Abstract
Multiple sclerosis (MS) is a T cell autoimmune, inflammatory, and demyelinating disease of the central nervous system (CNS). Currently available therapies have partially effective actions and numerous side reactions. Inosine, an endogenous purine nucleoside, has immunomodulatory, neuroprotective, and analgesic properties. Herein, we evaluated the effect of inosine on the development and progression of experimental autoimmune encephalomyelitis (EAE), an experimental model of MS. Inosine (1 or 10 mg/kg, i.p.) was administrated twice a day for 40 days. Immunological and inflammatory responses were evaluated by behavioral, histological, immunohistochemical, ELISA, RT-PCR, and Western blotting analysis. The administration of inosine exerted neuroprotective effects against EAE by diminishing clinical signs, including thermal and mechanical hyperalgesia, as well as weight loss typical of the disease. These beneficial effects of inosine seem to be associated with the blockade of inflammatory cell entry into the CNS, especially lymphocytes, thus delaying the demyelinating process and astrocytes activation. In particular, up-regulation of IL-17 levels in the secondary lymphoid tissues, a result of EAE, was prevented by inosine treatment in EAE mice. Additionally, inosine consistently prevented A2AR up-regulation in the spinal cord, likely, through an ERK1-independent pathway. Altogether, these results allow us to propose that this endogenous purine might be a putative novel and helpful tool for the prevention of autoimmune and neurodegenerative diseases, such as MS. Thus, inosine could have considerable implications for future therapies of MS, and this study may represent the starting point for further investigation into the role of inosine and adenosinergic receptors in neuroinflammation processes.

Preventive treatment with inosine inhibits the development and progression of EAE in C57Bl/6 mice. Furthermore, neuroinflammation and demyelinating processes were blocked by inosine treatment. Additionally, inosine consistently inhibited IL-17 levels in peripheral lymphoid tissue, as well as IL-4 levels and A2AR up-regulation in the spinal cord, likely, through an ERK1-independent pathway. EAE: experimental autoimmune encephalomyelitis; MS: multiple sclerosis; A2AR: adenosine A2A receptor; IL-17: interleukin-17; IL-4: interleukin-4
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and demyelinating disease of the central nervous system (CNS) with progressive damage to the myelin sheath and neurodegeneration initiated by an autoimmune response to self-antigens [1, 2]. MS affects more than 2.5 million people worldwide and is considered a classical neurological disease in young adults, which causes permanent disability and compromises quality of life [3, 4]. Although the cause of MS remains unknown, genetic and environmental factors have been shown to be implicated in the development of this disease [5, 6]. However, MS and its murine model, experimental autoimmune encephalomyelitis (EAE), have been characterized as a T cell-mediated disease [7]. These T cells are antigen (Ag)-specific, activated in peripheral lymphoid tissues, and cross the blood–brain barrier (BBB) into the CNS, where they are reactivated by resident Ag-presenting brain–spinal cord glial cells [8]. These reactivated T lymphocytes and glial cells secrete pro-inflammatory cytokines and chemokines, culminating in a progressive neuroinflammation, demyelinating lesions, and neuronal and oligodendrocytes death [9]. Previously, the primary effector T cell in the pathology of both EAE and MS was thought to be a Th1 cell, which requires IL-12 for its differentiation and is characterized by the secretion of IFN-γ, IL-2, and TNF [7]. However, Th17 cells and their major product, interleukin-17 (IL-17), have been shown to play a critical role in these Th cell-mediated disease [10, 11]. IL-17 is a pro-inflammatory cytokine that activates immune cells to produce a variety of inflammatory mediators, which together increase BBB permeability and perivascular cell infiltrates [12, 13].
A substantial number of individuals diagnosed with MS report persistent pain as a relevant clinical sign [14], and they might also experience cognitive and mood impairment, like fatigue and anxiety, detectable even before a definitive diagnosis of MS is made [15, 16]. In MS patients, previous studies have described this pain as central neuropathic pain [17] that can be manifested through mechanical and thermal hyperalgesic pain-like behavior in EAE [18]. The activation of glial cells and secretion of pro-nociceptive mediators like IL-17 are related to pain symptoms [19, 20].
Inosine, an endogenous purine nucleoside, is formed by the breakdown of adenosine by the enzyme adenosine deaminase. It has been demonstrated that inosine has neuroprotective, anti-inflammatory, and analgesic effects. Previous data show that it regulates the expression of genes involved in axon outgrowth and promotes recovery in rodent models of stroke and CNS injury [21–24]. Inosine reduces the levels of pro-inflammatory cytokines in animal models of inflammatory diseases and suppresses macrophage, lymphocyte, and neutrophil activation [25–27]. Moreover, it has been shown that inosine has a pronounced effect against pain-like behavior in rodent models [28]. The protective effects of inosine are associated with its actions through G-protein-coupled adenosine receptors (especially, A1, A2A, and A3) [26, 29, 30], which are expressed in the brain, spinal cord, spleen, and, mainly, in leukocytes [31]. In this context, inosine effect is due, in part, to intracellular signaling pathways including mitogen-activated protein kinase (MAPK) [32, 33]. This pathway is involved in the regulation of inflammatory responses [34], in which ERK1 can modulate autoimmune disorders like EAE [35]. Earlier studies have demonstrated that inosine treatment is well-tolerated and benefits patients with MS through improvements in the clinical score and a reduction in lesion activity [36, 37]. In addition, adenosine A1 and A2A receptors are involved in the development and progression of EAE disease [11, 38]. However, the specific mechanisms of inosine action in MS are not well-established. For these reason, we aimed to investigate the effects of inosine on the progression of EAE by assessing clinical scores, as well as to provide further evidence on the underlying signaling pathways related to the immunomodulatory and neuroprotective effects of inosine in the EAE model. We also verified the effects of inosine in the pre-motor signs of the disease, like hyperalgesia, anxiety-like behavior, and fatigue.
Material and Methods
Experimental Animals
Experiments were conducted using female C57BL/6 mice, 6 to 12 weeks old. The mice were kept in groups of four to six animals per cage, maintained under controlled temperature (22 ± 1 °C) with a 12-h light/dark cycle (lights on at 7:00 a.m.), and were given free access to food and water. All procedures used in the present study followed the “Principles of laboratory animal care” (NIH publication no. 85–23) and were approved by the Animal Ethics Committee of the Universidade Federal de Santa Catarina (CEUA-UFSC, protocol number PP00811 and PP00956).
EAE Induction
Experimental autoimmune encephalomyelitis (EAE) was induced by subcutaneous (s.c.) immunization into the flanks with 200 μl of an emulsion containing 200 μg of MOG35–55 peptide and 500 μg of Mycobacterium tuberculosis extract H37Ra (Difco Laboratories, Detroit, MI, USA) in incomplete Freund’s adjuvant oil (Sigma Chemical Co., St. Louis, MO, USA). This procedure was repeated after 7 days to increase the incidence of EAE, as previously described [39]. In addition, the animals received by the i.p. route 300 ng of Pertussis toxin (Sigma Chemical Co., St. Louis, MO, USA) on day 0 and day 2 post-immunization. Non-immunized (naïve) and EAE animals were used as the negative and positive control groups, respectively. Mice were weighed and observed daily for clinical signs of EAE for up to 40 days post-immunization. The neurological impairment of EAE was quantified using a clinical scale according to the following scores: 0, no signs of disease; 1, loss of tone in the tail; 2, hindlimb paresis; 3, hindlimb paralysis; 4, tetraplegia; and 5, moribund and/or death.
