Abstract
Currently no effective therapies are available for the treatment of traumatic brain injury (TBI). Early intervention that specifically provides neuroprotection is of most importance which profoundly influences the outcome of TBI. In the present study, we adopted a closed-skull mild TBI model to investigate potential roles of omega-3 polyunsaturated fatty acids (ω-3 PUFAs) in protecting against TBI. Using two-photon laser scanning microscopy (2PLSM), parenchymal cell death and reactive oxidative species (ROS) expression were directly observed and recorded after TBI through a thinned skull bone window. Fat-1 mice with high endogenous ω-3 PUFAs significantly inhibited ROS expression and attenuated parenchymal cell death after compression injury during the early injury phase. Elevated generation of glutathione (GSH) and neuroprotectin D1 (NPD1) in the parenchyma of fat-1 mice could be the contributor to the beneficial role of ω-3 PUFAs in TBI. The results of the study suggest that ω-3 PUFAs is an effective neuroprotectant as an early pharmacological intervention for TBI and the information derived from this study may help guide dietary advice for those who are susceptible to repetitive mild TBI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) represents the leading cause of death and neurological impairment in children and adults under the age of 45 years. It is classified into mild, moderate, and severe injury to the brain and involves the primary and secondary injury phase [1, 2]. While the primary injury results from mechanical forces applied to the skull and brain at the time of impact, leading to either focal or diffuse injury patterns such as laceration, contusion, intracranial hemorrhage, and diffuse axonal injury, the secondary injury represents a consequence of a cascade of biochemical changes initiated by the primary insult such as oxidative stress [3, 4], loss of ionic homeostasis [5, 6], excitotoxicity [7], inflammation [8–10], and mitochondrial dysfunction [11]. Despite advances in research in the last decade, currently no FDA-approved pharmacological therapies are available for TBI.
There is an unmet need to develop an effective treatment for TBI. Because the primary injury can only be prevented by avoiding injury itself, nearly all the therapeutic measures are designed to target the secondary injury. It is reported that early intervention after TBI, especially the treatment in the early minutes after injury that specifically provides neuroprotection, is the key factor of the most importance which profoundly influences the outcome of TBI patients [12, 13].
Recent studies suggest that the long-chain omega-3 polyunsaturated fatty acids (ω-3 PUFAs) such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) play key roles in neuroprotection and functional recovery after various traumatic injuries to the brain and spinal cord. It was reported that exogenous administration of ω-3 PUFAs attenuated cellular damage and improved neurological and histological outcomes in the focal cerebral ischemia model [14–18]. Lim and his colleagues reported that both exogenous and endogenous ω-3 PUFAs provided robust protection against spinal cord injury and significantly improved functional recovery in mice [19, 20]. Our studies also showed that enriched endogenous ω-3 PUFAs in fat-1 mice conferred potent neuroprotection against ischemia injury [21, 22]. More importantly, a number of ω-3 PUFAs-derived metabolites such as neuroprotectin D1 (NPD1) act as a potent neuroprotectant that evokes anti-inflammatory and pro-survival repair signaling pathways in the brain [23–26]. All these studies indicate that it is of great value to investigate therapeutic potentials of ω-3 PUFAs in TBI. Notably, ω-3 PUFAs can be used as a prophylactic strategy under various conditions, which highlights the feasibility of applying ω-3 PUFAs as an early pharmacological intervention for the treatment of TBI.
In the present study, we adopted a closed-skull mild TBI model in which parenchymal cell death and reactive oxidative species (ROS) expression could be directly observed and recorded after TBI through a thinned skull bone window using two-photon laser scanning microscopy (2PLSM) [27]. Fat-1 mice expressing the Caenorhabditis elegans fat-1 gene that converts ω-6 to ω-3 were used in the present study [28]. When fat-1 mice are maintained on a diet high in ω-6 PUFAs, they are capable of producing ω-3 PUFAs from the ω-6 type, leading to a higher level of ω-3 PUFAs and a higher ω-3 PUFAs/ω-6 PUFAs ratio in their tissues compared with the wild-type (WT) mice. Thus, fat-1 mice provide a desired animal model to study the role of ω-3 PUFAs in neurological protection [29]. Our study showed that fat-1 mice with high endogenous ω-3 PUFAs significantly inhibited ROS expression and attenuated parenchymal cell death after compression injury during the early injury phase, suggesting that ω-3 PUFAs could be an effective neuroprotectant as an early pharmacological intervention for TBI.
