Abstract
N-acetylcysteine (NAC), a precursor of glutathione that reduces reperfusion-induced injury, has been shown protection when it was administered pre-ischemia. However, less is known about the effect when it was given post-ischemia and there is no positive result associated with anti-oxidant in clinical trials. This study investigated the neuro- and vaso-protection of post-ischemia NAC administration as well as combining NAC with normobaric hyperoxia (NBO). Male Sprague–Dawley rats were exposed to NBO or normoxia during 2-h occlusion of the middle cerebral artery, followed by 48-h reperfusion. NAC or vehicle was intraperitoneally administered to rats immediately before reperfusion onset. NAC and NBO treatments produced 1.2 and 30 % reduction of infarction volume, respectively, and combination treatment showed greater reduction (59.8 %) as well as more decrease of hemispheric swelling volume. Of note, combination therapy showed improved neurological assessment and motor function which were sustained for 7 days after reperfusion. We also determined that the combination therapy showed greater inhibitory effects on tight junction protein degradation accompanied by Evan’s blue extravasation, hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF) induction, and poly ADP-ribose polymerase (PARP)-1 activation in ischemic brain tissue. Our results showed that although post-ischemia NAC administration had limited protection, combination treatment of NAC plus NBO effectively prevented blood–brain barrier (BBB) damage and significantly improved the outcome of brain injury, providing new evidence to support the concept that “cocktail” treatment targeting different stages provides better neuro- and vaso-protection than current individual treatment that has all failed in their clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The primary goal of current treatment for acute ischemic stroke is to salvage the ischemic penumbra [1], which will progress to irreversible damage if reperfusion could not be attained. This damage was primarily driven by ischemia-induced hypoxia and the subsequent bioenergetic failure [2], followed by reperfusion-induced injury [3]. This penumbra concept has inspired the development of drugs to interrupt cell death pathways [4].
Neuro-protection alone without restoration of tissue perfusion and vascular integrity may not be adequate for treatment of acute stroke [5]. However, when reperfusion is attained, additional irreversible damage can also develop following reperfusion due to a mechanism of reperfusion injury including inflammation, oxidative stress, and excitatory injury [3, 6]. N-acetylcysteine (NAC), a precursor of glutathione and used as mucolyticum in clinic, acts as an anti-oxidant and is also a scavenger of oxygen free radicals. Preconditioning (pre-ischemia) with NAC has been shown to reduce the reperfusion injury and infarct size in animal models of transient and permanent middle cerebral artery occlusion (MCAO) mainly by its anti-inflammatory potential [7, 8]. However, little is known about the effect of post-ischemia NAC administration in focal ischemia.
Clinical trials of neuro-protection drugs have been very disappointing, and there is no positive result associated with anti-oxidant [9]. The multi-stage progression of ischemic brain damage motivated the scientists to seek combinational therapeutic approaches targeting injury at different stages to treat acute ischemic stroke [10, 11].
Tissue damage was mainly induced by oxygen and energy loss during ischemia, a simplistic but plausible strategy to reduce ischemic injury is to improve tissue oxygenation, and normobaric hyperoxia (NBO) has been shown to effectively reduce tissue infarction and protect the blood–brain barrier (BBB) in animal ischemic stroke models [12–14]. The neuro- and vaso-protection makes NBO a promising approach to extend the narrow time window of the reperfusion therapies for ischemic stroke [13, 15]. NBO has been used to expand the neuro-protection effect of cilostazol [16], edaravone [17], minocycline [12], melatonin [18], and ethanol [19, 20].
In this study, we investigated the neuro- and vaso-protection of post-ischemia NAC administration as well as combining NAC (targeting reperfusion) with normobaric hyperoxia (NBO, targeting ischemia). Furthermore, underlying molecular mechanisms of the protection were investigated.
Materials and Methods
Animal Model of Focal Cerebral Ischemia and Reperfusion
Sprague–Dawley rats (SLAC Company, Shanghai, China) weighing 270 to 290 g were anesthetized with isoflurane (4 % for induction, 1.75 % for maintenance) and subjected to MCAO surgery using suture occlusion model, as we previously described [21]. No rats died because of stroke or surgical complications. The University Committee on Animal Care of Soochow University approved all experimental protocols, and all animal procedures were performed according to the NIH Guide for the Care and Use of Laboratory Animals.
