Abstract
Dopamine D2 receptor (D2R) activation triggers both G protein- and β-arrestin-dependent signaling. Biased D2R ligands activating β-arrestin pathway have been proposed as potential antipsychotics. The ability of D2R to heteromerize with adenosine A2A receptor (A2AR) has been associated to D2R agonist-induced β-arrestin recruitment. Accordingly, here we aimed to demonstrate the A2AR dependence of D2R/β-arrestin signaling. By combining bioluminescence resonance energy transfer (BRET) between β-arrestin-2 tagged with yellow fluorescent protein and bimolecular luminescence complementation (BiLC) of D2R/A2AR homomers and heteromers, we demonstrated that the D2R agonists quinpirole and UNC9994 could promote β-arrestin-2 recruitment only when A2AR/D2R heteromers were expressed. Subsequently, the role of A2AR in the antipsychotic-like activity of UNC9994 was assessed in wild-type and A2AR−/− mice administered with phencyclidine (PCP) or amphetamine (AMPH). Interestingly, while UNC9994 reduced hyperlocomotion in wild-type animals treated either with PCP or AMPH, in A2AR−/− mice, it failed to reduce PCP-induced hyperlocomotion or produced only a moderate reduction of AMPH-mediated hyperlocomotion. Overall, the results presented here reinforce the notion that D2R/A2AR heteromerization facilitates D2R β-arrestin recruitment, and furthermore, reveal a pivotal role for A2AR in the antipsychotic-like activity of the β-arrestin-biased D2R ligand, UNC9994.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is a severe mental disorder that affects approximately 1% of the general population [1]. It is characterized by positive (e.g., delusions, hallucinations), negative (e.g., social withdrawal, lack of emotional expression), and cognitive symptoms, which are typically of lifelong duration [2]. Current treatment is based on dopamine D2 receptor (D2R) antagonists or weak partial agonists, which may block the excessive dopaminergic activity in the mesolimbic pathway thought to underlie positive symptoms [3]. Antipsychotics are classified into typical and atypical (or first and second generation, respectively) drugs, based on their side-effect profiles. Typical antipsychotics are effective in reducing positive, but not negative, symptoms, but prone to cause severe Parkinson-like motor side effects, so-called extrapyramidal symptoms. Atypical antipsychotics have lower propensity to disturb motor function, but while effective against positive symptoms, still fail to adequately address negative and cognitive symptoms [2, 3].
D2R is coupled to several intracellular signaling pathways, including the classical Gαi/o pathway and the more recently discovered β-arrestin pathway, each of which can be activated to varying extents by different D2R ligands [4]. Recent studies found that current antipsychotics are antagonists or partial agonists at both the Gαi/o- and the β-arrestin pathways [5, 6]. It was further suggested that antipsychotic efficacy might be conferred by modulation of β-arrestin signaling, while a reduction of Gαi/o-pathway activity would be responsible for extrapyramidal symptoms. Interestingly, by modifying the scaffold of the partial agonist antipsychotic, aripiprazole, a new series of D2R selective ligands, the “UNC family,” was recently developed [7]. These ligands act as partial agonists for β-arrestin recruitment, without eliciting G protein-dependent signaling, and show antipsychotic-like efficacy and low propensity for motor inhibition in preclinical animal models assessing potential antipsychotic activity [8, 9]. Since β-arrestin expression is higher in cortex compared to the striatum, it was suggested that these ligands, by means of their partial agonist activity at β-arrestin recruitment, may exert agonist activity preferentially in cortical areas [9], offering a potential avenue towards simultaneously treating the striatal hyperdopaminergia and cortical hypodopaminergia believed to underlie positive and negative symptoms, respectively.
It is well established that direct receptor-receptor interactions between D2R and A2AR occur in striatal medium spiny neurons, which modulate the output of striatal circuitry [10, 11]. In addition, D2R/A2AR oligomerization has been shown to favor β-arrestin recruitment in heterologous systems [12, 13]. Hence, here we aimed to test whether the antipsychotic-like effects of one member of the UNC family, UNC9994, might involve A2AR-dependent, D2R-biased signaling. Accordingly, we first designed a robust methodology, based on bimolecular luminescence complementation (BiLC) and bioluminescence resonance energy transfer (BRET) to test the impact of A2AR expression on D2R signaling bias. and thereafter, we examined the effects of UNC9994 in pharmacological mouse models of psychosis both in wild-type (WT) and A2AR-deficient (A2AR−/−) mice.