Material and Methods
Rodents and Diets
We obtained fat-1 breeders based on a C57BL/6 background from Dr. Jing X. Kang (Harvard Medical School) and raised them in the Laboratory Animal Center, University of Macau. Mice were housed in a temperature-controlled, 12:12 light/dark room with free access to water and food. The phenotypes of each animal were characterized using isolated genomic DNA and fatty acid composition analyses from mouse tails. Both fat-1 and littermate WT mice were fed a modified diet containing 10 % corn oil (TROPHIC Animal Feed High-tech Co., Ltd, China), with a fatty acid profile high in ω-6 PUFAs (mainly linoleic acid) and low in ω-3 PUFAs (around 0.1 % of the total fat supplied).
Fatty Acid Analysis
To examine whether the expression of the fat-1 gene and the dietary regime altered the PUFA composition in the cortex, the cortex of fat-1 mice and WT mice was isolated and processed for fatty acid analysis by using gas chromatography–mass spectrometry (GC-MS). Briefly, tissue samples were ground to powder under liquid nitrogen and subjected to fatty acid methylation by 14 % boron trifluoride-methanol reagent at 100 °C for 1 h. Fatty acid methyl esters were analyzed using an Agilent GC-MS system (Agilent Technologies, Palo Alto, CA) consisting of an Agilent 6890 gas chromatography and an Agilent 5973 mass spectrometer. Fatty acids were identified in forms of their methyl esters by three means: (i) searching potential compounds from NIST MS Search 2.0 database, (ii) comparing retention time with those of reference compounds (Nu-Chek Prep, Elysian, MN) eluted under the identical chromatographic condition, and (iii) comparing their mass spectra plots with those of authentic standards. Quantification was performed by normalizing individual peak area as the percentage of total fatty acids.
Skull Thinning and TBI Model
A total of 66 mice (fat-1 mice and their littermate WT mice weighing between 20 and 25 g and aging between 8 and 12 weeks) fed a high ω-6 PUFAs diet was used in the study. In addition, 15 adult WT mice with a normal diet were also used in the study and evenly divided into three groups: one group were served baseline controls, another group received both daily intraperitoneal injection of NPD1 (550 μg/kg body weight) for three consecutive days prior to TBI and transcranial administration of glutathione (GSH) immediately after TBI, and the last group received vehicle treatment only. All studies were conducted in accordance with prevailing laws on animal experiments and were approved by the ethical committee of the University of Macau. Animals were deeply anesthetized with 0.035 ml/10 g 10 % chloral hydrate and the rectal temperature was maintained at 37 ± 0.5 °C using a regulated heating pad with a rectal probe (TR-200, FST, CA, USA) throughout the surgery. Surgical incision was performed on the skull scalp and the skull bone was exposed after clearing the external periosteum with sterilized tampons (left, 3 mm; rostral, 1.4 mm; from Bregma). The skull bone was thinned to approximately 30 μm with a microdrill and a compression injury was induced as described with minor modifications [27]. Briefly, once the skull bone was thinned, the blunt end (1000 μm in diameter) of a surgical stereotaxic instrument (RWD, life science) was used to gently press the thinned skull bone downward to promote concavity. The downward pressure applied was minimal and only performed until there was a visible dent.
GSH Measurement
To evaluate the generation of glutathione (GSH), we harvested the cortex with the injury site at 30 min post TBI after quick perfusion with 0.9 % saline and extracted the sample with 10 mg:100 μl tissue lysates in a vacuum broken instrument (SANYO, MSS.150-CX15). After mixed with vortex and centrifuged at 15,000 rpm for 3–5 min, the supernatant was obtained and diluted with tissue lysates, then the concentration of GSH was evaluated by measurement of the absorbance at 320 nm for GSH (Cellular Glutathione Peroxidase Assay Kit S0056).
LC-MS/MS for NPD1
Mouse cortices were harvested at 30 min post injury and homogenized by adding 0.5 mL cold methanol. Homogenate was supplemented with deuterated internal standards and purified by solid-phase extraction. Methyl formate fractions were then used for LC-MS/MS with an Agilent 1100 HPLC (Agilent Technologies) equipped with an Agilent Eclipse Plus C-18 column (4.6 mm×50 mm×1.8 μm) paired with an ABI Sciex Instruments 5500 QTRAP linear ion trap triple quadrupole mass spectrometer (Applied Biosystems). The AnalystTM 1.5 software (Applied Biosystems) was used to perform instrument control and data acquisition. The mobile phase consisted of methanol/water/acetic acid (55/45/0.01; v/v/v) and moved to 88/12/0.01 (v/v/v) after 10 min, 100/0/0.01 (v/v/v) after 18 min, and 55:45:0.01 (v/v/v) after 1 min to wash and equilibrate the column. Mass spectrometry analyses were carried out in negative ion mode using multiple reaction monitoring (MRM) of established specific transitions for NPD1 (m/z 359>153). The criteria used for identification of NPD1 were matched as previously reported [30, 31]. Quantification was performed using a standard calibration curve for NPD1. Recovery was calculated using deuterated internal standards.