Treatment Protocol
After MCAO, animals were randomly assigned into four different treatment groups: (1) air plus vehicle group (Air+Veh), (2) air plus N-acetylcysteine (NAC) group (Air+NAC), (3) NBO plus NAC group (NBO+NAC), and (4) normobaric hyperoxia (NBO) plus vehicle group (NBO+Veh). NBO or air treatment: 5 min after the onset of MCAO, anesthesia was discontinued, and the rats were put into an air-tight box which was ventilated (3 L/min) with air (21 % O2, air) or a gas mixture of 95 % O2 + 5 % CO2 (NBO) until the end of 2-h MCAO. (2) NAC or vehicle treatment: NAC (Sigma) was dissolved in saline to the final concentration of 20 mg/mL. Five minutes before reperfusion onset, NAC at 60 mg/kg body weight or vehicle was administered to rats via intraperitoneal injection. Detail is shown in Fig. 1.
Behavioral Tests
Two types of behavioral functional tests were performed at 48 h or 7 days after reperfusion. The observer was blinded to the experimental conditions. (1) Neurological deficits were scored as previously described [21]. (2) Forelimb foot-fault-placing test was performed as described [22]. Each animal was tested individually.
Analysis of Ischemic Brain Damage
At 7 days after MCAO, rat was killed and brain was photographed using a digital camera to allow us to observe the changes on the brain surface. To quantify the degree of atrophy, surface area shrinkage (percentage of contralateral corresponding brain region) was used [16]. For quantifying acute 48-h infarct volumes, we used triphenyl-2,3,4-tetrazolium chloride (TTC) staining as we described [12]. Infarction volume was quantitated and expressed as a percentage of infarcted tissue as compared to the total volume, as we described previously [21].
For 7-day infarct volumes, we used neutral red staining for seven 40-μm coronal sections at +4.7, +2.7, +0.7, −1.3, −3.3, −5.3, and −7.3 mm from bregma [23]. Sections were digitalized and infarct area was measured using ImageJ.
Measurement of Brain Hemispheric Swelling
At the end of 48-h reperfusion, rats were transcardially perfused with cold PBS to remove intravascular blood. Brain hemispheric swelling was quantitated and expressed as a relative increase of the brain area in the ischemic hemisphere (I) versus the nonischemic hemisphere (NI), as we described previously [12].
EB Leakage Detection
Evan’s blue (EB) dye (2 % wt/vol in PBS) was intravenously administered (3 mL/kg) via the femoral vein at 47 h after reperfusion. At the end of reperfusion, the rats were transcardially perfused with PBS to remove intravascular EB. The brains were then removed, and BBB disruption was quantitatively assessed by measuring EB contents as we have reported [13].
Immunofluorescence for HIF-1α, VEGF, Claudin-5, and PAR
The 20-μm-thick cryosection was fixed with 4 % paraformaldehyde (PFA) for immunofluorescence analysis as we described previously [24]. In brief, sections were incubated overnight with primary antibody against hypoxia-inducible factor-1α (HIF-1α) (1:200 dilution, Novus), vascular endothelial growth factor (VEGF) (1:200 dilution, Abcam), claudin-5 (1:200 dilution, Invitrogen), or poly(ADP-ribose (PAR) (1:100 dilution, Axxora, LLC, San Diego, CA, diluted to 10 μg/mL) at 4 °C. The latter was followed by incubation with 488- or Cy3-conjugated secondary antibody (anti-rabbit, 1:800 dilution; anti mouse, 1:800 dilution) for 1.5 h at room temperature. Immunostaining was visualized under an LSM 700 microscope (Zeiss), and images were taken from the ischemic region and the mirrored region on the nonischemic hemisphere.
Western Blot Analysis for Claudin-5, Occludin, HIF-1α, VEGF, and PARP-1
Ischemic and nonischemic tissues were collected from the fourth coronal section. Tissue was homogenized with lysis buffer, and protein concentrations in the homogenates were determined using BCA protein assay kit (Beyotime). Aliquots of homogenates were subjected to western blot analysis. Homogenate aliquots (40 μg of total protein) were boiled and then electrophoresed in 10 % SDS-PAGE acrylamide gels, transferred onto PVDF membranes (Millipore), and incubated for 2 h in Tris-buffered saline and 0.1 % Tween 20 (TBS-T) containing 5 % nonfat milk. Membranes were then incubated overnight at 4 °C with primary antibodies against occludin (1:300, Invitrogen), claudin-5 (1:500, Invitrogen), HIF-1α (1:300, Novus), VEGF (1:500, Abcam), and poly ADP-ribose polymerase (PARP)-1 (1:2500, Cell Signaling Technology); washed in TBS-T; and then incubated for 2 h at room temperature with corresponding horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse antibodies (1:3000; Boster). The membranes were developed with the SuperSignal West Pico HRP substrate kit (Pierce) and photographed. Protein band intensities were quantitated after normalization to Coomassie blue staining of the same membrane.