Materials and Methods
Reagents
The ligands used were amphetamine (AMPH), CGS21680, quinpirole, phencyclidine (PCP) from Tocris Bioscience (Bristol, UK), and UNC9994 from Axon Medchem B.V. (Groningen, the Netherlands).
Plasmid Constructs
To perform BiLC, we used two complementary fractions of the Rluc8 (Renilla luciferase 8) protein (L1 and L2) kindly provided by Dr. J.A. Javitch (University of Columbia, NY, USA). Both fractions were extracted from its template by digestion with XhoI and XbaI restriction enzymes, and inserted in a pcDNA3.1 vector (pcDNA3.1-L1 and pcDNA3.1-L2). Subsequently, the complementary DNA (cDNA) encoding D2R [14] and A2AR [15] were amplified by a polymerase chain reaction using the following primers: FD2RHind (5′-GCCAAGCTTATGGTCCTTCTGTTGATCCTGTCAG-3′) and RD2REco (5′-CCGGAATTCGGCAGTGGAGGATCTTCAG-3′) and FA2AHind (5′-GCCAAGCTTATGGTCCTTCTGTTGATCCTGTCAG-3′) and RA2AXho (5′-GCGCTCGAGAGGACACTCCTGCTCCATCC-3′). Finally, the different PCR products were subcloned into the HindIII/EcoRI or HindIII/XhoI sites (for D2R and A2AR, respectively) of the above-mentioned pcDNA3.1-L1 and pcDNA3.1-L2 vectors. On the other hand, for BRET experiments, we also used G-proteins and β-arrestin-2 constructs containing a yellow fluorescent protein (YFP): Gαs YFP and Gαi YFP, kindly provided by Dr. J.P. Vilardaga (University of Pittsburgh, Pittsburgh, USA), and β-arrestin-2YFP [12].
Cell Culture and Transfection
Human embryonic kidney (HEK)-293 T cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, Saint Louis, MO, USA) supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 100 U/mL streptomycin, 100 mg/mL penicillin, and 5% (v/v) fetal bovine serum at 37 °C and in an atmosphere of 5% CO2. HEK-293 T cells growing in 25-cm2 flasks or six-well plates containing 18-mm coverslips were used for BRET or fluorescence imaging, respectively. Cells were transiently transfected with the cDNA encoding the specified proteins using polyethylenimine (Polysciences Inc., Warrington, PA, USA).
Bioluminescence Resonance Energy Transfer Measurements
BRET experiments in HEK-293 T cells were performed as previously described [16]. In brief, HEK-293 T expressing the indicated constructs were rapidly washed, detached, and resuspended in HBSS buffer (137 mM NaCl, 5 mM KCl, 0.34 mM Na2HPO4, 0.44 mM KH2PO4, 1.26 mM CaCl2, 0.4 mM MgSO4, 0.5 mM MgCl2, 10 mM HEPES, pH 7.4) containing 10 mM glucose. Cell suspensions (20 μg of protein) were distributed in 96-well microplate plates, incubated with the corresponding ligands, and finally, BRET was determined, after adding 5 μM h-coelenterazine (NanoLight Technology, Pinetop, AZ, USA), in a POLARstar Optima plate reader (BMG Labtech, Durham, NC, USA) as previously described [16].
Animals
CD-1 mice (Charles River Laboratories) and A2AR−/− mice developed in a CD-1 genetic background [17] (animal facility of University of Barcelona) of around 3 months old were used. The University of Barcelona Committee on Animal Use and Care approved the protocol. Animals were housed and tested in compliance with the guidelines described in the Guide for the Care and Use of Laboratory Animals [18] and following the European Union directives (2010/63/EU). All efforts were made to minimize animal suffering and the number of animals used. All animals were housed in groups of five in standard cages with ad libitum access to food and water and maintained under 12-h dark/light cycle (starting at 7:30 AM), 22 °C temperature, and 66% humidity (standard conditions). Behavioral testing was performed in mice aged 2–3 months and between 2 and 7 PM.
