doi.org/10.1038/nature10423
Rupp V, Rauf S, Naveed I, Windpassinger C, Mir A (2014) A novel single base pair duplication in WDR62 causes primary microcephaly. BMC Med Genet 15:107. https://doi.org/10.1186/s12881-014-0107-4
Naseer MI, Rasool M, Sogaty S, Chaudhary RA, Mansour HM, Chaudhary AG, Abuzenadah AM, Al-Qahtani MH (2017) A novel WDR62 mutation causes primary microcephaly in a large consanguineous Saudi family. Ann Saudi Med 37(2):148–153. https://doi.org/10.5144/0256-4947.2017.148
Xu D, Zhang F, Wang Y, Sun Y, Xu Z (2014) Microcephaly-associated protein WDR62 regulates neurogenesis through JNK1 in the developing neocortex. Cell Rep 6(1):104–116. https://doi.org/10.1016/j.celrep.2013.12.016
Cohen-Katsenelson K, Wasserman T, Darlyuk-Saadon I, Rabner A, Glaser F, Aronheim A (2013) Identification and analysis of a novel dimerization domain shared by various members of c-Jun N-terminal kinase (JNK) scaffold proteins. J Biol Chem 288(10):7294–7304. https://doi.org/10.1074/jbc.M112.422055
Cohen-Katsenelson K, Wasserman T, Khateb S, Whitmarsh AJ, Aronheim A (2011) Docking interactions of the JNK scaffold protein WDR62. Biochem J 439(3):381–390. https://doi.org/10.1042/BJ20110284
Novorol C, Burkhardt J, Wood KJ, Iqbal A, Roque C, Coutts N, Almeida AD, He J et al (2013) Microcephaly models in the developing zebrafish retinal neuroepithelium point to an underlying defect in metaphase progression. Open Biol 3(10):130065. https://doi.org/10.1098/rsob.130065
Sgourdou P, Mishra-Gorur K, Saotome I, Henagariu O, Tuysuz B, Campos C, Ishigame K, Giannikou K et al (2017) Disruptions in asymmetric centrosome inheritance and WDR62-Aurora kinase B interactions in primary microcephaly. Sci Rep 7:43708. https://doi.org/10.1038/srep43708
Lim NR, Shohayeb B, Zaytseva O, Mitchell N, Millard SS, Ng DC, Quinn LM (2017) Glial-specific functions of microcephaly protein WDR62, and interaction with the mitotic kinase AURKA, are essential for Drosophila brain growth. Stem Cell Rep S2213-6711(17):30222–30229. https://doi.org/10.1016/j.stemcr.2017.05.015
Florio M, Huttner WB (2014) Neural progenitors, neurogenesis and the evolution of the neocortex. Development 141(11):2182–2194. https://doi.org/10.1242/dev.090571
Pollen AA, Nowakowski TJ, Chen J, Retallack H, Sandoval-Espinosa C, Nicholas CR, Shuga J, Liu SJ et al (2015) Molecular identity of human outer radial glia during cortical development. Cell 163(1):55–67. https://doi.org/10.1016/j.cell.2015.09.004
Cunningham CL, Martinez-Cerdeno V, Noctor SC (2013) Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33(10):4216–4233. https://doi.org/10.1523/JNEUROSCI.3441-12.2013
Fietz SA, Huttner WB (2011) Cortical progenitor expansion, self-renewal and neurogenesis-a polarized perspective. Curr Opin Neurobiol 21(1):23–35. https://doi.org/10.1016/j.conb.2010.10.002
Lesage B, Gutierrez I, Marti E, Gonzalez C (2010) Neural stem cells: the need for a proper orientation. Curr Opin Genet Dev 20(4):438–442. https://doi.org/10.1016/j.gde.2010.04.013
Manzini MC, Walsh CA (2011) What disorders of cortical development tell us about the cortex: one plus one does not always make two. Curr Opin Genet Dev 21(3):333–339. https://doi.org/10.1016/j.gde.2011.01.006