Drug Treatment Protocol
Inosine was kept in amber glass, maintained at 4 °C, and diluted in the vehicle saline (0.9 % NaCl solution) to the desired dose (1 and 10 mg/kg) just before use. Inosine or vehicle was administered intraperitoneally (i.p.) twice a day (8:00 a.m. and 5:00 p.m.) for 40 days (starting on day 0 until day 40 post-immunization), 1 h before immunization and the behavioral tests, which were carried out in the morning. The choice of dose was based on previous data described in the literature [28, 29].
Behavioral Experiments
Mechanical Hyperalgesia
For the evaluation of mechanical hyperalgesia, mice were placed individually in clear Plexiglas boxes (9 × 7 × 11 cm) on elevated wire mesh platforms to allow access to the ventral surface of the right hind paw. The withdrawal response frequency (%) was measured following 10 applications (3 s each) of von Frey hairs (VFH, Stoelting, Chicago, IL, USA). Stimuli were applied on the plantar surface of the right hind paw with enough force to elicit a slight bend in the filament. A withdrawal of the paw was considered a positive response (10 % response frequency). Importantly, 0.4 and 0.6 VFH filaments produce a mean withdrawal frequency of about 15 %, which is considered an adequate value for the measurement of mechanical hyperalgesia [40]. The animals were acclimatized for 30 min before behavioral testing, and to determine the basal tactile thresholds, all the groups were evaluated before disease induction. All animals were evaluated at different time points until day 14 because, after this time point, the clinical signs of EAE, such as locomotor deficits, were visible.
Cold Thermal Hyperalgesia
For the evaluation of cold thermal hyperalgesia, the acetone method was adapted from Walczak, Beaulieu [41]. The mice were placed in clear Plexiglas boxes (9 × 7 × 11 cm) on elevated wire mesh platforms and acclimatized for 30 min. The acetone technique consisted of placing 20 μl of acetone on the plantar surface of the right hind paws. The time the animal spent lifting, licking, or shaking, the paw was recorded for 20 s. Three measurements were done and recorded at intervals of 15 min. In order to determine the baseline threshold, all the groups were tested before immunization. Cold thermal hyperalgesia was evaluated on alternate days, starting on day 1 until day 13 after immunization.
Hot Thermal Hyperalgesia
The hot plate test was used to measure response latencies. The apparatus consists of a metal plate (AVS system, cold-hot plate, São Paulo, SP, Brazil) maintained at 50 ± 1 °C. Animals were placed into the heated surface and the time between placement and jumping, shaking, or licking of the paws was recorded as the index of response latency. An automatic 30-s cutoff was used to prevent possible injuries caused by hot exposure for a long period of time to the animal paw. All animals were evaluated at days 11, 13, and 15 p.i. since, after this day, the clinical signs of EAE, such as locomotor deficit, were visible.
Elevated Plus-Maze Test (EPM)
Twelve days after immunization, mice were evaluated in the elevated plus-maze test, which is a recognized behavioral paradigm for the assessment of anxiolytic- and anxiogenic-like effects of procedures and drugs in rodents [42]. The maze consists of a central platform (6 × 6 cm), with two open arms (30 × 6 cm) aligned perpendicularly to two closed arms (30 × 6 × 16 cm). The open arms had a 1-cm high Plexiglas rim to prevent falls. The maze was elevated 50 cm above the floor. Mice were placed on the central platform facing a closed arm and allowed to explore the maze for 5 min. The videos were analyzed visually. The time spent in the open and closed arms was used to calculate the percentage of time spent in the open arms. Any decrease in this parameter or in the number of entries in open arms represents anxiety-like behavior, and the total number of entries in the closed arms is a measure of locomotor activity.
Weight Load Swimming Test (WLST)
Fourteen days after immunization, mice were evaluated using the weight load swimming test adapted from Su et al. [43] for the assessment of fatigue-like behavior. Mice were placed individually in an open cylindrical container (diameter, 10 cm; height, 25 cm) with water at a depth of 17 cm maintained at 28 ± 1 °C. A weight equivalent to 5 % of body weight was attached to the tail root, and the endurance for each mouse was measured as swimming time recorded from placement in the pool to exhaustion. The swimming period (30 min) was considered the time spent struggling and making necessary movements until exhaustion and possible drowning. When mice were unable to remain at the water surface (for 7 s), they were considered exhausted.
Histological Analysis
Forty days after EAE induction, animals were euthanized and each portion of the lumbar spinal cord (L3–L5) was removed and fixed immediately in 4 % paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 24 h and stored in 70 % alcohol until paraffin processing. Samples were dehydrated in ethanol for removal of tissue water followed by diaphanization with xylene for removal resident alcohol and complete permeation of paraffin. After that, tissues were embedded in paraffin and sections (5 μm) were mounted on glass slides and deparaffinized. The histological analysis of inflammation and demyelination was performed using hematoxylin-eosin (H&E) and luxol fast blue (LFB), respectively. The settings used for image acquisition were identical for both control and experimental tissues. Five to six ocular fields per section (three to four mice per group) were captured and a threshold optical density that best discriminated the nuclear staining of inflammatory cells (H&E) or myelin (LFB) was obtained using NIH ImageJ 1.36b imaging software (NIH, Bethesda, MD, USA) and applied to all experimental groups. The total pixel intensity was determined, and the data are expressed as optical density (O.D.).
Immunohistochemical Analysis
Immunohistochemical analysis was performed on paraffin-embedded lumbar spinal cord tissue sections mounted on glass slides and deparaffinized, as described above. Slices (5 μm) were stained with a monoclonal mouse anti-glial fibrillary acidic protein (GFAP, 1:100). High-temperature antigen retrieval was performed by immersion of the slides in a water bath at 95–98 °C in 10 mM trisodium citrate buffer of pH 6.0 for 45 min. After overnight incubation at 4 °C with the primary antibody, the slices were washed with PBS and incubated with the secondary antibody, and immune complexes were visualized with 0.05 % 3,3′-diaminobenzidine tetrahydrochloride (DAB: Dako Cytomation, Glostrup, Denmark) + 0.03 % H2O2 in phosphate buffer saline (PBS) (for an exact amount of time: 1 min). The reaction was stopped by washing in water and counterstaining with Harris’ hematoxylin. Images of the dorsal and ventral horns of spinal cord sections stained with antibodies to anti-GFAP were obtained by using a Q-imaging digital camera connected to an Olympus Bx41 microscope. Settings for image acquisition were identical for control and experimental tissues. Five to six ocular fields per section (3–4 mice per group) were captured and a threshold optical density that best discriminated staining from the background was obtained using NIH ImageJ 1.36b imaging software (NIH, Bethesda, MD, USA) using a counting grid at ×100 and ×400 magnification. The total pixel intensity was determined and the data are expressed as optical density (O.D.).