Locomotor Activity
Horizontal locomotor activity was studied in an open-field arena measuring 30 × 30 cm, made from plywood, and painted black. Following i.p. injection with UNC9994 dissolved in physiological saline supplemented with 10% Tween-80 and 7.3% DMSO, or vehicle (VEH), animals were placed in the arena for an initial habituation period of 30 min, after which the animals were injected (i.p.) with 6 mg/kg PCP or 3 mg/kg AMPH (both dissolved in saline) and immediately returned to the arena for another 60 min. Locomotion was recorded by a camera placed above the arena and analyzed using ImageJ (National Institutes of Health, Bethesda, MD) together with an automated tracking plug-in (SpotTracker; Ecole Polytechnique Fédérale de Lausanne, Switzerland).
Statistics
The number of samples/animals (n) in each experimental condition is indicated in figure legends. Statistical analysis was performed by Student’s t test and by two-way ANOVA followed by Bonferroni’s multiple comparison post hoc test. Statistical significance is indicated for each experiment.
Results
A number of strategies have been designed to study biased GPCR signaling [19]. Here, we developed a novel approach to assay Gαi/o- and β-arrestin signaling from the D2R/A2AR oligomer. Accordingly, we used a BiLC/BRET assay, in which a BRET process between G-proteins/β-arrestin and receptors takes place only when receptors interact in close proximity (Fig. 1). Noteworthy, this kind of approach was first described as complemented donor-acceptor resonance energy transfer (CODA-RET), in which BRET was engaged after complementation of Rluc (the two complementary halves of Rluc separately fused to two different receptors) and YFP fused to a Gα subunit [20]. We first assessed bicomplementation of the donor molecules by determining luminescence after transfection of the corresponding D2R and/or A2AR forms containing complementary RLuc fragments. Thus, we transfected increasing concentrations of RLuc1 and RLuc2-containing proteins (A2ARL1/A2ARL2, D2RL1/D2RL2, A2ARL1/D2RL2) in a 1:1 ratio and observed a BRET saturation bell-shaped curve in all cases (Supplementary Fig. 1a). Similarly, we examined acceptor molecules (Gαs YFP, Gαi YFP, β-arrestin-2YFP) to achieve similar fluorescence levels (Supplementary Fig. 1b). Once donor and acceptor molecules had been characterized, we performed the BiLC/BRET assay by co-transfecting the constructs and challenging transfected cells with selective agonists (Fig. 1). First, we examined the ability of A2AR/A2AR and D2R/D2R homodimers to interact with Gαs and Gαi proteins, respectively. Both the adenosine A2AR agonist CGS21680 (100 nM) and D2R agonist quinpirole (100 nM) could recruit Gαs and Gαi proteins to A2ARL1/A2ARL2 and D2RL1/D2RL2 homodimers, respectively (Fig. 2a, b). Of note, the former single doses of CGS21680 and quinpirole were chosen as those eliciting similar emission levels (Supplementary Fig. 2), and used for subsequent experiments. Next, we assessed whether the effects of selective agonists were affected upon D2R/A2AR oligomerization. We observed that CGS21680-induced Gαs YFP and quinpirole-induced Gαi YFP recruitment produced emission levels like those obtained with the respective receptor homodimers (Fig. 2a, b), indicating that G-protein signaling was not modified when receptors heterodimerized. We then followed the same approach to evaluate the interaction of both homodimers and heterodimers with β-arrestin-2. Notably, both the A2ARL1/A2ARL2 homodimer and the A2ARL1/D2RL2 heterodimer recruited β-arrestin-2 when challenged with CGS21680, inducing a comparable BRET signal (Fig. 2c). Thus, although it cannot be excluded that the ability of A2AR to recruit β-arrestin-2 could be affected upon D2R expression, we did not observe significant changes in the present conditions. Conversely, only the A2ARL1/D2RL2 oligomer but not the D2RL1/D2RL2 homodimer interacted with β-arrestin-2 when challenged with quinpirole (Fig. 2d). Overall, these results indicate that A2AR co-expression increased the ability of D2R to signal via the β-arrestin pathway.