In Vivo Labeling with Fluorescent Dyes
To visualize brain vasculature, mice were intravenously injected with 0.3 ml FITC-dextran tracker in PBS (0.2 mg/ml; Sigma) at 10 min before imaging. Cell death was visualized by incubating the thinned skull with 1.0 mM propidium iodide (PI) in aCSF for 30 min, which was followed by a single wash with aCSF and then imaging. ROS were visualized and imaged by applying Amplex Red (Invitrogen Life Technologies) transcranially for 10 min.
ROS Test
To further quantify the expression of ROS (represented by H2O2), the cortex with the injury site at 3, 6, and 12 h post injury was harvested after quick perfusion with 0.9 % saline and extracted with 10 mg:100 μl tissue lysates in a vacuum broken instrument (SANYO, MSS.150-CX15). After mixed with vortex and centrifuged at 15,000 rpm for 3–5 min, the supernatant was obtained and diluted with tissue lysates, then the concentration of H2O2 was evaluated by measurement of the absorbance at 560 nm of H2O2 (Beyotime, Hydrogen Peroxide Assay Kit S0038).
In Vivo Two-Photon Imaging
Anesthetized mice with a thinned and compressed skull bone were imaged using a Leica SP5 two-photon imaging system (Leica Microsystems) equipped with a one-way (8000 Hz) and a two-way (16,000 Hz) resonant scanner, a ×20/1.0 NA dipping objective, and two Mai Tai HP DeepSee Lasers (SpectraPhysics) at 809 wavelength and a laser-scanning system (coherent company, America) equipped with a water-immersion objective (×25). Stacks of images were acquired using a step size of 1.0 mm (single stacks) or 2.5 mm (time-lapse videos) to a depth of 300 μm. For all imaging studies, the ×25 lens was submerged directly into artificial cerebral spinal fluid (aCSF) placed on the top of the thinned skull.
Three-Dimensional Image Analyses of Parenchymal Dead Cells
We performed the quantification of parenchymal dead cells by three-dimensional imaging data analyses with the Leica software (LAS AF Lite). Quantification was processed from reconstructed 600 × 500 × 300 μm (xyz) three-dimensional image stacks obtained at 3, 6, and 12 h post compression. Considering the cells between 0–50 μm beneath the compressed skull bone as the meningeal cells, only PI-positive dead cells from 50 to 300 μm were counted and considered as parenchymal. The number of dead cells was divided by the volume analyzed and represented as cells per cubic millimeter.
Immunohistochemistry
Immunohistochemistry was performed to characterize the identity of dead cells in the brain parenchyma. Dead cells were labeled by transcranial PI application, and the mice were perfused with 4 % paraformaldehyde (PFA) at 12 h after injury. After post fixed in 4 % PFA for 8 h and then stored in 30 % sucrose for another 24 h, 50 μm serial coronal brain sections containing the injury site were cut with a vibratome, selected, and blocked with 0.3 % triton and 10 % anti-donkey serum for 1.5 h at room temperature. The sections were incubated with rabbit polyclonal antibody (pAb) Iba-1 (1:200, AbCam), NeuN (1:200, Millipore), and monoclonal pAb GFAP (1:100, AbCam) for 24 h at 4 °C in dark, and then incubated with secondary antibodies at room temperature for 1 h in the dark. The expression of Iba-1, NeuN, and GFAP was examined and imaged using the Leica TCS SP5MP confocal fluorescence microscope.
TUNEL Staining
TUNEL staining was performed on coronal brain sections containing the injury site with the in situ cell death detection, Fluorescein kit (Roche Applied Science), according to the manufacturer’s instructions. Briefly, sections were treated with 0.3 % triton and 10 % anti-donkey serum for 1 h at room temperature. Subsequently, the sections were incubated with the TUNEL reaction mixture for 1 h at 37 °C in dark. After washing with PBS, the sections were incubated with the polyclonal antibody NeuN (1:500, Millipore) overnight at 4 °C in dark. Then the sections were incubated with the secondary antibody Alexa Fluor® 555 donkey anti-rabbit IgG (1:500, Invitrogen) for 1 h at room temperature in dark and washed with PBS again. Cells expressing NeuN with red fluorescence were considered to be surviving neurons and cells with green fluorescence in the nucleus were considered to be the apoptotic cells under microscope. Total TUNEL-positive cells at the injured site were calculated under the microscope with ×200 magnifications.