Determination of Cytokine Levels
Forty days after EAE induction, animals were killed and inguinal lymph nodes, spleen, and spinal cord were removed. All tissue samples were weighed, frozen in liquid nitrogen, and stored at −70 °C. For cytokine assays, samples were homogenized in phosphate buffer containing 0.05 % Tween 20, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 0.1 mM benzethonium chloride, 10 mM EDTA, and 2 ng/ml aprotinin. The homogenates were centrifuged at 3000g for 10 min (4 °C) and the supernatants stored at −80 °C until assays for the determination of levels of the cytokines IL-17, IL-10, and IL-4. The amount of protein in each sample was measured using the Bradford method using bovine serum albumin as the standard. The levels of cytokines were measured in 100 μl of sample using mouse cytokine ELISA kits, according to the manufacturer’s instructions. The level of the cytokines was estimated by interpolation from a standard curve by colorimetric measurements at 450 nm (correction wavelength 540 nm) on an ELISA plate reader (Berthold Technologies, Apollo 8, LB 912, KG, Germany). All results are expressed as picograms per milligram protein (pg/mg).
Real-Time Quantitative PCR
Lumbar spinal cords were removed 40 days p.i. from 4 to 6 animals/group, and the total RNA was extracted using the Trizol protocol. The reverse transcription assay was carried out as described in the M-MLV Reverse Transcriptase Kit (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. Real-time quantitative PCR analysis was performed in StepOnePlusTM using the TaqMan1 Universal PCR Master Mix Kit (Applied Biosystems, Foster City, CA, USA) for quantification of the amplicons. Fifty nanograms of total RNA was used for cDNA synthesis. The cDNA was amplified in duplicate using specific TaqMan1 Gene Expression target genes, the 30 quencher MGB and FAM-labelled probes for A1R (Mm01308023_m1), A2AR (Mm00802075_m1) and GAPDH (NM_008084.2), the latter used as endogenous control for normalization. The PCRs were performed in a 96-well Optical Reaction Plate (Applied Biosystems, Foster City, CA). The thermocycler parameters were as follows: 50 °C for 2 min, 95 °C for 10 min, 50 cycles of 95 °C for 15 s, and 60 °C for 1 min. Expression of the target genes was calibrated against conditions found in control animals (i.e., animals that received vehicle, 0.9 % NaCl solution).
Immunoblotting Analysis
Forty days after EAE induction, animals were euthanized and the lumbar spinal cords were rapidly removed to subsequent A1R, A2AR, ERK1, and p-ERK analysis by Western blotting assay. During dissection, spinal cords were maintained in ice-cold Krebs-Ringer bicarbonate buffer (KRB) with the following composition (in mM): 122 NaCl, 3 KCl, 1.2 MgSO4, 1.3 CaCl2, 0.4 KH2PO4, 25 NaHCO3, and 10 d-glucose. The buffer was bubbled with 95 % O2 and 5 % CO2 up to pH 7.4. The spinal cord was solubilized with SDS-stopping solution (4 % SDS, 2 mM EDTA, 8 % β-mercaptoethanol, and 50 mM Tris, pH 6.8, 2 mM PMSF). Samples (60 μg of total protein/lane) were separated by 10 % SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 2 % albumin (1 h) in TBS (10 mM Tris, 150 mM NaCl, pH 7.5), followed by three washes with TBS-T (10 mM Tris, 150 mM NaCl, 0.05 % Tween-20, pH 7.5). Membranes were incubated with the following primary antibodies: anti-A1R (Abcam, ab82477, 1:250), A2AR (Santa Cruz Biotechnology, sc-32261, 1:250), ERK (Cell Signaling®, sc-93, 1:250), p-ERK (Cell Signaling®, sc-7976, 1:250), or β-actin (1:1000; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) overnight, at 4 °C, and then were exposed to appropriate peroxidase-conjugated secondary antibodies (1:1000 anti-goat and 1:250 anti-rabbit; Santa Cruz Biotechnology) for 1 h at room temperature. Immunocomplexes were visualized using an enhancing chemiluminescence detection system (GE Healthcare). Densitometric analysis was performed for the quantification of immunoblotting using Scion Image Software (Scion Corporation). The level of total immunocontent was determined as the ratio of optic densitometry (O.D.). The β-actin band was used as the loading control. The level of phosphorylation was determined as the ratio of the O.D. of the phosphorylated protein band over the O.D. of the total protein band. The data are expressed as a percentage of the value in the control group for each membrane (100 %). Total protein level was evaluated by Peterson’s method using bovine serum albumin as the standard.
Drugs and Reagents
Inosine, Pertussis toxin, phosphate buffered saline (PBS), incomplete Freund’s adjuvant oil, H&E, H2O2, and BSA were obtained from Sigma Chemical Co. (St. Louis, MO, USA); MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNG) from EZBiolab (Carmel, IN, USA); M. tuberculosis extract H37Ra from Difco Laboratories (Detroit, MI, USA); Trizol and M-MLV reverse transcriptases were purchased from Invitrogen (Carlsbad, CA, USA). The primers and probes for A1R, A2AR, and GAPDH were purchased from Applied Biosystems (Foster City, CA, USA); monoclonal mouse anti-GFAP from Cell Signaling Technology (Danvers, MA, USA); monoclonal goat anti-A2AR, polyclonal rabbit anti-ERK sc-93, polyclonal goat anti-pERK sc-7976, and secondary antibodies (anti-goat and anti-rabbit) from Santa Cruz Biotechnology® (Santa Cruz, CA, USA); polyclonal rabbit anti-adenosine A1R and β-actin from Abcam® (Cambridge, MA, USA); anti-mouse IL-10 and IL-4 ELISA MAX Deluxe Sets from BioLegend (San Diego, CA, USA); anti-mouse-IL-17 Duo Set kits from R&D Systems (Minneapolis, MN, USA). The other reagents used were of analytical grade and obtained from different commercial sources.
Statistical Analysis
All data are presented as mean ± SEM: clinical score, weight and hyperalgesia tests, 4–6 mice per group; EPM and WLST, 6–11 mice per group; ex vivo tests, 3–6 mice per group. The results are representative of one or two independent experiments. A statistical comparison of the data was performed by one or two-way ANOVA followed by the Bonferroni or Newman–Keuls post hoc test, depending on the experimental protocol. P < 0.05, 0.01, and 0.001 were considered significant. The statistical analyses were performed using GraphPad Prism 4 software (GraphPad Software Inc., San Diego, CA, USA).