In Situ Detection of Superoxide Anion Production
Superoxide anion was detected with the use of hydroethidine (Het) as previously described [21] with some modifications. Rats were administered intravenously 0.5 mg Het (Molecular Probes) 5 min before induction of ischemia, killed 48 h after reperfusion, and transcardially perfused with PBS and 4 % PFA. Brains were removed and fixed in 4 % PFA for 24 h and then sectioned into 40-μm-thick sections. The sections were photographed with a fluorescent microscope, and pictures were taken from the ischemic penumbra.
Statistical Analysis
The data are presented as means ± SEM. Statistical analysis was carried out with ANOVA followed by LSD post hoc test. A value of P < 0.05 was considered statistically significant.
Results
Effects of NAC and Combination Treatment on Brain Injury Induced by Two-Hour Ischemia and Forty-Eight Hour Reperfusion
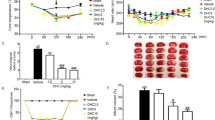
As expected, brain swelling was observed in the ischemic hemisphere of the Air+Veh group (Fig. 2c). NAC alone showed limited and NBO showed 10.9 % reduction of hemispheric enlargement; remarkably, their combination led to a 50.4 % reduction in hemispheric swelling volume compared to the Air+Veh group (P < 0.05).
Effects of NAC and combination treatment of NAC with NBO on infarction and hemispheric swelling. a Representative TTC-stained coronal sections showed tissue infarction in the ischemic (right) hemisphere of each group. Veh vehicle, NAC N-acetylcysteine. b Combination therapy group showed greater neuro-protection than mono treatment. c The combination therapy showed more reduction in hemispheric swelling formation. *P < 0.05 versus Air+Veh. Data are expressed as mean ± SEM, n = 6–9
TTC staining of the 2-mm-thick brain sections showed obvious infarction in the ischemic hemispheres (consistently seen in cortex and subcortex) of all groups of rats (Fig. 2a). NAC or NBO demonstrated a 1.2 and 30 % reduction of infarction volume (P > 0.05 compared to the Air+Veh). Their combination resulted in a greater reduction (59.8 %) of infarction volume (Fig. 2b, P < 0.05 versus Air+Veh).
Effects of NAC and Combination Treatment on Superoxide Production and PARP-1 Cleavage
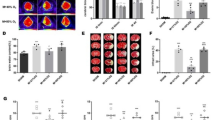
The fluorescent dye, Het, reacts with superoxide to produce ethidium (Et), which has been widely used as a biomarker of superoxide production [21]. As shown in Fig. 3a, signal for Het staining was markedly increased and lit up the shape of neuronal bodies in the ischemic hemisphere in Air+Veh group, suggesting the production of superoxide. Compared with Air+Veh group, NAC or NBO treatment made no difference in the intensity of the signal; however, combination treatment showed a marked decrease of signal (Fig. 3a).
Effects of NAC and combination treatment of NAC with NBO on superoxide generation, PARP-1 cleavage, and PAR accumulation. a Representative photomicrographs of fluorescent staining of oxidized hydroethidine (Het) signals from cortex and subcortex hemispheric tissue. Scale bar = 100 μm. b A representative western blot revealed cleaved PARP-1 changes in nonischemic (NI) and ischemic (I) hemisphere of each group. Cleaved PARP-1 protein was quantitated after normalization to the intensity of Coomassie blue staining and expressed as hemispheric ratio. Cerebral ischemia and reperfusion induced a 1.66-fold increase, and combination treatment showed better effect in inhibiting this increase. **P < 0.01 versus Air+Veh. Data are expressed as mean ± SEM, n = 6. c Immunofluorescence of brain sections stained with PAR antibody. Scale bar = 50 μm
PARP-1 plays an important role in cell death regulation, and PARP-1 cleavage is a marker for apoptotic cell death. Cleaved PARP-1 (89 kDa) was significantly increased in Air+Veh group, and combination but not NAC treatment showed significant reduction of cleaved PARP-1, and significant difference could be detected between combination group and Air+Veh group (Fig. 3b).