Statistical Analysis
Statistical differences between two groups were determined by two-tailed Student’s t test. Multiple group comparisons were made by one-way ANOVA and followed with Tukey post hoc test. Each group included five animals and data were presented as mean ± SEM. Significance levels were set to 0.05 for all comparisons. All graphs and statistical analyses were performed using the GraphPad Prism 5.
Results
Fatty Acid Profiles of the Cortex in Fat-1 Mice and WT Mice
The fatty acid profile in the cortex from fat-1 mice and their WT littermates which were all fed with a high ω-6 PUFAs diet had been analyzed by using GC-MS of PUFAs (Table 1). A significantly elevated level of ω-3 docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA) was detected in the fat-1 mice cortex compared with their WT littermates. Meanwhile, a concomitant decrease in the expression of ω-6 DTA, DPA, and AA was found in fat-1 mice, leading to a dramatically lower ratio of ω-6/ω-3 PUFAs compared with WT mice.
Attenuated Parenchymal Cell Death in Fat-1 Mice Compared with WT Mice
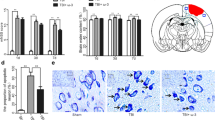
A closed-skull TBI model was used in the present study. The intact mouse skull bone is composed of three layers: cortical, cancellous, cortical, which forms the cranial cavity containing the brain with its covering meninges. The underlying meninges and brain parenchyma can be directly observed and imaged using 2PLSM by thinning the mouse skull bone to around 30 μm. The mild compression injury was induced by applying minimal downward pressure for 1 min to promote concavity in the thinned skull region, leading to the cell death in the meninges and parenchyma (Fig. 1a). After intravenously injected FITC-dextan, the brain vasculature could be clearly seen and imaged through the intact thinned skull window by using 2PLSM. Using the Leica software (LAS AF Lite), stacks of images were reconstructed into a three-dimensional vascular network which distinctly distinguishes the meningeal vascular network (0–50 μm beneath the compressed bone) and the parenchymal vascular network (below 50 μm from the compressed bone) (Fig. 1b).
Attenuated parenchymal cell death was detected in fat-1 mice after mild compression injury. a Schematic showing a closed-skull mild TBI model used in the study. The compression injury was induced by applying minimal downward pressure for 1 min, which promotes concavity in the thinned region. b A representative three-dimensional brain vascular network produced by two-photon laser microscope which was used to identify dead cells in the meningeal layer and parenchymal layer. c A representative image showed the compression injury area. Vascular network was displayed by FITC-dextran (200 kD) and the compression injury site (white dash circle) consisted of dead cells labeled by transcranially applied propidium iodide (PI). d Representative reconstructed three-dimensional vascular networks showing cell death (PI+ cells; red) in the brain parenchyma at 3, 6, and 12 h after mild compression injury. e Quantification of cell death in the parenchyma at 3, 6, and 12 h after mild compression injury (mean ± SEM). The number of PI+ dead cells (per mm3) in the parenchyma was significantly reduced in fat-1 mice compared with controls (*P < 0.001). All data were representative of five mice per group. Scale bar: 300 μm for (c) and 250 μm for (d)
Cell death could be transcranially labeled by PI [27]. Cell death in the meninges was immediately observed following compression injury (Fig. 1c). Then it gradually spread to the parenchyma over time. Using the reconstructed three-dimensional vasculature images, PI-positive dead cells were clearly found to be present in the meningeal and parenchymal vascular network after compression injury, which indicates the meningeal cell death and parenchymal cell death, respectively (Fig. 1d). In vivo recordings on the spread of cell death in the parenchyma were made at 3, 6, and 12 h after compression injury. PI-positive cells were found in the parenchymal vascular network of all the animals at 3 h after compression injury and increased over time (Fig. 1d). Approximately 1000, 1400, and 1900 PI-positive cells per cubic millimeter were detected in the parenchyma of WT mice fed high ω-6 PUFAs at 3, 6, and 12 h after compression injury, respectively (Fig. 1d, e). No statistical differences in the number of PI-positive cells were found in the parenchyma at each time point between WT mice fed high ω-6 PUFAs and WT mice fed a normal diet, suggesting that a diet rich in high ω-6 PUFAs does not seem to have a detrimental effect on the cell survival (Fig. 1d, e). Notably, the number of PI-positive cells was dramatically reduced in the parenchyma of fat-1 mice (Approximately 280, 610, and 720 PI-positive cells per cubic millimeter were detected at each examined time point.) (Fig. 1d, e; P < 0.001).