Results
Inosine Attenuates the Clinical Signs of EAE
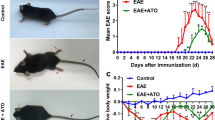
To evaluate the efficacy of inosine, EAE was induced in C57BL/6 mice using MOG35–55 peptide. As expected, clinical symptoms, such as tail atony and clumsy gait, appeared on day 12 post-MOG-injections and peaked around day 36 on untreated EAE group (Fig. 1a). There was just one death during the course of EAE development. To test the preventive effect of inosine in the EAE model, this compound was given intraperitoneally as 1 and 10 mg/kg from day 0 of immunization to day 40, the end of the analysis. Compared with the untreated EAE group, animals treated with inosine at both 1 and 10 mg/kg presented significant protection against disease induction, delayed onset of clinical signs, and attenuated clinical scores (Fig. 1a, b). Animals treated with 1 mg/kg inosine showed the first EAE typical symptoms only on day 30, with a maximum clinical score of 1 on the last day of analysis, an 88 % inhibition based on the area under the curve (AUC) (Fig. 1a, b). Moreover, although the clinical signs appeared on day 20 in animals treated with 10 mg/kg inosine, the maximum clinical score achieved was also 1, indicating maximal inhibition of 77 % based on the AUC (Fig. 1a, b). In addition, a progressive loss of body weight paralleled the severity of the disease (Fig. 1c). At the peak of disease (day 22 post-induction), mice treated with 1 mg/kg inosine were protected against marked loss of body weight and recovered a healthy appearance similar to naïve mice (Fig. 1d). However, treatment with 10 mg/kg inosine failed to inhibit weight loss induced by EAE (Fig. 1d). Based on these results, a dose of 1 mg/kg of inosine was used in subsequent experiments to investigate some of the mechanisms underlying its immunomodulatory and neuroprotective effects.
Inosine reduces EAE severity and prevents weight loss after immunization. Mice were immunized on days 0 and 7 with MOG35–55/CFA and monitored daily for clinical signs for 40 days. The treatment was given at 8 a.m. and 5 p.m. by i.p. route with inosine (1 or 10 mg/kg) or vehicle (0.9 % NaCl solution) from day 0 to day 40. The EAE group had remarkably higher clinical scores compared to the naïve group starting on day 14 and treatment with inosine delayed the onset and attenuated the clinical scores (a) and area under the curve (AUC) (b). Mice were monitored daily for weight gain/loss from the beginning of the experiment. Body weight changes (c) and body weight gain/loss at the peak of disease (day 22 post-induction) (d). Data are presented as mean ± SEM of 4–6 mice/group. *p < 0.05 and ***p < 0.001 versus the naïve group, # p < 0.05 and ### p < 0.001 versus the EAE group (one-way ANOVA followed by Newman-Keuls)
The Effect of Inosine on Mechanical and Thermal Hyperalgesia Following EAE
It is recognized that neuropathic pain is a relevant symptom that precedes motor dysfunction in MS, contributing to greatly reduced quality of life in patients [44]. Therefore, in the next set of experiments, we investigated the effect of inosine pre-treatment on mechanical and thermal hyperalgesia during the EAE pre-symptomatic phase. The mechanical hyperalgesia produced following EAE induction was characterized by a pronounced and long-lasting increase in the response frequency to stimulation with von Frey hairs (0.4 and 0.6 g) on the right hind paw (Fig. 2a–d). This hyperalgesic behavior was observed starting on day 4, reached a maximum on day 12 after immunization, and remained elevated for up to 14 days (Fig. 2a). Significantly, 1 mg/kg inosine was effective in reducing mechanical hyperalgesia with 65 ± 13 and 79 ± 10 % inhibition (0.4 and 0.6 g filament, respectively) based on the AUC (Fig. 2b, d). We also assessed whether inosine is effective in preventing cold and hot thermal hyperalgesia. The treatment with inosine (1 mg/kg) showed a significantly lower time response to the cold stimuli than the untreated EAE group, with 84 ± 9 % inhibition based on the AUC (Fig. 2e, f). Furthermore, a reduction in paw withdrawal latency to heat stimulus was obtained by inosine treatment compared to untreated EAE group in the acute phase of disease (Fig. 2g–i).
Inosine reduces hyperalgesia associated with EAE. Mice were immunized on days 0 and 7 with MOG35–55/CFA. The figure shows time course and its respective area under the curve (AUC) from mechanical hyperalgesia (assessed by the von Frey hair test) and cold thermal hyperalgesia (assessed by the acetone test), which were evaluated during the induction phase of EAE (0 to 14 days p.i.); the hot thermal hyperalgesia at 50 °C (assessed by hot plate test) was evaluated in three different points: 11, 13, and 15 days p.i. Treatment with inosine (1 mg/kg, i.p.) or vehicle (0.9 % NaCl solution) was performed twice a day and throughout the test period. Hyperalgesia upon mechanical stimulation with von Frey filaments 0.4 g (a) plus AUC (b), filament 0.6 g (c) plus AUC (d), cold thermal stimulation (e) plus AUC (f), and hot thermal stimulation 11 (g), 13 (h), and 15 (i) days p.i. INO 1 inosine 1 mg/kg, Dpi days post-immunization. Data are presented as mean ± SEM of 4–6 mice/group. *p < 0.05, **p < 0.01, and ***p < 0.001 versus the naïve group, # p < 0.05, ## p < 0.01, and ### p < 0.001 versus the EAE group (two-way ANOVA followed by Bonferroni’s test for a, c, and e and one-way ANOVA followed by Newman-Keuls for b, d, and f–i)
Inosine Decreases the Neuroinflammatory Process, Myelin Loss, and Reactive Astrogliosis in the Spinal Cord after EAE Induction
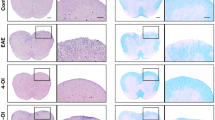
Inflammatory cells trafficking to the CNS during EAE are predominantly Ag-specific T lymphocytes; the expression of cellular adhesion molecules [18, 45] and chemokines by these cells contributes to neuroinflammation and demyelination [46]. We next evaluated the effect of inosine on the intensity of inflammatory cell infiltration induced by EAE and if it could preserve the integrity of myelin in the spinal cord 40 days post-immunization. It was found that the number of inflammatory foci in the spinal cord was significantly higher in the EAE group compared with the naïve group (Fig. 3a, b). In contrast, mice treated with inosine (1 mg/kg) showed a significantly lower number of inflammatory foci compared with the untreated EAE group (Fig. 3a, b). In addition, the demyelination index was significantly higher in the EAE group whereas treatment with inosine (1 mg/kg) significantly prevented myelin loss (Fig. 3c, d). Of great relevance, inosine (1 mg/kg) prevented demyelination, maintaining tissue integrity similar to that observed in the naïve group (Fig. 3c, d). In addition, reactive astrogliosis contributes to the extent of CNS damage in EAE [47]. In the next step, we assessed whether treatment with inosine exerts a role on astrocytic cell activation in the spinal cord 40 days post-immunization. GFAP immunoreactivity was up-regulated in the untreated EAE group when compared with the naïve group (Fig. 3e, f). Remarkably, treatment with inosine (1 mg/kg) was also effective in preventing reactive astrogliosis (Fig. 3e, f).