Accumulation of PAR is considered a surrogate marker of PARP-1 activation [22]. Therefore, we next explored the PAR levels. PAR-positive cells were not detectable in NI hemisphere; however, there is a dramatic increase of PAR-positive cells in the ischemic tissue (Fig. 3c). Combination but not NAC treatment showed significant reduction of PAR accumulation, suggesting that PARP-1 contributed to ischemic brain damage and only combination treatment could inhibit PARP-1 activation.
Effects of NAC and Combination Treatment on BBB Damage
EB was used as a marker in evaluating BBB permeability. EB leakage increased significantly in the brain from rats of Air+Veh group. Our results showed that NAC could partially reduce BBB damage and combination treatment showed a greater reduction of EB leakage (P < 0.05), indicating a better protection of combination treatment on BBB integrity (Fig. 4a).
Effects of NAC and combination treatment of NAC with NBO on BBB damage and tight junction protein degradation. a EB leakage was quantitated, and combination treatment significantly decreased ischemia–reperfusion-induced EB leakage (P < 0.05, n = 6). b Immunostaining results revealed that claudin-5 was degraded, and combination treatment could inhibit the claudin-5 degradation. Scale bar = 50 μm. Representative western blot revealed claudin-5 (c) and occludin (d) degradation. The band intensity of claudin-5 (c) and occludin (d) was quantitated after normalization and expressed as hemispheric ratio. Cerebral ischemia and reperfusion led to 46 and 42 % reduction in claudin-5 (c) and occludin (d), respectively, and the combination therapy significantly inhibited claudin-5 (c) and occludin (d) degradation in the ischemic tissue (*P < 0.05, **P < 0.01 versus Air+Veh), while no significant effect was observed for NBO or NAC alone. Data are expressed as mean ± SEM, n = 6
Immunofluorescence results showed loss of claudin-5 in ischemic cortex and subcortex in Air+Veh group. Combination but not NAC treatment could significantly inhibit the loss of claudin-5 (Fig. 4b). The band intensity of the claudin-5 and occludin protein was quantitated and expressed as hemispheric ratio (ischemic/nonischemic) after normalizing to total protein (Coomassie blue). Claudin-5 (Fig. 4c) and occludin (Fig. 4d) protein levels were reduced in ischemic tissue of Air+Veh group, and this degradation was significantly inhibited by the combination but not NAC therapy (P < 0.05 versus Air+Veh group). No significant difference was observed in occludin and claudin-5 levels in the contralateral tissues across animal groups.
Effects of NAC and Combination Treatment on HIF-1α and VEGF
VEGF and HIF-1α have been shown to increase BBB permeability [25, 26]. Here, we examined the effects of NAC, their combination, and NBO on VEGF and HIF-1α expression. Immunofluorescence results showed that HIF-1α was significantly increased in the ischemic cortex and subcortex and combination treatment could significantly decrease the expression of HIF-1α (Fig. 5a) and VEGF (Fig. 5c). The effect was further confirmed by western blot results. Compared to its low level in the contralateral tissue, HIF-1α (Fig. 5b) and VEGF (Fig. 5d) proteins were significantly increased in the Air+Veh group. Combination therapy showed greater effect than each individual treatment alone and significantly inhibited HIF-1α and VEGF upregulation (P < 0.05). There were no detectable differences in HIF-1α or VEGF levels in the contralateral tissues across all animal groups.
Effects of NAC and combination treatment of NAC with NBO on HIF-1α and VEGF induction in the ischemic brain. Immunofluorescence (IF) was used to detect the spatial distribution, and western blot was used to detect the HIF-1α and VEGF in the NI and I hemispheric tissue. a IF showed the spatial distribution of HIF-1α. Scale bar = 100 μm. b The combination therapy led to a greater inhibition than NAC and NBO alone. A representative western blot showed that the HIF-1α was significantly increased in I tissue. NAC, NBO, or their combination significantly reduced the level of HIF-1α in the ischemic tissue, and a greater reduction was seen for the combination therapy. c IF showed the spatial distribution of VEGF. Scale bar = 100 μm. d The combination therapy of NAC and NBO reduced more VEGF expression than alone. A representative western blot showed VEGF levels in NI and I hemispheric tissue. *P < 0.05, **P < 0.01 versus Air+Veh, n = 6
Effects of NAC and Combination Treatment on Brain Injury Induced by Two-Hour Ischemia and Seven-Day Reperfusion
On day 7, obvious atrophy in the ischemic hemisphere could be seen in the gross photographs of whole brains (arrows in Fig. 6a) and in brain sections stained with neutral red. Combination but not NAC treatment showed reduction of the shrinkage because of brain atrophy (P < 0.05, Fig. 6b). In the coronal slices, combined but not NAC causes a significant reduction of lesion area at 7 days after ischemia (P < 0.05, Fig. 6c).