At 12 h after TBI, 50 μm serial coronal brain sections containing the injury site were cut with a vibratome and the total number of PI-positive cells was counted in every other section. Consistent with the quantification of parenchymal cell death by the reconstructed three-dimensional in vivo imaging system, histological results confirmed that the total number of PI-positive cells was significantly reduced in fat-1 mice compared with WT mice (Fig. 2a, b). TUNEL staining further confirmed the neuroprotection shown in fat-1 mice in that approximately 345 ± 15 TUNEL-positive cells were found in the injured site, which was significantly lower than 906 ± 67 TUNEL-positive cells found in WT mice (Fig. 2d, e; P < 0.001). We then further characterized the cell type of dead cells. Immunostaining on the coronal brain section at 12 h post injury showed that cell death was non-selective, as the lesion site in both fat-1 mice (Fig. 2c) and WT mice (data not shown) was largely devoid of neuronal (NeuN+), astrocytic (GFAP+), and microglia (Iba1+) staining.
Histological analyses on parenchymal cell death using PI labeling and TUNEL staining. a Representative images of PI labeling on the coronal brain slice section at 12 h after injury. b Histological results showed that the total number of PI-positive cells was significantly reduced in fat-1 mice compared with WT mice (*P < 0.001). Data were representative of five mice per group. c Non-selective cell loss in the parenchyma after mild TBI, as the lesion site was largely devoid of neuronal (NeuN+), astrocytic (GFAP+), and microglia (Iba1+) staining. Dead cells were labeled by transcranially applied PI. d Representative images of TUNEL staining on the coronal brain slice section at 12 h after injury. e Quantification showed that the total number of TUNEL-positive cells was significantly reduced in fat-1 mice compared with WT mice (*P < 0.001). Data were representative of five mice per group. Scale bar: 400 μm for (a), 50 μm for (c), and 350 μm for (d)
Elevated Generation of GSH and NPD1 in the Parenchyma of Fat-1 Mice
We then sought to explore the mechanisms by which fat-1 mice attenuated parenchymal cell death after TBI. Oxidative damage is one of the best validated secondary injury mechanisms in TBI [1, 32, 33]. Using 2PLSM, we documented in vivo ROS expression (profiles by transcranially applying Amplex Red, starting from 10 min after compression injury. Within 30 min, ROS was detected in the whole meningeal layer and spread the parenchyma as deep as nearly 75 μm in WT mice, while its expression was confined to the meningeal layer and only a very small amount of ROS was detected in the surface area of the parenchyma in fat-1 mice (Fig. 3a). To further quantify the expression of ROS, we used a hydrogen peroxide assay kit to measure the concentration of H2O2 in the injured parenchyma at 3, 6, and 12 h post compression. The results showed H2O2 concentration in the injured parenchyma was reduced nearly by half in fat-1 mice compared with WT mice at the each examined time point (Fig. 3b; P < 0.001). Moreover, expression of GSH, a potent ROS scavenger, in the parenchyma of fat-1 mice was pronouncedly increased as compared to WT mice at 3, 6, and 12 h post compression (Fig. 3c; P < 0.001). We then transcranially applied GSH to WT mice immediately after compression injury. As expected, transcranial administration of GSH significantly inhibited ROS expression and decreased H2O2 concentration in the parenchyma of WT mice after TBI (Fig. 3b, c; P < 0.001). This finding was highly consistent with a recent study reporting that DHA is an antioxidant that modulates neuronal defenses in neuroprotection via activating GSH expression in brain cells [34].
Elevated generation of GSH and NPD1 in the parenchyma of fat-1 mice. a Two-photon imaging system recorded in vivo ROS expression after compression injury by transcranially applying Amplex Red. b Upregulated GSH was found in fat-1 mice compared with WT mice. (*P < 0.0001) c Hydrogen peroxide assay kit was used to quantify H2O2 concentration. The concentration of H2O2 was significantly reduced in fat-1 mice or WT mice with transcranially applied GSH compared with the control animals at 3, 6, and 12 h post compression (*P < 0.001). c Elevated generation of NPD1 was found in fat-1 mice compared with WT mice (*P < 0.0001). d WT mice which received both daily intraperitoneal injection of NPD1 for 3 days prior to TBI and transcranial administration of GSH immediately after TBI significantly improved parenchymal cell survival as compared to the control (*P < 0.001). All the results are representative of five animals per group. Scale bar: 100 μm
Neuroinflammation is another well-defined secondary injury mechanism following TBI [8, 10]. NPD1, a metabolite derived from DHA, could function as a potent endogenous mediator of neuroprotection and anti-inflammatory resolution [25, 35]. We performed LC-MS/MS to measure the expression level of NPD1 in the cortex of fat-1 mice and their WT littermates. As shown in Fig. 3d, NPD1 levels were remarkably higher in fat-1 mice compared to their WT littermates at 3, 6, and 12 h post compression (P < 0.0001). Furthermore, WT mice which received both daily intraperitoneal injection of NPD1 for three consecutive days prior to TBI and transcranial administration of GSH immediately after TBI significantly improved parenchymal cell survival as compared to the control (Fig. 3e; P < 0.001). Therefore, our results collectively suggest that higher expression of GSH and NPD1 in fat-1 mice could be the key contributors to reducing parenchymal cell death in TBI.