Inosine ameliorates EAE pathology. The lumbar spinal cords were analyzed on day 40 p.i. in different experimental groups for neuroinflammation by H&E staining (a and b), for demyelination by luxol fast blue staining (c and d) and astroglial activation by GFAP immunoreactivity (e and f). Specifically, four 5-μm sections of lumbar spinal cord white matter were obtained between L4 and L6 from the naïve group, EAE group, and mice pre-treated with inosine (1 mg/kg). The EAE group exhibited a higher inflammatory cells infiltration (a and b), demyelination area (c and d), and astroglial activation (e and f) when compared with naïve group, and inosine pre-treatment reduced significantly neuroinflammation (a and b), demyelination (c and d), and astrogliosis (e and f) when compared with the EAE group. Magnification correspond ×400. O.D. optical density, INO 1 inosine 1 mg/kg. Data are presented as mean ± SEM of 4–6 mice/group. **p < 0.01 and ***p < 0.001 versus the naïve group, # p < 0.05 and ### p < 0.001 versus the EAE group (one-way ANOVA followed by Newman-Keuls)
Inosine Inhibits IL-17 Cytokine Production in the Peripheral Lymphoid Tissue
Previous studies have suggested a strong association between IL-17 levels and MS severity [11], which pathogenic potential is associated with a marked neuroinflammatory process [48]. To provide evidence as to whether inosine treatment could suppress IL-17 cytokine expression, we examined inguinal lymph node and spinal cord IL-17 levels 40 days post-immunization. Initially, IL-17 level was not altered in the spinal cord (Fig. 4a), but these values were markedly increased in the inguinal lymphocytes of untreated EAE group compared with naïve group (Fig. 4d). Inosine (1 mg/kg) significantly inhibited the levels of IL-17 in the inguinal lymph nodes after EAE induction (Fig. 4d). To investigate whether the immunomodulatory effects of inosine were linked to the up-regulation of anti-inflammatory cytokines, we measured the IL-4 and IL-10 levels in the spleen and spinal cord after EAE induction. Importantly, pronounced increase in IL-4 and down-regulation of IL-10 levels was observed in the spinal cord and spleen, respectively, after EAE-immunization (Fig. 4b, f). Inosine markedly inhibited the up-regulation of IL-4 in the CNS (Fig. 4b) and spleen (Fig. 4e), after EAE induction; however, IL-10 production was not significantly affected by treatment with inosine in the spinal cord (Fig. 4c). Together, our data suggest that inosine inhibits EAE development and progression, as well as neuroinflammation and demyelination through a reduction in the expansion, activation and/or proliferation of encephalitogenic lymphocytes, especially Th17 cells, which are the main producers of IL-17 in peripheral lymphoid tissues during autoimmune neuroinflammatory disease.
Preventive treatment with inosine reduces IL-17 level in the peripheral lymphoid tissue after EAE-induction. Mice were immunized on days 0 and 7 with MOG35–55/CFA and spinal cord (a–c), lymph nodes (d), and spleen (e and f) were collected on day 40 p.i. Level of IL-17 (a and d), IL-4 (b and e), and IL-10 (c and f) were determined by ELISA assay and expressed in pg/mg of protein. INO 1 inosine 1 mg/kg. Data are presented as mean ± SEM of 4–6 mice/group. *p < 0.05 and **p < 0.01 versus naïve group, ## p < 0.01 and ### p < 0.001 versus EAE group (one-way ANOVA followed by Newmann–Keuls)
Immunomodulatory Effect of Inosine During EAE Correlates with the Adenosine A2A Receptor
Several previous studies have shown that A1R and A2AR expression is involved in EAE severity [49, 50]. In addition, immunomodulatory effects of inosine may depend at least in part on adenosinergic receptors signaling [25]. Herein, a new set of experiments were conducted to explore whether or not inosine could interfere with adenosinergic receptors A1 and A2A expression in the spinal cord after EAE immunization. Indeed, as can be seen in Fig. 5, pronounced up-regulation of A2AR mRNA and protein levels were observed in the EAE group (Fig. 5b–e), while A1R mRNA and protein significantly down-regulated in white-matter of the untreated EAE group compared to the naïve group (Fig. 5a–d). Importantly, inosine (1 mg/kg) administration resulted in strikingly reduced A2AR mRNA and protein levels (Fig. 5b–e), and up-regulated A1R mRNA expression in the CNS 40 days after EAE induction (Fig. 5a). However, inosine treatment failed to increase A1R protein levels in the spinal cord when compared to the untreated EAE group (Fig. 5c, d).
Inosine modulates adenosinergic receptors expression/levels without affecting phosphorylation of ERK1 in spinal cord during EAE pathology. Total mRNA and protein levels were extracted from the lumbar spinal cord (L4–L6) of mice in the naïve group, untreated EAE group, and mice pre-treated with inosine (1 mg/kg) on day 40 post-immunization. The mRNA levels of A1R (a) and A2AR (b) were measured by RT-PCR. The housekeeping gene GAPDH mRNA was used to normalize the relative amounts of mRNA. The protein expression levels of A1R (c and d), A2AR (c and e), ERK1 (f and g), and p-ERK1 (f and h) were determined by Western blotting analysis. The levels of protein were expressed as ratio of signal intensity for the target proteins relative to that for β-actin. N naïve group, Veh untreated EAE group, INO 1 inosine 1 mg/kg. Data are presented as mean ± SEM of 4–6 mice/group. *p < 0.05, **p < 0.01, and ***p < 0.001 versus naive group, # p < 0.05 and ## p < 0.01 versus EAE group (one-way ANOVA followed by Newmann–Keuls)
Pre-treatment with Inosine Does Not Suppress the Increase of p-ERK Expression Following EAE
It was previously reported that p-ERK-1 is increased in the spinal cord after established EAE and is related to the incidence and severity of disease [51]. The results demonstrate higher expression of p-ERK in the spinal cord of the untreated EAE group 40 days after immunization compared to the naïve group (Fig. 5f, h). However, p-ERK and total ERK levels were not significantly altered by inosine (1 mg/kg) treatment when compared to the untreated EAE group (Fig. 5f–h). Thus, our data suggest that the immunomodulatory and analgesic effects of inosine during EAE model depend, at least in part, on adenosine A2A receptor modulation by ERK1-independent pathways.
Inosine Does Not Reverse EAE-Induced Fatigue and Anxiety-Like Behavior
In addition to motor and sensory symptoms, fatigue is another disability associated with MS progression [52]. Therefore, to evaluate fatigue-like behavior in the course of EAE, mice were submitted to a weight load swimming test (WLST) on the 14th day post-induction. Figure 6a shows that EAE induction led to a shorter swimming time in mice. Compared with the untreated EAE group, inosine treatment (1 mg/kg) provided no significant reversal in EAE-induced fatigue behavior during the pre-motor stage of the disease (Fig. 6a). Another common symptom in MS patients is anxiety, and it has been reported that anxious-like behavior sometimes overlaps with the manifestation of EAE motor signs [53, 54]. In this study, we submitted mice to the elevated plus maze test (EPM) to evaluate the effect of inosine on EAE-induced anxiety-like behavior on day 12 during the pre-motor phase. As shown in Fig. 6b, there was no significant difference in the number of entries into the closed arms among the experimental groups, which confirms no locomotor debility during the EAE pre-motor phase. Importantly, the untreated EAE mice demonstrated a decrease in the percentage of entries into the open arms and spent significantly less time in the open arms when compared to naïve mice (Fig. 6c, d). However, mice treated with inosine (1 mg/kg) did not show a higher percentage of entries nor did they spend more time in the open arms than the untreated EAE group (Fig. 6c, d).