Schematic representation of the BiLC/BRET assay. Agonist (blue triangles) binding to A2AR/D2R heterodimer complementing two halves of the RLuc8 protein (L1 and L2) prompted YFP-tagged G-protein or β-arrestin recruitment. The A2AR/D2R heterodimer interaction with either G-protein or β-arrestin was monitored by BiLC/BRET. Red circles indicate the luciferase substrate coelenterazine
A2AR-dependent biased activation of the D2R. a, c BRET was measured in HEK293T cells co-expressing either A2ARL1/A2ARL2 homodimers or A2ARL1/D2RL2 heterodimers as donors and Gαs YFP or β-arrestin-2YFP proteins as acceptors (as indicated in each panel), and challenged with the selective A2AR agonist CGS21680 (100 nM). b, d BRET was measured in HEK293T cells co-expressing either D2RL1/D2RL2 homodimers or A2ARL1/D2RL2 heterodimers as donors and Gαi YFP or β-arrestin-2YFP proteins as acceptors (as indicated in each panel), and challenged with the selective D2R agonist quinpirole (100 nM). Data (expressed as arbitrary units (AUs)) represent the mean ± SEM of at least three independent experiments. Statistical significance was assessed using a paired Student’s t test (*P < 0.05)
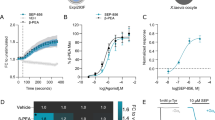
As described above, a series of β-arrestin-biased D2R ligands, namely, the UNC family, has been shown to have antipsychotic-like activity [8, 9]. Therefore, we aimed to elucidate whether the activity of these UNC compounds is A2AR dependent. To this end, we selected UNC9994 as it was recently demonstrated to be the most arrestin-selective drug, both in terms of its incapacity to antagonize G protein-dependent D2R signaling in vitro, and its antipsychotic-like inefficacy in mice lacking β-arrestin-2 in D2R-expressing neurons [7]. Accordingly, we evaluated the UNC9994 ability to selectively recruit β-arrestin-2 upon A2AR expression by using our BiLC/BRET assay. Interestingly, UNC9994 (300 nM) [7] produced a significant (P < 0.05) BRET signal between receptors and β-arrestin-2 only upon D2R-A2AR heteromerization (Fig. 3). On the other hand, no BRET signal was observed between D2R containing homodimers and heterodimers with Gαi YFP (Fig. 3), as previously reported [7, 9]. Overall, UNC9994 β-arrestin-2-biased signaling was also shown to depend on A2AR expression in our heterologous system. Subsequently, we next aimed to correlate the observed in vitro functional selectivity with potential in vivo therapeutic effects in pharmacological mouse models of psychosis. To this end, we examined the ability of UNC9994 to reduce hyperlocomotion induced by PCP (6 mg/kg) or AMPH (3 mg/kg) in WT and A2AR−/− mice. Of note, PCP- and AMPH-induced hyperactivity have become frequently used rodent models of psychosis, and its reversal by drugs is considered a useful measure for predicting clinical antipsychotic activity [21,22,23]. Interestingly, while 10 mg/kg UNC9994 (i.p.) robustly reduced PCP-induced hyperlocomotion in WT animals (Fig. 4a), it was ineffective in A2AR−/− mice (Fig. 4b). Thus, when examining the cumulative distance traveled (Fig. 4c), the two-way ANOVA analysis revealed a significant effect of UNC9994 treatment [F (1, 37) = 17.54, P < 0.001], but not of genotype, and a significant interaction between genotype and drug treatment [F (1, 37) = 6.63, P < 0.05]. Also, Bonferroni-corrected pairwise comparisons detected a significant difference between UNC9994-pretreated WT and A2AR−/− animals (*P < 0.05), whereas no differences were observed between vehicle (VEH)-pretreated WT and A2AR−/− animals. Overall, only upon A2AR expression, an effect of UNC9994 on reducing PCP-induced hyperlocomotion was observed. On the other hand, we also assessed the effects of AMPH on hyperlocomotion. Interestingly, UNC9994 (10 mg/kg, i.p.) significantly reduced AMPH-induced hyperlocomotion both in WT and A2AR−/− mice (Fig. 5a, b). However, a close view of the results obtained showed that the effect of UNC9994 in A2AR−/− mice was lower than in WT mice. Accordingly, when analyzing the cumulative distance traveled (Fig. 5c), the two-way ANOVA revealed significant main effects of UNC9994 treatment (F (1, 36) = 32.25, P < 0.001) and genotype (F (1, 36) = 7.74, P < 0.01), but no significant interaction between genotype and drug treatment. Also, Bonferroni-corrected pairwise comparisons detected a significant difference between UNC9994-pretreated WT and A2AR−/− animals (*P < 0.05), whereas no such difference was observed between VEH-pretreated WT and A2AR−/− animals. Overall, the ability of UNC9994 to block AMPH-induced hyperlocomotion was reduced in the absence of A2AR expression, thus supporting the notion that A2AR may lead to the biased activity of the D2R ligand.