Discussion
In the present study, we investigated the protective effects of ω-3 PUFAs on brain cell survival after TBI. After transcranially applied PI and Amplex Red, cell death and ROS activation were in vivo observed and recorded through the intact thinned murine skull bone with 2PLSM. Significantly improved parenchymal cell survival was found in fat-1 mice with enriched endogenous ω-3 PUFAs compared with WT mice at the early stage of compression injury. The higher generation of GSH and NPD1 in fat-1 mice could be the key contributor to reducing parenchymal cell death in TBI.
Two-photon microscopy offers the advantage in real-time recordings on brain activities including blood flow and cellular activity [36–39]. Using 2PLSM, we could temporally record the spread of brain cell death after TBI and quantify the cell death in a three-dimensional imaging system. The result acquired by this in vivo quantification of parenchymal dead cells is highly consistent with histological analyses on brain slices. Using 2PLSM, we could also detect ROS activation after TBI and document the change of its expression due to pharmacological intervention. All these results suggest that this in vivo mapping and recording system by 2PLSM is a convenient and reliable technique to study cellular and molecular changes after pharmacological manipulations in TBI.
The management of TBI at the moment is mainly supportive and aims at treating brain edema, reducing intracranial pressure, and combating complications such as hypoxia and hypotension [40]. Intensive efforts have been put to develop pharmacological agents targeting the secondary injuries for the treatment of TBI in the past decades; however, no conclusively proven drugs have been found in clinical trials. The secondary events which exacerbate the devastating neurological effects is initiated at the very early time following the primary injury, suggesting that there is a need of very early intervention, even in the first minutes after injury. Our study provides evidence that early intervention by inhibiting ROS activation is an effective approach for mitigating neurological loss in TBI. We further detected an increased generation of GSH, a potent ROS scavenger, in the brain of fat-1 mice, indicating that ω-3 PUFAs could activate the generation of GSH. This finding supports the conclusion in a recent study that DHA is an antioxidant that modulates neuronal defenses in neuroprotection via activating GSH expression in brain cells [34].
It is worth noting that ω-3 PUFAs have a multitude of action mechanisms. Many studies reported that ω-3 PUFAs exert anti-inflammatory effects via activating peroxisome proliferator-activated receptors (PPARs) [41], stimulating G-protein coupled receptor 120 (GPR120) [42], and giving rise to a family of active metabolites such as E- and D-series of resolvins and protectins which display strong anti-inflammatory and anti-apoptotic actions [43–45]. In addition, it is reported that ω-3 PUFAs can control the glutamate overflow associated with TBI [46]. Thus, ω-3 PUFAs can be used as a sustained intervention targeting multiple secondary injury mechanisms for the treatment of TBI.
A number of studies reported the beneficial effects of ω-3 PUFAs on functional recovery after TBI. Dietary supplementation of ω-3 PUFAs for more than 4 weeks could normalize BDNF levels and counteract learning disability after TBI in rats [47], maintain rat brain homeostasis after TBI [48], and reduce axonal dysfunction, microglial activation, and caspase-3 levels with the concomitant behavioral improvement in a rodent head injury model [49]. It is proposed that an intervention targeting a combination of damaging mechanisms may be more effective for TBI. In the present study, we demonstrate that a remarkable upregulation of GSH and NPD1 was detected in the parenchyma of fat-1 mice with enriched endogenous ω-3 PUFAs during the early injury phase, suggesting that ω-3 PUFAs could offer the advantage of this poly-target approach. Although further investigations on the beneficial effects of ω-3 PUFAs in moderate or severe TBI and other action mechanisms underlying their beneficial effects in TBI are necessary, the information derived from this study may help guide dietary advice for those who are susceptible to repetitive mild TBI.