Inosine treatment fails to reduce fatigue and anxiogenic-like behavior induced by EAE. Mice were immunized on days 0 and 7 with MOG35–55/CFA. Animals were tested during the induction phase of EAE (0 to 14 days p.i.). The weight-loaded swimming test (WLST) performed on day 14 p.i. The EAE group showed reduced endurance performance; however, preventive treatment with inosine (1 mg/kg, i.p.) did not improve symptoms when compared with the EAE group (a). The elevated plus maze (EPM) test was performed on day 12 p.i. The EAE group showed no change in entries in the closed arm (b), but exhibited a reduction in time spent in the open arm (c). The inosine treatment group displayed a reduction in the number of entries into the open arm (d). INO 1 inosine 1 mg/kg. Data are presented as mean ± SEM of 8–11 mice/group. *p < 0.05 and ***p < 0.001 and versus the naïve group (one-way ANOVA followed by Newman-Keuls)
Discussion
MS is an autoimmune CNS disease for which no cure is presently known [7]. New medicines for MS have emerged, but the most widely used therapies have limitations associated with side effects and, most importantly, do not effectively change the course of the disease [44, 55]. So, additional therapeutic options are urgently needed. A less detrimental compound is required that provides effective and early immunosuppression to stabilize the cascade of events that culminates in neurodegeneration and irreversible disability; this may limit disease progression [8]. In this study, we focused on investigating the effects of inosine in EAE progression and its impact on the severity of clinical scores. We also aimed to understand the mechanisms involved and sought to improve the pre-motor signs of the disease, such as hyperalgesia, anxiety, and fatigue.
The results of the present study show the effectiveness of inosine (1 and 10 mg/kg) in blocking the development of EAE in C57BL/6 mice, a validated experimental model to study MS. In agreement with previous reports in which inosine treatment improved clinical scores in MS patients [36, 37], the treatment of EAE mice with inosine had beneficial actions in modulating the onset of disease and preventing its progression by reducing the severity of clinical signs. Moreover, administration of inosine induced pronounced and long-lasting effects against mechanical and thermal hyperalgesia after EAE immunization. The protective role of inosine was mediated by its anti-inflammatory effects through the inhibition of inflammatory cell infiltration into the CNS and a reduction in peripheral lymphoid tissue IL-17 levels, as well as by preventing demyelination lesions and suppressing astrogliosis in the spinal cord after treatment (see proposed scheme in Fig. 7).
Preventive treatment with inosine inhibits the development and progression of EAE in C57Bl/6 mice. Furthermore, neuroinflammation, and demyelinating processes were blocked by inosine treatment. Additionally, inosine consistently inhibited IL-17 levels in peripheral lymphoid tissue, as well as IL-4 levels and A2AR up-regulation in the spinal cord, likely, through an ERK1-independent pathway. EAE experimental autoimmune encephalomyelitis, MS multiple sclerosis, A2AR adenosine A2A receptor, IL-17 interleukin-17, IL-4 interleukin-4
The pathogenesis of MS and the EAE model is a complex process, involving increased migration of encephalitogenic lymphocytes across the BBB, successively inducing inflammatory and demyelination lesions with oligodendrocyte and axon depletion throughout the CNS [9]. The results reported here indicate, for the first time to our knowledge, that inosine preventive treatment leads to a marked reduction in inflammatory cell recruitment into the spinal cord and effectively prevents demyelination areas in EAE mice after the established phase of disease. Therefore, these results suggest a neuroprotective effect of inosine, which can be observed in reduced EAE development and decreased severity of clinical scores.
Several findings have demonstrated a relationship between adenosine receptors and MS [56, 57]. In EAE model, A1R protein expression has been shown necessary to prevent neuroinflammation and demyelination [49] since A1AR expression was concomitantly reduced in brains of MS patients relative to non-MS brains [57]. Moreover, previous studies have shown A2AR protein up-regulation in brain [56] and lymphocytes from MS patients [50], as well as throughout the choroid plexus in EAE model [38]. The A2AR protein up-regulation may occur, per example, on microglial cells due to inflammatory stimuli, to the high inflammatory cells infiltration into the CNS and to inflammatory cytokines [45]. Importantly, Mills et al. [38] have reported that lack of A2AR in CNS from EAE mice can block lymphocyte infiltration. Earlier study also have demonstrated a role of inosine in prevent cell migration during animal model of inflammatory disease [26]. On the other side, extracellular inosine can access the intracellular space through nucleoside transporters and it can be degraded to uric acid (UA). Kean et al. [58] demonstrated in EAE model that UA acts protecting the BBB integrity through suppression of inflammatory cells into CNS. Furthermore, inosine was used to increase UA levels in MS patients, thereby blocking neuronal lesion and improving clinical scores [36]. Herein, inosine treatment down-regulates both A2AR mRNA and protein levels in spinal, as well as recovered A1R mRNA levels, although the same results was not observed for A1R protein level. Therefore, our data confirm and largely extend these previous data, by demonstrating that inosine showed immunomodulatory effects during EAE progression, and that this is associated with A2AR down-regulation in the spinal cord. However, to date, we are not able to confirm whether: (i) inosine acts by directly activating A2A receptors, or (ii) the mouse diet is deficient in purines and inosine is converted to inosine monophosphate (IMP) and then to AMP to produce ATP and/or adenosine, or (iii) inosine is metabolized to hypoxanthine which is then metabolized to AMP and then to ATP and/or adenosine. For this reason, future studies are needed to clarify this hypothesis.
Accumulated evidence has suggested a potential pathogenic role of IL-17 in MS development, with higher expression of this cytokine found in T cells from MS lesions; Th17 cells are associated with active plaques and increased clinical severity of disease [11, 48]. These data are correlated to the involvement of IL-17 in Th17 infiltration across the BBB, thereby facilitating neuroinflammation and consequent CNS injury [59]. It has been shown that high levels of pro-inflammatory cytokines like IL-17 are expressed in peripheral lymphoid tissues of EAE-induced mice [46, 60], corroborating our results in the untreated EAE group 40 days after immunization. Herein, we demonstrate that inosine treatment pronouncedly reduced IL-17 levels in the inguinal lymph nodes of EAE mice, as well as inhibited the up-regulation of IL-4 in the CNS and spleen after EAE induction, albeit IL-10 levels did not differ between the two groups in the spinal cord. Taken together, we suggest that the protective effects of inosine on neuroinflammation are associated, in part, with IL-17 suppression. This may be due to a decrease in the differentiation and/or proliferation of Th17 cells, i.e., IL-17 producers, in secondary lymphoid tissues. Therefore, future studies will need to investigate whether inosine could inhibit these cells in CNS after EAE induction, as well as examine whether or not inosine directly modulates the Th2 (CD3+CD4+IL-4+GATA3+) and/or Treg (CD3+CD4+IL-10+TGF-β+FoxP3+) during development of EAE.
In established EAE, after the migration of pro-inflammatory T cells across the BBB, disease progression is also affect by astroglial activation [46, 61], which contributes to the secretion of soluble pro-inflammatory mediators [11]; this exacerbates neuroinflammation and the death of oligodendrocytes and, consequently, drives demyelination [1]. Our results show that EAE induced significant astroglial activation in the spinal cord after immunization, which was markedly reduced by inosine treatment. As previously demonstrated, IL-17 induces astroglial activation [62]. Since inosine reduced IL-17 levels in peripheral lymphoid tissue, associated with the inhibition of neuroinflammation, this could be the pathway by which inosine reduces astroglial activation.