UNC9994 as a β-arrestin-2-biased D2R ligand in the BiLC/BRET assay. BRET was measured in HEK293T cells co-expressing either D2RL1/D2RL2 homodimers or A2ARL1/D2RL2 heterodimers as donors and Gαi YFP or β-arrestin-2YFP proteins as acceptors (as indicated in each panel), and challenged with UNC9994 (300 nM). Data (expressed as arbitrary units (AUs)) represent the mean ± SEM of at least three independent experiments. Statistical significance was assessed using a paired Student’s t test (*P < 0.05)
Effects of the β-arrestin-2-biased D2R ligand, UNC9994, on PCP-induced hyperlocomotion in WT and A2AR−/− mice. a, b PCP-induced locomotor activity was assessed in WT and A2AR−/− mice (n = 10–12) either pretreated with vehicle (VEH) or UNC9994 (UNC). VEH or UNC (10 mg/kg, i.p.) was administered immediately prior to introducing the animals to the arena, whereas PCP (6 mg/kg, i.p.) was given at 30 min. c Quantified locomotor activity in WT and A2AR−/− animals pretreated with VEH or UNC after PCP administration. Significant differences were found between UNC-pretreated WT and A2AR−/− animals (*P;0.05, two-way ANOVA followed by Bonferroni-corrected pairwise comparisons)
Effects of the β-arrestin-biased D2R ligand, UNC9994, on AMPH-induced hyperlocomotion in WT and A2AR−/− mice. a, b AMPH-induced locomotor activity was assessed in WT and A2AR−/− mice (n = 10) pretreated with vehicle (VEH) or UNC9994 (UNC). VEH or UNC (10 mg/kg, i.p.) was administered immediately prior to introducing the animals to the arena, whereas AMPH (3 mg/kg, i.p.) was given at 30 min. c Quantified locomotor activity in WT and A2AR−/− animals pretreated with UNC or VEH after AMPH administration. Significant differences were found between UNC-pretreated WT and A2AR−/− animals (*P < 0.05, two-way ANOVA followed by Bonferroni-corrected pairwise comparisons)
Discussion
The development of drugs displaying antipsychotic efficacy without extrapyramidal side effects is a major goal in drug discovery. Biased D2R agonists triggering β-arrestin activation without G-protein coupling have been postulated to be potential antipsychotic candidates [7,8,9]. Interestingly, the concept of functional selectivity or biased signaling has emerged in recent years as a novel mechanism to optimize drug therapeutic actions. Thus, functionally selective ligands, by promoting distinct conformational rearrangements and preferential activation of signaling pathways, may lead to different receptors’ signaling outcomes (for review, see [24,25,26]). Accordingly, these kinds of drugs, by discriminating mechanisms leading to therapeutic or undesired effects, may potentially permit to achieve better benefit/risk balances. Here, we assessed the impact of A2AR in the antipsychotic-mediated effects of UNC9994, a D2R/β-arrestin bias compound. Our hypothesis was based on previous results indicating that striatal allosteric D2R-A2AR interactions favor D2R β-arrestin-2 recruitment [12, 13].