Alshawaf A, Antonic A, Skafidas E, Ng DCH, Dottori M (2017) WDR62 regulates early neural and glial progenitor specification from human pluripotent stem cells. Stem Cells Int 2017:7848932. https://doi.org/10.1155/2017/7848932
Koyano S, Ito M, Takamatsu N, Shiba T, Yamamoto K, Yoshioka K (1999) A novel Jun N-terminal kinase (JNK)-binding protein that enhances the activation of JNK by MEK kinase 1 and TGF-beta-activated kinase 1. FEBS Lett 457(3):385–388
Stirnimann CU, Petsalaki E, Russell RB, Muller CW (2010) WD40 proteins propel cellular networks. Trends Biochem Sci 35(10):565–574. https://doi.org/10.1016/j.tibs.2010.04.003
Macia MS, Halbritter J, Delous M, Bredrup C, Gutter A, Filhol E, Mellgren AE, Leh S et al (2017) Mutations in MAPKBP1 cause juvenile or late-onset cilia-independent nephronophthisis. Am J Human Genet 100(2):323–333. https://doi.org/10.1016/j.ajhg.2016.12.011
Hadad M, Aviram S, Darlyuk-Saadon I, Cohen-Katsenelson K, Whitmarsh AJ, Aronheim A (2015) The association of the JNK scaffold protein, WDR62, with the mixed lineage kinase 3, MLK3. MAP Kinase 4(1):5307
Zhang F, Yu J, Yang T, Xu D, Chi Z, Xia Y, Xu Z (2016) A novel c-Jun N-terminal kinase (JNK) signaling complex involved in neuronal migration during brain development. J Biol Chem 291(22):11466–11475. https://doi.org/10.1074/jbc.M116.716811
Kodani A, Yu TW, Johnson JR, Jayaraman D, Johnson TL, Al-Gazali L, Sztriha L, Partlow JN (2015) Centriolar satellites assemble centrosomal microcephaly proteins to recruit CDK2 and promote centriole duplication. eLife 4, e07519. https://doi.org/10.7554/eLife.07519
Lim NR, Yeap YY, Ang CS, Williamson NA, Bogoyevitch MA, Quinn LM, Ng DC (2016) Aurora A phosphorylation of WD40-repeat protein 62 in mitotic spindle regulation. Cell Cycle 15(3):413–424. https://doi.org/10.1080/15384101.2015.1127472
Lim NR, Yeap YY, Zhao TT, Yip YY, Wong SC, Xu D, Ang CS, Williamson NA et al (2015) Opposing roles for JNK and Aurora A in regulating the association of WDR62 with spindle microtubules. J Cell Sci 128(3):527–540. https://doi.org/10.1242/jcs.157537
Bogoyevitch MA, Ngoei KR, Zhao TT, Yeap YY, Ng DC (2010) c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim Biophys Acta 1804(3):463–475. https://doi.org/10.1016/j.bbapap.2009.11.002
Guarguaglini G, Duncan PI, Stierhof YD, Holmstrom T, Duensing S, Nigg EA (2005) The forkhead-associated domain protein Cep170 interacts with Polo-like kinase 1 and serves as a marker for mature centrioles. Mol Biol Cell 16(3):1095–1107. https://doi.org/10.1091/mbc.E04-10-0939
Rome P, Montembault E, Franck N, Pascal A, Glover DM, Giet R (2010) Aurora A contributes to p150(glued) phosphorylation and function during mitosis. J Cell Biol 189(4):651–659. https://doi.org/10.1083/jcb.201001144
Kettenbach AN, Schweppe DK, Faherty BK, Pechenick D, Pletnev AA, Gerber SA (2011) Quantitative phosphoproteomics identifies substrates and functional modules of Aurora and Polo-like kinase activities in mitotic cells. Sci Signal 4(179):rs5. https://doi.org/10.1126/scisignal.2001497
Barr AR, Gergely F (2007) Aurora-A: the maker and breaker of spindle poles. J Cell Sci 120(Pt 17):2987–2996. https://doi.org/10.1242/jcs.013136