It has been demonstrated that ERK1 modulates autoimmune disorders like EAE, which suggests that a deficiency in ERK1 in vivo increases susceptibility to EAE [35]. Previous studies have shown that elevated p-ERK following EAE occurs due to the neuroinflammatory process, and ERK inhibition suppresses the clinical signs of disease [63]. Our results show significant ERK phosphorylation in the untreated EAE group 40 days after immunization; while inosine treatment did not significantly modulate ERK phosphorylation.
Several patients with MS suffer chronic pain, which is often a major symptom of the disease [64]. Prior to the motor phase and associated with inflammatory and neuropathic processes, a previous study revealed that the EAE model is characterized by marked mechanical and thermal hyperalgesia [19], in agreement with our results. Here, there were no significant differences between the 0.4 and 0.6 g von Frey filament response, a measure of moderate mechanical hyperalgesia [40]. Inosine has been considered an A1R agonist [31] and the involvement of A1 and A2A receptors on its analgesic effects have been reported [28, 29]. Herein, our data show that preventive treatment with inosine produced significant inhibition of mechanical and thermal (cold and hot) hyperalgesia during EAE progression. In addition, hyperalgesia can be modulated by increased IL-17 levels due to astrocytes reactivity in the spinal cord after EAE induction [19, 65]. Therefore, it is also possible that the analgesic effect of inosine is related to the inhibition of peripheral IL-17 levels and astroglial activity.
These results demonstrate the protective effect of inosine in the EAE model. Based on these findings, we further investigated the effect of inosine on anxiety and fatigue-like signs in EAE mice. Anxiety-like behavior reported in EAE mice may overlap or not with the manifestation of the motor signs of disease [53, 54]. In this study, we demonstrated significant anxiety-like behavior in untreated EAE mice during the pre-symptomatic phase of disease compared to the naïve group. Recently, an anxiolytic-like effect of inosine has been reported in a depression model by Muto et al. [66]; however, our findings did not show any prevention of anxiety-like behavior by inosine treatment after EAE induction. Corroborating our data, the involvement of adenosinergic receptors in the modulation of anxiety is controversial and the central mechanisms involved remain to be established [67, 68]. Finally, the fatigue symptoms of MS could reflect varying combinations of factors following disease [69] and may be present regardless the amount of exertion and even with no exertion at all [16]. Supporting previous MS human studies [70], our data demonstrate fatigue-like behavior in mice after EAE induction during the pre-motor phase. However, inosine was not effective in reversing fatigue-like behavior following EAE induction Rather, inosine treatment improved factors that could be determinants of a fatigue state, such as the inhibition of hyperalgesia and the prevention of corporal mass loss.
Nowadays, it is not known if inosine is safe or what the possible side effects might be, although kidney stone formation was observed in 4/16 subjects with relapsing–remitting MS (RRMS), after oral administration of inosine during a 1-year randomized, double-blind trial [36]. In addition, unused inosine is converted by the body to UA, which may be hazardous to people at risk for chronic asymptomatic hyperuricemia and, consequently, gout, coronary heart diseases, and urethral and kidney stones. Therefore, further studies are necessary to prove the treatment’s efficacy and safety.
Conclusion
In summary, the data presented here demonstrate that inosine preventive treatment inhibited the development of neurological signs of EAE and disease progression, which was associated with the blockade of immune cell migration and demyelinating processes in the CNS. It should be noted that inosine showed a relevant effectiveness in EAE-induced inflammation, since it also inhibited astroglial activity in the spinal cord and down-regulated IL-17 levels in peripheral lymphoid tissues, which may be related to its effect on thermal and mechanical hyperalgesia. In addition, our data also indicate that the effect of inosine on EAE, possibly, occurs through the down-regulation of A2AR in the spinal cord via an ERK1-independent pathway. Altogether, our findings support previous studies and provide new evidence that inosine, an endogenous purine nucleoside, represents an attractive molecule for the development of a new disease-modifying therapy for MS (Fig. 7). However, additional studies will need to assess the effect of inosine in different signaling pathways related to A2A adenosine receptors.
References
Nylander A, Hafler DA (2012) Multiple sclerosis. J Clin Invest 122(4):1180–1188. doi:10.1172/JCI58649
Sawcer S, Franklin RJ, Ban M (2014) Multiple sclerosis genetics. Lancet Neurol 13(7):700–709. doi:10.1016/S1474-4422(14)70041-9
Blahova Dusankova J, Kalincik T, Dolezal T, Kobelt G, Havrdova E (2012) Cost of multiple sclerosis in the Czech Republic: the COMS study. Mult Scler 18(5):662–668. doi:10.1177/1352458511424422
Federation MSI (2013) Atlas of MS 2013. http://www.atlasofms.org/. Accessed 23 de maio 2014
Ascherio A, Munger KL, Simon KC (2010) Vitamin D and multiple sclerosis. Lancet Neurol 9(6):599–612. doi:10.1016/S1474-4422(10)70086-7
Goodin DS (2009) The causal cascade to multiple sclerosis: a model for MS pathogenesis. PLoS One 4(2):e4565. doi:10.1371/journal.pone.0004565
McFarland HF, Martin R (2007) Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 8(9):913–919. doi:10.1038/Ni1507
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372(9648):1502–1517. doi:10.1016/S0140-6736(08)61620-7
Comabella M, Khoury SJ (2012) Immunopathogenesis of multiple sclerosis. Clin Immunol 142(1):2–8. doi:10.1016/j.clim.2011.03.004
Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y (2006) IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 177(1):566–573
Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L (2008) Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 172(1):146–155. doi:10.2353/ajpath.2008.070690
Moseley TA, Haudenschild DR, Rose L, Reddi AH (2003) Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev 14(2):155–174
Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM (2008) Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med 14(3):337–342. doi:10.1038/nm1715
O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH (2008) Pain associated with multiple sclerosis: systematic review and proposed classification. Pain 137(1):96–111. doi:10.1016/j.pain.2007.08.024