Reference
Cornelius C, Crupi R, Calabrese V, Graziano A, Milone P, Pennisi G et al (2013) Traumatic brain injury: oxidative stress and neuroprotection. Antioxid Redox Signal 19(8):836–853
Loane DJ, Faden AI (2010) Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci 31(12):596–604
Hall ED, Andrus PK, Yonkers PA (1993) Brain hydroxyl radical generation in acute experimental head injury. J Neurochem 60(2):588–594
Kontos HA, Wei EP (1986) Superoxide production in experimental brain injury. J Neurosurg 64(5):803–807
Bramlett HM, Dietrich WD (2004) Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab 24(2):133–150
Kanemaru K, Kubota J, Sekiya H, Hirose K, Okubo Y, Iino M (2013) Calcium-dependent N-cadherin up-regulation mediates reactive astrogliosis and neuroprotection after brain injury. Proc Natl Acad Sci U S A 110(28):11612–11617
Bullock R, Zauner A, Myseros JS, Marmarou A, Woodward JJ, Young HF (1995) Evidence for prolonged release of excitatory amino acids in severe human head trauma. Relationship to clinical events. Ann N Y Acad Sci 765:290–297, discussion 298
Gyoneva S, Ransohoff RM (2015) Inflammatory reaction after traumatic brain injury: therapeutic potential of targeting cell-cell communication by chemokines. Trends Pharmacol Sci 36(7):471–480
Lenzlinger PM, Morganti-Kossmann MC, Laurer HL, McIntosh TK (2001) The duality of the inflammatory response to traumatic brain injury. Mol Neurobiol 24(1–3):169–181
Witcher KG, Eiferman DS, Godbout JP (2015) Priming the inflammatory pump of the CNS after traumatic brain injury. Trends Neurosci 38(10):609–620
Signoretti S, Marmarou A, Tavazzi B, Dunbar J, Amorini AM, Lazzarino G et al (2004) The protective effect of cyclosporin A upon N-acetylaspartate and mitochondrial dysfunction following experimental diffuse traumatic brain injury. J Neurotrauma 21(9):1154–1167
Jennings JS, Gerber AM, Vallano ML (2008) Pharmacological strategies for neuroprotection in traumatic brain injury. Mini Rev Med Chem 8(7):689–701
Michael-Titus AT, Priestley JV (2014) Omega-3 fatty acids and traumatic neurological injury: from neuroprotection to neuroplasticity? Trends Neurosci 37(1):30–38
Belayev L, Khoutorova L, Atkins KD, Bazan NG (2009) Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke 40(9):3121–3126
Kelly L, Grehan B, Chiesa AD, O’Mara SM, Downer E, Sahyoun G et al (2011) The polyunsaturated fatty acids, EPA and DPA exert a protective effect in the hippocampus of the aged rat. Neurobiol Aging 32(12):2311–2318
Zhang W, Hu X, Yang W, Gao Y, Chen J (2010) Omega-3 polyunsaturated fatty acid supplementation confers long-term neuroprotection against neonatal hypoxic-ischemic brain injury through anti-inflammatory actions. Stroke 41(10):2341–2347
Lalancette-Hebert M, Julien C, Cordeau P, Bohacek I, Weng YC, Calon F et al (2011) Accumulation of dietary docosahexaenoic acid in the brain attenuates acute immune response and development of postischemic neuronal damage. Stroke 42(10):2903–2909
Hong SH, Belayev L, Khoutorova L, Obenaus A, Bazan NG (2014) Docosahexaenoic acid confers enduring neuroprotection in experimental stroke. J Neurol Sci 338(1–2):135–141
Lim SN, Huang W, Hall JC, Michael-Titus AT, Priestley JV (2013) Improved outcome after spinal cord compression injury in mice treated with docosahexaenoic acid. Exp Neurol 239:13–27
Lim SN, Gladman SJ, Dyall SC, Patel U, Virani N, Kang JX et al (2013) Transgenic mice with high endogenous omega-3 fatty acids are protected from spinal cord injury. Neurobiol Dis 51:104–112
Luo C, Ren H, Wan JB, Yao X, Zhang X, He C et al (2014) Enriched endogenous omega-3 fatty acids in mice protect against global ischemia injury. J Lipid Res 55(7):1288–1297
Shi Z, Ren H, Luo C, Yao X, Li P, He C et al. (2015) Enriched endogenous omega-3 polyunsaturated fatty acids protect cortical neurons from experimental ischemic injury. Mol Neurobiol (In Press).