Lee CY, Andersen RO, Cabernard C, Manning L, Tran KD, Lanskey MJ, Bashirullah A, Doe CQ (2006) Drosophila Aurora-A kinase inhibits neuroblast self-renewal by regulating aPKC/Numb cortical polarity and spindle orientation. Genes Dev 20(24):3464–3474. https://doi.org/10.1101/gad.1489406
Wang H, Somers GW, Bashirullah A, Heberlein U, Yu F, Chia W (2006) Aurora-A acts as a tumor suppressor and regulates self-renewal of Drosophila neuroblasts. Genes Dev 20(24):3453–3463. https://doi.org/10.1101/gad.1487506
Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH (2012) Astrocytes and disease: a neurodevelopmental perspective. Genes Dev 26(9):891–907. https://doi.org/10.1101/gad.188326.112
Sfakianos AP, Whitmarsh AJ, Ashe MP (2016) Ribonucleoprotein bodies are phased in. Biochem Soc Trans 44(5):1411–1416. https://doi.org/10.1042/BST20160117
Antoniou X, Borsello T (2012) The JNK signalling transduction pathway in the brain. Front Biosci 4:2110–2120
Sabapathy K (2012) Role of the JNK pathway in human diseases. Prog Mol Biol Transl Sci 106:145–169. https://doi.org/10.1016/B978-0-12-396456-4.00013-4
Sabapathy K, Jochum W, Hochedlinger K, Chang L, Karin M, Wagner EF (1999) Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech Dev 89(1–2):115–124
Westerlund N, Zdrojewska J, Padzik A, Komulainen E, Bjorkblom B, Rannikko E, Tararuk T, Garcia-Frigola C et al (2011) Phosphorylation of SCG10/stathmin-2 determines multipolar stage exit and neuronal migration rate. Nat Neurosci 14(3):305–313. https://doi.org/10.1038/nn.2755
Barr AR, Kilmartin JV, Gergely F (2010) CDK5RAP2 functions in centrosome to spindle pole attachment and DNA damage response. J Cell Biol 189(1):23–39. https://doi.org/10.1083/jcb.200912163
Knoblich JA (2008) Mechanisms of asymmetric stem cell division. Cell 132(4):583–597. https://doi.org/10.1016/j.cell.2008.02.007
Siller KH, Doe CQ (2008) Lis1/dynactin regulates metaphase spindle orientation in Drosophila neuroblasts. Dev Biol 319(1):1–9. https://doi.org/10.1016/j.ydbio.2008.03.018
Januschke J, Nathke I (2014) Stem cell decisions: a twist of fate or a niche market? Semin Cell Dev Biol 34:116–123. https://doi.org/10.1016/j.semcdb.2014.02.014
Insolera R, Bazzi H, Shao W, Anderson KV, Shi SH (2014) Cortical neurogenesis in the absence of centrioles. Nat Neurosci 17(11):1528–1535. https://doi.org/10.1038/nn.3831
Marjanovic M, Sanchez-Huertas C, Terre B, Gomez R, Scheel JF, Pacheco S, Knobel PA, Martinez-Marchal A et al (2015) CEP63 deficiency promotes p53-dependent microcephaly and reveals a role for the centrosome in meiotic recombination. Nat Commun 6:7676. https://doi.org/10.1038/ncomms8676
Kim S, Lehtinen MK, Sessa A, Zappaterra MW, Cho SH, Gonzalez D, Boggan B, Austin CA et al (2010) The apical complex couples cell fate and cell survival to cerebral cortical development. Neuron 66(1):69–84. https://doi.org/10.1016/j.neuron.2010.03.019
Higginbotham H, Guo J, Yokota Y, Umberger NL, Su CY, Li J, Verma N, Hirt J et al (2013) Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nat Neurosci 16(8):1000–1007. https://doi.org/10.1038/nn.3451
Tong CK, Han YG, Shah JK, Obernier K, Guinto CD, Alvarez-Buylla A (2014) Primary cilia are required in a unique subpopulation of neural progenitors. Proc Natl Acad Sci U S A 111(34):12438–12443. https://doi.org/10.1073/pnas.1321425111