Effects of NAC and combination treatment of NAC with NBO on brain injury and functional outcomes after 2-h MCAO and 7 days of reperfusion. a Photograph of whole brains 7 days after ischemia. Arrows indicated lesion area and brain atrophy. Photograph of brain slices stained with neutral red. The hypocelluar or neutrophil infiltration areas were judged by the lesion area. The rectangle enclosed the lesion area. b Cortical atrophy and c lesion area were quantitated, and only combination treatment showed significant effect. Data are expressed as mean ± SEM. *P < 0.05 versus Air+Veh, n = 6. Neurological deficit score and foot fault placing test (d) were measured 48 h or 7 days after reperfusion. a Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 versus Air+Veh, n = 6–10
Next, we investigated neurological outcome of mono and combination treatment in the ischemic rats using two independent methods. Compared with Air+Veh group, treatment with NAC or NBO had a limited effect on neurological deficit scores assessed by Rogers’ eight-point neurological scale, whereas combination treatment caused a great improvement of the performance which sustained up to 7 days (P < 0.05, Fig. 6d). Foot-fault-placing test was recruited to assess the neurological function, and 2-h MCAO and 48-h reperfusion induced severe functional deficits of the left forelimb (Fig. 6d). Combination but not mono treatment markedly improved the behavioral deficits and enhanced the functional recovery when compared with Air+Veh group, and the effect sustained up to 7 days. These results suggest that combination but not NAC treatment improved performance in ischemia reperfusion-induced behavioral dysfunction.
Discussion
We investigated the protection of post-ischemia NAC and combining treatment with NAC (targeting reperfusion) and NBO (targeting ischemia) in a rat model of transient focal cerebral ischemia. Our results showed that post-ischemia NAC administration had limited effect. However, combination therapy induces greater reductions in tissue infarction and vascular damage. Furthermore, rescuing the brain tissue with combination treatment resulted in much improved functional outcomes sustained up to 7 days. The greater neuro- and vaso-protection is associated with inhibition on HIF-1α and VEGF induction, tight junction protein degradation, activation of PARP-1, and free radical scavenge.
Neuro-protection effect of NAC in stroke has been studied by several research groups. For example, rodents receiving NAC (pre-ischemia) showed obvious reduction in brain infarct volume [7, 8, 27], neuronal cell death, and improvement in neurological function [8]. However, in the present study, NAC administration (during reperfusion) showed limited reduction in infarction volume, but if rats received NBO during ischemia, NAC could produce greater reduction in infarction volume and vascular damage. Two factors may explain the discrepancy: first, pre-ischemia treatment would have better effect than post-ischemia; second, ischemia duration is an important factor determining the outcome. In our study, 2-h ischemia is more serious than they used (30- or 45-min ischemia).
When used as a mono therapy, NBO has neuro- and vaso-protection and could extend the tPA thrombolytic window [13, 15, 28, 29]. NBO delivered during ischemia could be viewed as a prior interruption to reperfusion, which, in turn, increases the conditioning capacity to reperfusion injury. However, in this study, we found that NBO alone showed limited (29.5 % decrease) infarction volume reduction following 48-h reperfusion and we have showed NBO’s neuro-protection effect earlier in the same MCAO model but with shorter (90 min) ischemia duration or shorter (3 or 22.5 h) reperfusion durations [12, 28], suggesting that NBO’s effect was diminished if the ischemia/reperfusion duration was extended. In a recent study, we have observed that tissue infarction was not appreciable in 1-h MCAO rats but was clearly seen after 2- or 3-h ischemia [30]. Brain injury with temporal evolution is critical as 1.9 million neurons loss/min [31], and it has been shown that when the ischemia duration was extended, NBO-afforded early cerebro-protection could diminish over time during reperfusion [16].
In clinical trials, there is no positive result associated with anti-oxidant. This may because the mono treatment only targeted either stage of ischemia reperfusion. Strategy of combination treatment targeting both ischemia stage and reperfusion stage is needed in future clinical trials. Previous work has examined the combination of NBO with radical scavengers edaravone [17] and minocycline [12]; however, they are not widely used in clinic. N-acetylcysteine (NAC), a precursor of glutathione, has been used as mucolyticum in clinic. Therefore, NAC has an advantage to be used in clinic to treat acute ischemia stroke and has more translational significance.