Antony R, Lukiw WJ, Bazan NG (2010) Neuroprotectin D1 induces dephosphorylation of Bcl-xL in a PP2A-dependent manner during oxidative stress and promotes retinal pigment epithelial cell survival. J Biol Chem 285(24):18301–18308
Bazan NG, Calandria JM, Serhan CN (2010) Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res 51(8):2018–2031
Bazan NG (2012) Neuroinflammation and proteostasis are modulated by endogenously biosynthesized neuroprotectin D1. Mol Neurobiol 46(1):221–226
Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K et al (2005) A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest 115(10):2774–2783
Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB (2014) Transcranial amelioration of inflammation and cell death after brain injury. Nature 505(7482):223–228
Kang JX, Wang J, Wu L, Kang ZB (2004) Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature 427(6974):504
Das UN, Puskás LG (2009) Transgenic fat-1 mouse as a model to study the pathophysiology of cardiovascular, neurological and psychiatric disorders. Lipids Health Dis 8:61
Orr SK, Palumbo S, Bosetti F, Mount HT, Kang JX, Greenwood CE et al (2013) Unesterified docosahexaenoic acid is protective in neuroinflammation. J Neurochem 127(3):378–393
Yang R, Chiang N, Oh SF, Serhan CN (2011) Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes. Curr Protoc Immunol Chapter 14(14):26
Bains M, Hall ED (2012) Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophys Acta 1822(5):675–684
Hall ED, Vaishnav RA, Mustafa AG (2010) Antioxidant therapies for traumatic brain injury. Neurotherapeutics 7(1):51–61
Casanas-Sanchez V, Perez JA, Fabelo N, Herrera-Herrera AV, Fernandez C, Marin R et al (2014) Addition of docosahexaenoic acid, but not arachidonic acid, activates glutathione and thioredoxin antioxidant systems in murine hippocampal HT22 cells: potential implications in neuroprotection. J Neurochem 131(4):470–483
Bazan NG (2014) Is there a molecular logic that sustains neuronal functional integrity and survival? Lipid signaling is necessary for neuroprotective neuronal transcriptional programs. Mol Neurobiol 50(1):1–5
Nikolenko V, Poskanzer KE, Yuste R (2007) Two-photon photostimulation and imaging of neural circuits. Nat Methods 4(11):943–950
Schaffer CB, Friedman B, Nishimura N, Schroeder LF, Tsai PS, Ebner FF et al (2006) Two-photon imaging of cortical surface microvessels reveals a robust redistribution in blood flow after vascular occlusion. PLoS Biol 4(2):e22
Shih AY, Driscoll JD, Drew PJ, Nishimura N, Schaffer CB, Kleinfeld D (2012) Two-photon microscopy as a tool to study blood flow and neurovascular coupling in the rodent brain. J Cereb Blood Flow Metab 32(7):1277–1309
Wolf S, Supatto W, Debrégeas G, Mahou P, Kruglik SG, Sintes JM et al (2015) Whole-brain functional imaging with two-photon light-sheet microscopy. Nat Methods 12(5):379–380
Roozenbeek B, Maas AI, Menon DK (2013) Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol 9(4):231–236
Moraes LA, Piqueras L, Bishop-Bailey D (2006) Peroxisome proliferator-activated receptors and inflammation. Pharmacol Ther 110(3):371–385
Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W et al (2010) GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142(5):687–698
Bazan NG (2006) Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci 29(5):263–271
Kohli P, Levy BD (2009) Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol 158(4):960–971
Schwab JM, Chiang N, Arita M, Serhan CN (2007) Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447(7146):869–874
Vreugdenhil M, Bruehl C, Voskuyl RA, Kang JX, Leaf A, Wadman WJ (1996) Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proc Natl Acad Sci U S A 93(22):12559–12563
Wu A, Ying Z, Gomez-Pinilla F (2004) Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma 21(10):1457–1467
Wu A, Ying Z, Gomez-Pinilla F (2007) Omega-3 fatty acids supplementation restores mechanisms that maintain brain homeostasis in traumatic brain injury. J Neurotrauma 24(10):1587–1595
Mills JD, Hadley K, Bailes JE (2011) Dietary supplementation with the omega-3 fatty acid docosahexaenoic acid in traumatic brain injury. Neurosurgery 68(2):474–481
Acknowledgments
This study was supported by the Macao Science and Technology Development Fund (063/2015/A1), multi-year research grant, University of Macau, MYRG122 (Y1-L3)-ICMS12-SHX and MYRG110 (Y1-L2)-ICMS13-SHX, the National Natural Scientific Foundation of China (31270992 and 30800215), Zhu Jiang Science and Technology New Star of Guangzhou City (2013 J2200019), Guangdong Province Science and Technology Plan (2013B021800275), and the Fundamental Research Funds for the Central Universities in Sun Yat-Sen University (13ykpy24).
Author Contributions
HR and HS designed the study; RH, ZY, CL, HZ, PL, CH, JK, JW, and HS performed and analyzed the results; and all authors wrote the draft together and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Huixia Ren and Zhen Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ren, H., Yang, Z., Luo, C. et al. Enriched Endogenous Omega-3 Fatty Acids in Mice Ameliorate Parenchymal Cell Death After Traumatic Brain Injury. Mol Neurobiol 54, 3317–3326 (2017). https://doi.org/10.1007/s12035-016-9931-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9931-1