We developed a new BiLC/BRET assay allowing the study of D2R/A2AR heteromer signaling. Thus, by using our BiLC/BRET approach, we unequivocally demonstrated that D2R agonist-mediated β-arrestin-2 recruitment was A2AR dependent. While our results are in agreement to those showing D2R/A2AR heteromer-dependent, D2R agonist-mediated β-arrestin-2 recruitment [12, 13], we further demonstrated for the first time the D2R/A2AR/β-arrestin-2 trimeric formation upon agonist challenge. Indeed, it has been reported that upon certain experimental conditions (e.g., high D2R agonist concentrations or different D2R/β-arrestin-2 ratios), the D2R is able to signal through β-arrestin in an A2AR-independent manner [9]. Nevertheless, the A2AR dependencies of D2R/β-arrestin-signaling under physiological conditions are yet unexplored. Thus, we assessed the impact of A2AR in D2R/β-arrestin signaling in vivo by evaluating the UNC9994-mediated antipsychotic-like effect in A2AR-deficient mice. Interestingly, our behavioral data point to a scenario in which the striatal D2R/A2AR-β-arrestin-2 module would be involved in the antipsychotic-like effects of UNC9994. Needless to say, although A2AR has a very restrictive localization in the brain, thus being mainly expressed in the striatum and olfactory bulb [27], it cannot be excluded the possibility that allosteric A2AR-D2R interactions in other brain areas may participate in modulating the effects of UNC9994. In the striatum, the A2AR exerts a fine-tuning regulation of D2R activity [28], which among other mechanisms may involve the enhancement of β-arrestin-2 recruitment. In order to ascertain whether the in vivo actions of a β-arrestin-biased ligand (i.e., UNC9994) was effectively dependent on A2AR expression, we evaluated the effects of UNC9994 on PCP- and AMPH-induced hyperlocomotion in WT and A2AR−/− mice. In agreement with previous reports [7, 9], UNC9994 significantly reduced both PCP- and AMPH-induced hyperactivity in WT mice. Conversely, in A2AR−/− mice, UNC9994 failed to block PCP-mediated locomotor effects and partially inhibited AMPH-induced hyperlocomotion when compared to WT animals.
Our findings may be considered within the framework of the recent Urs and collaborator’s work [7]. Importantly, these authors demonstrated that the action of UNC9994 was dependent on β-arrestin-2 expression both in cortical and striatal regions. Thus, although UNC9994 efficacy against PCP-induced hyperlocomotion persisted in mice where β-arrestin-2 had been selectively deleted in A2AR-expressing neurons, a loss of efficacy against AMPH-induced hyperlocomotion was observed in such animals, seemingly conflicting with the present findings. However, these authors further demonstrated a dual involvement of striatal and cortical mechanisms in mediating the behavioral effects of both PCP and UNC9994. Thus, PCP-induced hyperlocomotion was itself reduced when D2R was deleted in A2AR-expressing neurons, and UNC9994 activity against PCP-induced hyperlocomotion persisted also when β-arrestin-2 was selectively deleted in prefrontal cortex. Hence, some of the discrepancies observed between the present study and that of Urs and collaborators may be explained by the fact that several different brain regions (including cortical and striatal) are involved in mediating the behavioral output. On the other hand, it should be noted that although from our data it might be inferred that the effects of UNC9994 regulating locomotor activity are dependent both on A2AR and β-arrestin-2 expressed at GABAergic striatopallidal neurons, A2AR expression is not only circumscribed to the former neurons. In such way, A2ARs are also located at corticostriatal terminals and astroglia tightly regulating glutamate transport activity; thus, they may be involved on the control of psychostimulant or psychomimetic-induced effects, as previously shown [29, 30].
In conclusion, the present study extended previous findings regarding the influence of the A2AR over D2R signaling, and found evidence for an important role of the A2AR in enabling the arrestin-biased D2R ligand, UNC9994, to mediate antipsychotic-like effects in two pharmacological mouse models of psychosis. Indeed, these findings increase our understanding of the role of D2R-A2AR interactions and may be valuable for future drug development efforts targeting these receptors.