Ischemia–reperfusion triggers oxidative DNA damage, which leads to PARP-1 activation [22, 32]. Our current results showed that combination treatment could decrease the free radical. PARP-1 is an important protein in cell death regulation, and PARP-1 cleavage is a marker for apoptotic cell death. During apoptosis, PARP-1 is cleaved by caspase-3[33] and our previous study showed that NBO could reduce expression of caspase-3 induced by ischemia and reperfusion [12]. Our current results further showed that cleaved PARP-1 level could be significantly inhibited by a combination of NAC with NBO.
It has been shown that activated PARP-1 repairs DNA damage through the formation of PAR, accumulation of PAR is considered a surrogate marker of PARP-1 activation [33], and PAR was detected as a measure of PARP-1 activity [22]. Our current study showed that neither NAC nor NBO could decrease the accumulation of PAR, but combination treatment could significantly decrease the accumulation of PAR.
HIF-1α and VEGF have been shown to play important roles in BBB damage [34, 35], and HIF-1α-upregulated VEGF led to a disruption of the BBB [36]. In addition, VEGF has been shown to increase BBB permeability by disruption of tight junction protein occludin and claduin-5 [37, 38], which degradation is frequently seen in the ischemic brain to cause BBB disruption [39–41]. Cerebral GSH plays an important role in maintaining the functional BBB integrity [42]. NAC has been shown to reduce BBB breakdown indicated by decreased tight junction protein degradation [43, 44], and as an antioxidant, NAC alters the cellular redox environment, which plays a critical role in regulating normal cellular functions and HIF-1α level. NAC has been shown to prevent HIF-1α stabilization under hypoxia in vitro [45]. Our results provide further evidence showing that combination treatment with NAC plus NBO significantly inhibited occludin and claudin-5 degradation by decreasing ischemia and reperfusion-induced HIF-1α/VEGF upregulation.
Besides neuro-protection, NBO can also protect the BBB against ischemic damage [12, 13]. NBO treatment during cerebral ischemia has been shown to reduce BBB disruption and edema formation [28]. However, consistent with our previous study [12], our current results showed that NBO alone was not able to reduce edema volume following 48-h of reperfusion, which is different from what we observed earlier in the same MCAO model but with shorter (3 or 22.5 h) reperfusion durations [28]. The mechanism of reperfusion BBB injury may explain this discrepancy [3], in which NBO-afforded early BBB protection could diminish over time during reperfusion.
Conclusion and Clinical Implications
NAC in combination with NBO results in greater neuro- and vaso-protection than mono therapy. Given the facts that (1) NBO has a superior advantage as an early stroke treatment, (2) NAC can work as a delayed treatment strategy to reduce reperfusion injury, and (3) both agents have good safety profiles, the overall concept could serve as a model for designing future more effective “cocktails” that would provide better neuro-protection than current individual treatment that has all failed in clinical trials.
Abbreviations
- BBB:
-
Blood–brain barrier
- EB:
-
Evan’s blue
- Et:
-
Ethidium
- Het:
-
Hydroethidine
- HIF-1α:
-
Hypoxia-inducible factor-1α
- I:
-
Ischemic hemisphere
- MCAO:
-
Middle cerebral artery occlusion
- NAC:
-
N-acetylcysteine
- NBO:
-
Normobaric hyperoxia
- NI:
-
Nonischemic hemisphere
- PAR:
-
Poly ADP-ribose
- PARP:
-
Poly ADP-ribose polymerase
- PFA:
-
Paraformaldehyde
- TTC:
-
Triphenyl-2,3,4-tetrazolium-chloride
- VEGF:
-
Vascular endothelial growth factor
References
Ramos-Cabrer P, Campos F, Sobrino T, Castillo J (2011) Targeting the ischemic penumbra. Stroke 42(1 Suppl):S7–S11
Dirnagl U, Iadecola C, Moskowitz MA (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22(9):391–397
Schaller B, Graf R (2004) Cerebral ischemia and reperfusion: the pathophysiologic concept as a basis for clinical therapy. J Cereb Blood Flow Metab 24(4):351–371
Reza Noorian A, Nogueira R, Gupta R (2011) Neuroprotection in acute ischemic stroke. J Neurosurg Sci 55(2):127–138