Paridaen JT, Wilsch-Brauninger M, Huttner WB (2013) Asymmetric inheritance of centrosome-associated primary cilium membrane directs ciliogenesis after cell division. Cell 155(2):333–344. https://doi.org/10.1016/j.cell.2013.08.060
Pelletier L, Yamashita YM (2012) Centrosome asymmetry and inheritance during animal development. Curr Opin Cell Biol 24(4):541–546. https://doi.org/10.1016/j.ceb.2012.05.005
Conduit PT, Raff JW (2010) Cnn dynamics drive centrosome size asymmetry to ensure daughter centriole retention in Drosophila neuroblasts. Curr Biol 20(24):2187–2192. https://doi.org/10.1016/j.cub.2010.11.055
Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH (2009) Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature 461(7266):947–955. https://doi.org/10.1038/nature08435
Zhang Y, Tian Y, Yu JJ, He J, Luo J, Zhang S, Tang CE, Tao YM (2013) Overexpression of WDR62 is associated with centrosome amplification in human ovarian cancer. J Ovar Res 6(1):55. https://doi.org/10.1186/1757-2215-6-55
Liang Y, Gao H, Lin SY, Peng G, Huang X, Zhang P, Goss JA, Brunicardi FC et al (2010) BRIT1/MCPH1 is essential for mitotic and meiotic recombination DNA repair and maintaining genomic stability in mice. PLoS Genet 6(1):e1000826. https://doi.org/10.1371/journal.pgen.1000826
Pulvers JN, Bryk J, Fish JL, Wilsch-Brauninger M, Arai Y, Schreier D, Naumann R, Helppi J et al (2010) Mutations in mouse Aspm (abnormal spindle-like microcephaly associated) cause not only microcephaly but also major defects in the germline. Proc Natl Acad Sci U S A 107(38):16595–16600. https://doi.org/10.1073/pnas.1010494107
Zaqout S, Bessa P, Kramer N, Stoltenburg-Didinger G, Kaindl AM (2017) CDK5RAP2 is required to maintain the germ cell pool during embryonic development. Stem Cell Rep 8(2):198–204. https://doi.org/10.1016/j.stemcr.2017.01.002
Shinmura K, Kato H, Kawanishi Y, Igarashi H, Inoue Y, Yoshimura K, Nakamura S, Fujita H et al (2017) WDR62 overexpression is associated with a poor prognosis in patients with lung adenocarcinoma. Mol Carcinog 56(8):1984–1991. https://doi.org/10.1002/mc.22647
Zeng S, Tao Y, Huang J, Zhang S, Shen L, Yang H, Pei H, Zhong M et al (2013) WD40 repeat-containing 62 overexpression as a novel indicator of poor prognosis for human gastric cancer. Eur J Cancer 49(17):3752–3762. https://doi.org/10.1016/j.ejca.2013.07.015
Acknowledgements
DN acknowledges funding support from the National Health and Medical Research Council (APP1046032), Australian Research Council (FT120100193) and Cancer Council (APP1101931). NL was a recipient of a Melbourne International Research Scholarship from the University of Melbourne and BS is a recipient of a UQ International Scholarship from the University of Queensland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shohayeb, B., Lim, N.R., Ho, U. et al. The Role of WD40-Repeat Protein 62 (MCPH2) in Brain Growth: Diverse Molecular and Cellular Mechanisms Required for Cortical Development. Mol Neurobiol 55, 5409–5424 (2018). https://doi.org/10.1007/s12035-017-0778-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0778-x