References
Janoutová J, Janácková P, Serý O et al (2016) Epidemiology and risk factors of schizophrenia. Neuro Endocrinol Lett 37:1–8
Sanger DJ (2004) The search for novel antipsychotics: pharmacological and molecular targets. Expert Opin Ther Targets. doi:10.1517/14728222.8.6.631
Miyamoto S, Miyake N, Jarskog LF et al (2012) Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry 17:1206–1227. doi:10.1038/mp.2012.47
Brust TF, Hayes MP, Roman DL et al (2015) Bias analyses of preclinical and clinical D2 dopamine ligands: studies with immediate and complex signaling pathways. J Pharmacol Exp Ther 352:480–493. doi:10.1124/jpet.114.220293
Masri B, Salahpour A, Didriksen M et al (2008) Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci U S A. doi:10.1073/pnas.0803522105
Klewe IV, Nielsen SM, Tarpø L et al (2008) Recruitment of beta-arrestin2 to the dopamine D2 receptor: insights into anti-psychotic and anti-parkinsonian drug receptor signaling. Neuropharmacology 54:1215–1222. doi:10.1016/j.neuropharm.2008.03.015
Urs NM, Gee SM, Pack TF et al (2016) Distinct cortical and striatal actions of a β-arrestin-biased dopamine D2 receptor ligand reveal unique antipsychotic-like properties. Proc Natl Acad Sci U S A. doi:10.1073/pnas.1614347113
Park SM, Chen M, Schmerberg CM et al (2016) Effects of β-arrestin-biased dopamine D2 receptor ligands on schizophrenia-like behavior in hypoglutamatergic mice. Neuropsychopharmacology 41:704–715. doi:10.1038/npp.2015.196
Allen JA, Yost JM, Setola V et al (2011) Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci U S A 108:18488–18493. doi:10.1073/pnas.1104807108
Fuxe K, Ferre S, Zoli M, Agnati LF (1998) Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res Res Rev 26:258–273
Fernández-Dueñas V, Taura JJ, Cottet M et al (2015) Untangling dopamine-adenosine receptor-receptor assembly in experimental parkinsonism in rats. Dis Model Mech. doi:10.1242/dmm.018143
Borroto-Escuela DO, Romero-Fernandez W, Tarakanov AO et al (2011) On the existence of a possible A2A-D2-beta-arrestin2 complex: A2A agonist modulation of D2 agonist-induced beta-arrestin2 recruitment. J Mol Biol 406:687–699. doi:10.1016/j.jmb.2011.01.022
Huang L, Wu D, Zhang L, Feng L (2013) Modulation of A2a receptor antagonist on D2 receptor internalization and ERK phosphorylation. Acta Pharmacol Sin 34:1292–1300. doi:10.1038/aps.2013.87
Ciruela F, Burgueno J, Casado V et al (2004) Combining mass spectrometry and pull-down techniques for the study of receptor heteromerization. Direct epitope-epitope electrostatic interactions between adenosine A2A and dopamine D2 receptors. Anal Chem 76:5354–5363. doi:10.1021/ac049295f
Cabello N, Gandia J, Bertarelli DC et al (2009) Metabotropic glutamate type 5, dopamine D(2) and adenosine A(2a) receptors form higher-order oligomers in living cells. J Neurochem 109:1497–1507. doi:10.1111/j.1471-4159.2009.06078.x
Ciruela F, Fernández-Dueñas V (2015) GPCR oligomerization analysis by means of BRET and dFRAP. Methods Mol Biol 1272:133–144
Ledent C, Vaugeois JM, Schiffmann SN et al (1997) Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388:674–678. doi:10.1038/41771
Clark JD, Gebhart GF, Gonder JC et al (1997) Special report: the 1996 guide for the care and use of laboratory animals. ILAR J 38:41–48
Denis C, Saulière A, Galandrin S et al (2012) Probing heterotrimeric G protein activation: applications to biased ligands. Curr Pharm Des 18:128–144
Urizar E, Yano H, Kolster R et al (2011) CODA-RET reveals functional selectivity as a result of GPCR heteromerization. Nat Chem Biol 7:624–630. doi:10.1038/nchembio.623
Nordquist RE, Risterucci C, Moreau JL et al (2008) Effects of aripiprazole/OPC-14597 on motor activity, pharmacological models of psychosis, and brain activity in rats. Neuropharmacology 54:405–416. doi:10.1016/j.neuropharm.2007.10.010