Zhang L, Zhang ZG, Chopp M (2012) The neurovascular unit and combination treatment strategies for stroke. Trends Pharmacol Sci 33(8):415–422
Pan J, Konstas AA, Bateman B, Ortolano GA, Pile-Spellman J (2007) Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology 49(2):93–102
Khan M, Sekhon B, Jatana M, Giri S, Gilg AG, Sekhon C, Singh I, Singh AK (2004) Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J Neurosci Res 76(4):519–527
Sekhon B, Sekhon C, Khan M, Patel SJ, Singh I, Singh AK (2003) N-Acetyl cysteine protects against injury in a rat model of focal cerebral ischemia. Brain Res 971(1):1–8
Lancet T (2006) Neuroprotection: the end of an era? Lancet 368(9547):1548
O’Collins VE, Macleod MR, Donnan GA, Howells DW (2012) Evaluation of combination therapy in animal models of cerebral ischemia. J Cereb Blood Flow Metab 32(4):585–597
Jin X, Liu J, Liu W (2014) Early ischemic blood brain barrier damage: a potential indicator for hemorrhagic transformation following tissue plasminogen activator (tPA) thrombolysis? Curr Neurovasc Res 11(3):254–262
Jin X, Liu J, Liu KJ, Rosenberg GA, Yang Y, Liu W (2013) Normobaric hyperoxia combined with minocycline provides greater neuroprotection than either alone in transient focal cerebral ischemia. Exp Neurol 240:9–16
Liang J, Qi Z, Liu W, Wang P, Shi W, Dong W, Ji X, Luo Y et al (2015) Normobaric hyperoxia slows blood–brain barrier damage and expands the therapeutic time window for tissue-type plasminogen activator treatment in cerebral ischemia. Stroke 46(5):1344–1351
Singhal AB, Wang X, Sumii T, Mori T, Lo EH (2002) Effects of normobaric hyperoxia in a rat model of focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab 22(7):861–868
Henninger N, Fisher M (2006) Normobaric hyperoxia—a promising approach to expand the time window for acute stroke treatment. Cerebrovasc Dis 21(1–2):134–136
Nonaka Y, Koumura A, Hyakkoku K, Shimazawa M, Yoshimura S, Iwama T, Hara H (2009) Combination treatment with normobaric hyperoxia and cilostazol protects mice against focal cerebral ischemia-induced neuronal damage better than each treatment alone. J Pharmacol Exp Ther 330(1):13–22
Nonaka Y, Shimazawa M, Yoshimura S, Iwama T, Hara H (2008) Combination effects of normobaric hyperoxia and edaravone on focal cerebral ischemia-induced neuronal damage in mice. Neurosci Lett 441(2):224–228
Beker MC, Caglayan AB, Kelestemur T, Caglayan B, Yalcin E, Yulug B, Kilic U, Hermann DM et al (2015) Effects of normobaric oxygen and melatonin on reperfusion injury: role of cerebral microcirculation., Oncotarget
Geng X, Fu P, Ji X, Peng C, Fredrickson V, Sy C, Meng R, Ling F et al (2013) Synergetic neuroprotection of normobaric oxygenation and ethanol in ischemic stroke through improved oxidative mechanism. Stroke 44(5):1418–1425
Geng X, Parmar S, Li X, Peng C, Ji X, Chakraborty T, Li WA, Du H et al (2013) Reduced apoptosis by combining normobaric oxygenation with ethanol in transient ischemic stroke. Brain Res 1531:17–24
Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ (2006) Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab 26(10):1274–1284
Zhao Y, Pan R, Li S, Luo Y, Yan F, Yin J, Qi Z, Yan Y et al (2014) Chelating intracellularly accumulated zinc decreased ischemic brain injury through reducing neuronal apoptotic death. Stroke 45(4):1139–1147
Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH (2006) Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 12(4):441–445
Liu J, Jin X, Liu KJ, Liu W (2012) Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood–brain barrier damage in early ischemic stroke stage. J Neurosci 32(9):3044–3057
Semenza GL (2014) Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol 9:47–71
Yan J, Zhou B, Taheri S, Shi H (2011) Differential effects of HIF-1 inhibition by YC-1 on the overall outcome and blood–brain barrier damage in a rat model of ischemic stroke. PLoS One 6(11), e27798
Zhang Z, Yan J, Taheri S, Liu KJ, Shi H (2014) Hypoxia-inducible factor 1 contributes to N-acetylcysteine’s protection in stroke. Free Radic Biol Med 68:8–21
Liu W, Hendren J, Qin XJ, Shen J, Liu KJ (2009) Normobaric hyperoxia attenuates early blood–brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia. J Neurochem 108(3):811–820
Singhal AB, Benner T, Roccatagliata L, Koroshetz WJ, Schaefer PW, Lo EH, Buonanno FS, Gonzalez RG et al (2005) A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke 36(4):797–802
Jin X, Liu J, Yang Y, Liu KJ, Liu W (2012) Spatiotemporal evolution of blood brain barrier damage and tissue infarction within the first 3h after ischemia onset. Neurobiol Dis 48(3):309–316
Saver JL (2006) Time is brain—quantified. Stroke 37(1):263–266
Yang Y, Candelario-Jalil E, Thompson JF, Cuadrado E, Estrada EY, Rosell A, Montaner J, Rosenberg GA (2010) Increased intranuclear matrix metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia. J Neurochem 112(1):134–149
van Wijk SJ, Hageman GJ (2005) Poly(ADP-ribose) polymerase-1 mediated caspase-independent cell death after ischemia/reperfusion. Free Radic Biol Med 39(1):81–90
Chen C, Hu Q, Yan J, Yang X, Shi X, Lei J, Chen L, Huang H et al (2009) Early inhibition of HIF-1alpha with small interfering RNA reduces ischemic-reperfused brain injury in rats. Neurobiol Dis 33(3):509–517
Chen C, Ostrowski RP, Zhou C, Tang J, Zhang JH (2010) Suppression of hypoxia-inducible factor-1alpha and its downstream genes reduces acute hyperglycemia-enhanced hemorrhagic transformation in a rat model of cerebral ischemia. J Neurosci Res 88(9):2046–2055
Semenza GL (2000) HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985) 88(4):1474–1480
Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR (2009) VEGF-mediated disruption of endothelial CLN-5 promotes blood–brain barrier breakdown. Proc Natl Acad Sci U S A 106(6):1977–1982
Yeh WL, Lu DY, Lin CJ, Liou HC, Fu WM (2007) Inhibition of hypoxia-induced increase of blood–brain barrier permeability by YC-1 through the antagonism of HIF-1alpha accumulation and VEGF expression. Mol Pharmacol 72(2):440–449
Liu J, Weaver J, Jin X, Zhang Y, Xu J, Liu KJ, Li W, Liu W (2015) Nitric oxide interacts with caveolin-1 to facilitate autophagy-lysosome-mediated claudin-5 degradation in oxygen-glucose deprivation-treated endothelial cells., Mol Neurobiol
Wang X, Liu Y, Sun Y, Liu W, Jin X (2016) Blood brain barrier breakdown was found in non-infarcted area after 2-h MCAO. J Neurol Sci 363:63–68
Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, Lo EH (2004) Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke 35(11 Suppl 1):2726–2730
Agarwal R, Shukla GS (1999) Potential role of cerebral glutathione in the maintenance of blood–brain barrier integrity in rat. Neurochem Res 24(12):1507–1514
Li W, Maloney RE, Circu ML, Alexander JS, Aw TY (2013) Acute carbonyl stress induces occludin glycation and brain microvascular endothelial barrier dysfunction: role for glutathione-dependent metabolism of methylglyoxal. Free Radic Biol Med 54:51–61
Beauchesne E, Desjardins P, Butterworth RF, Hazell AS (2010) Up-regulation of caveolin-1 and blood–brain barrier breakdown are attenuated by N-acetylcysteine in thiamine deficiency. Neurochem Int 57(7):830–837
Sceneay J, Liu MC, Chen A, Wong CS, Bowtell DD, Moller A (2013) The antioxidant N-acetylcysteine prevents HIF-1 stabilization under hypoxia in vitro but does not affect tumorigenesis in multiple breast cancer models in vivo. PLoS One 8(6), e66388
Acknowledgments
A patent application on therapeutic effects of combination NBO with NAC on ischemic stroke has been filed to China Intellectual Property Office. We thank the financial support from the Soochow University Research starting funds, Ministry of Education of China, Shenzhen Science and Technology Innovation Commission, and Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by Soochow University Research starting funds (Q 421500113), by Fund of Ministry of Education of China (K521507713), and by Shenzhen Science and Technology Innovation Commission (CXZZ20130516152706040 and ZDSY20140509173142601). This work was supported by A Project by the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
Additional information
Yushan Liu and Wen-Cao Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, Y., Liu, WC., Sun, Y. et al. Normobaric Hyperoxia Extends Neuro- and Vaso-Protection of N-Acetylcysteine in Transient Focal Ischemia. Mol Neurobiol 54, 3418–3427 (2017). https://doi.org/10.1007/s12035-016-9932-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9932-0