Large CH (2007) Do NMDA receptor antagonist models of schizophrenia predict the clinical efficacy of antipsychotic drugs? J Psychopharmacol 21:283–301. doi:10.1177/0269881107077712
Powell SB, Geyer Ma (2007) Overview of animal models of schizophrenia. Curr Protoc Neurosci Chapter 9:Unit 9.24. doi: 10.1002/0471142301.ns0924s39
Urban JD, Clarke WP, von Zastrow M et al (2006) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13. doi:10.1124/jpet.106.104463
Rozenfeld R, Devi LA (2007) Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J 21:2455–2465. doi:10.1096/fj.06-7793com
Guitart X, Navarro G, Moreno E et al (2014) Functional selectivity of allosteric interactions within G protein-coupled receptor oligomers: the dopamine D1–D3 receptor heterotetramer. Mol Pharmacol 86:417–429. doi:10.1124/mol.114.093096
Fredholm BB, IJ AP, Jacobson KA et al (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev 63:1–34. doi:10.1124/pr.110.003285
Fuxe K, Borroto-Escuela DO, Romero-Fernandez W et al (2014) Moonlighting proteins and protein-protein interactions as neurotherapeutic targets in the G protein-coupled receptor field. Neuropsychopharmacology 39:131–155. doi:10.1038/npp.2013.242
Matos M, Shen HY, Augusto E et al (2015) Deletion of adenosine A2A receptors from astrocytes disrupts glutamate homeostasis leading to psychomotor and cognitive impairment: relevance to schizophrenia. Biol Psychiatry 78:763–774. doi:10.1016/j.biopsych.2015.02.026
Shen HY, Canas PM, Garcia-Sanz P et al (2013) Adenosine a2A receptors in striatal glutamatergic terminals and GABAergic neurons oppositely modulate psychostimulant action and DARPP-32 phosphorylation. PLoS One. doi:10.1371/journal.pone.0080902
Acknowledgements
This work was supported by MINECO/ISCIII (SAF2014-55700-P and PIE14/00034), the Catalan Government (2014 SGR 1054), Fundació la Marató de TV3 (Grant 20152031), and FWO (SBO-140028) to FC. We thank Esther Castaño and Benjamín Torrejón from the Scientific and Technical Services (CCiT) group at the Bellvitge Campus of the University of Barcelona for their technical assistance. KS is a recipient of a postdoctoral grant from the Swedish Brain Foundation.
Author information
Authors and Affiliations
Contributions
FC and VF-D designed the research; KS, MG-S, MV, VF-D, ML-C, and JJT performed the research; KS MG-S, VF-D, and FC analyzed the data; and VF-D, KS, and FC wrote the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Electronic Supplementary Material
Supplementary Fig. 1
Characterization of the donor and acceptor molecules in the BiLC/BRET assay. (a) Bicomplementation of the D2R and A2AR oligomers (A2ARL1/A2ARL2, D2RL1/D2RL2; A2ARL1/D2RL2) was assessed by determining luminiscence in HEK293T transfected cells with increasing concentrations of the different constructs. (b) Similarly, increasing concentrations of the acceptor molecules (Gαs YFP, Gαi YFP or β-arrestin-2YFP) were transfected in HEK293T cells and fluorescence levels determined. Data (expressed as arbitrary units, AU) represent the mean ± s.e.m. of at least three independent experiments. (GIF 48 kb)
Supplementary Fig. 2
BiLC/BRET assay for the D2R and A2AR homodimers and the respective canonical G-proteins. (a) Dose-response curve for quinpirole activating HEK293T cells transfected with the D2RL1/D2RL2 homodimer and Gαi YFP. (b) Dose-response curve for CGS21680 activating HEK293T cells transfected with the A2ARL1/A2ARL2 homodimer and Gαs YFP. Data (expressed as arbitrary units, AU) represent the mean ± s.e.m. of at least three independent experiments. (GIF 30 kb)
Rights and permissions
About this article
Cite this article
Sahlholm, K., Gómez-Soler, M., Valle-León, M. et al. Antipsychotic-Like Efficacy of Dopamine D2 Receptor-Biased Ligands is Dependent on Adenosine A2A Receptor Expression. Mol Neurobiol 55, 4952–4958 (2018). https://doi.org/10.1007/s12035-017-0696-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0696-y