Goretti B, Viterbo RG, Portaccio E, Niccolai C, Hakiki B, Piscolla E, Iaffaldano P, Trojano M et al (2014) Anxiety state affects information processing speed in patients with multiple sclerosis. Neurol Sci 35(4):559–563. doi:10.1007/s10072-013-1544-0
Rubin SM (2013) Management of cognition and fatigue. Dis Mon 59(7):269–272. doi:10.1016/j.disamonth.2013.03.014
Gallien P, Gich J, Sanchez-Dalmau BF, Feneberg W (2014) Multidisciplinary management of multiple sclerosis symptoms. Eur Neurol 72(Suppl 1):20–25. doi:10.1159/000367620
Dutra RC, de Souza PR, Bento AF, Marcon R, Bicca MA, Pianowski LF, Calixto JB (2012) Euphol prevents experimental autoimmune encephalomyelitis in mice: evidence for the underlying mechanisms. Biochem Pharmacol 83(4):531–542. doi:10.1016/j.bcp.2011.11.026
Dutra RC, Bento AF, Leite DF, Manjavachi MN, Marcon R, Bicca MA, Pesquero JB, Calixto JB (2013) The role of kinin B1 and B2 receptors in the persistent pain induced by experimental autoimmune encephalomyelitis (EAE) in mice: evidence for the involvement of astrocytes. Neurobiol Dis 54:82–93. doi:10.1016/j.nbd.2013.02.007
Kim CF, Moalem-Taylor G (2011) Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J Pain 12(3):370–383. doi:10.1016/j.jpain.2010.08.003
Dachir S, Shabashov D, Trembovler V, Alexandrovich AG, Benowitz LI, Shohami E (2014) Inosine improves functional recovery after experimental traumatic brain injury. Brain Res 1555:78–88. doi:10.1016/j.brainres.2014.01.044
Kim D, Zai L, Liang P, Schaffling C, Ahlborn D, Benowitz LI (2013) Inosine enhances axon sprouting and motor recovery after spinal cord injury. PLoS One 8(12):e81948. doi:10.1371/journal.pone.0081948
Smith JM, Lunga P, Story D, Harris N, Le Belle J, James MF, Pickard JD, Fawcett JW (2007) Inosine promotes recovery of skilled motor function in a model of focal brain injury. Brain: J Neurol 130(Pt 4):915–925. doi:10.1093/brain/awl393
Zai L, Ferrari C, Subbaiah S, Havton LA, Coppola G, Strittmatter S, Irwin N, Geschwind D et al (2009) Inosine alters gene expression and axonal projections in neurons contralateral to a cortical infarct and improves skilled use of the impaired limb. J Neurosci 29(25):8187–8197. doi:10.1523/JNEUROSCI.0414-09.2009
Hasko G, Sitkovsky MV, Szabo C (2004) Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci 25(3):152–157. doi:10.1016/j.tips.2004.01.006
da Rocha Lapa F, de Oliveira AP, Accetturi BG, de Oliveira MI, Domingos HV, de Almeida CD, de Lima WT, Santos AR (2013) Anti-inflammatory effects of inosine in allergic lung inflammation in mice: evidence for the participation of adenosine A2A and A 3 receptors. Purinergic signal 9(3):325–336. doi:10.1007/s11302-013-9351-x
da Rocha LF, da Silva MD, de Almeida CD, Santos AR (2012) Anti-inflammatory effects of purine nucleosides, adenosine and inosine, in a mouse model of pleurisy: evidence for the role of adenosine A2 receptors. Purinergic Signal 8(4):693–704. doi:10.1007/s11302-012-9299-2
Nascimento FP, Figueredo SM, Marcon R, Martins DF, Macedo SJ Jr, Lima DA, Almeida RC, Ostroski RM et al (2010) Inosine reduces pain-related behavior in mice: involvement of adenosine A1 and A2A receptor subtypes and protein kinase C pathways. J Pharmacol Exp Ther 334(2):590–598. doi:10.1124/jpet.110.166058
Nascimento FP, Macedo-Junior SJ, Pamplona FA, Luiz-Cerutti M, Cordova MM, Constantino L, Tasca CI, Dutra RC et al (2014) Adenosine A1 receptor-dependent antinociception induced by inosine in mice: pharmacological. Genetic Biochem Aspects Mol Neurobiol. doi:10.1007/s12035-014-8815-5
Kaster MP, Budni J, Gazal M, Cunha MP, Santos AR, Rodrigues AL (2013) The antidepressant-like effect of inosine in the FST is associated with both adenosine A1 and A 2A receptors. Purinergic Signal 9(3):481–486. doi:10.1007/s11302-013-9361-8
Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53(4):527–552
Buckley S, Barsky L, Weinberg K, Warburton D (2005) In vivo inosine protects alveolar epithelial type 2 cells against hyperoxia-induced DNA damage through MAP kinase signaling. Am J Physiol Lung Cell Mol Physiol 288(3):L569–L575. doi:10.1152/ajplung.00278.2004
Tomaselli B, Nedden SZ, Podhraski V, Baier-Bitterlich G (2008) p42/44 MAPK is an essential effector for purine nucleoside-mediated neuroprotection of hypoxic PC12 cells and primary cerebellar granule neurons. Mol Cell Neurosci 38(4):559–568. doi:10.1016/j.mcn.2008.05.004
Zhang Y, Blattman JN, Kennedy NJ, Duong J, Nguyen T, Wang Y, Davis RJ, Greenberg PD et al (2004) Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature 430(7001):793–797. doi:10.1038/nature02764
Agrawal A, Dillon S, Denning TL, Pulendran B (2006) ERK1−/− mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J Immunol 176(10):5788–5796
Markowitz CE, Spitsin S, Zimmerman V, Jacobs D, Udupa JK, Hooper DC, Koprowski H (2009) The treatment of multiple sclerosis with inosine. J Altern Complement Med 15(6):619–625. doi:10.1089/acm.2008.0513
Toncev G (2006) Therapeutic value of serum uric acid levels increasing in the treatment of multiple sclerosis. Vojnosanitetski Pregled Military-Med Pharmaceut Rev 63(10):879–882
Mills JH, Kim DG, Krenz A, Chen JF, Bynoe MS (2012) A2A adenosine receptor signaling in lymphocytes and the central nervous system regulates inflammation during experimental autoimmune encephalomyelitis. J Immunol 188(11):5713–5722. doi:10.4049/jimmunol.1200545
Stromnes IM, Goverman JM (2006) Active induction of experimental allergic encephalomyelitis. Nat Protoc 1(4):1810–1819. doi:10.1038/nprot.2006.285
Lu J, Kurejova M, Wirotanseng LN, Linker RA, Kuner R, Tappe-Theodor A (2012) Pain in experimental autoimmune encephalitis: a comparative study between different mouse models. J Neuroinflammation 9:233. doi:10.1186/1742-2094-9-233
Walczak JS, Beaulieu P (2006) Comparison of three models of neuropathic pain in mice using a new method to assess cold allodynia: the double plate technique. Neurosci Lett 399(3):240–244. doi:10.1016/j.neulet.2006.01.058
Lappas CM, Rieger JM, Linden J (2005) A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol 174(2):1073–1080
Su KY, Yu CY, Chen YW, Huang YT, Chen CT, Wu HF, Chen YL (2014) Rutin, a flavonoid and principal component of saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model. Int J Med Sci 11(5):528–537. doi:10.7150/ijms.8220
Gajofatto A, Bacchetti P, Grimes B, High A, Waubant E (2009) Switching first-line disease-modifying therapy after failure: impact on the course of relapsing-remitting multiple sclerosis. Mult Scler 15(1):50–58. doi:10.1177/1352458508096687
Ouyang S, Hsuchou H, Kastin AJ, Mishra PK, Wang Y, Pan W (2014) Leukocyte infiltration into spinal cord of EAE mice is attenuated by removal of endothelial leptin signaling. Brain Behav Immun 40:61–73. doi:10.1016/j.bbi.2014.02.003
Dutra RC, Leite DF, Bento AF, Manjavachi MN, Patricio ES, Figueiredo CP, Pesquero JB, Calixto JB (2011) The role of kinin receptors in preventing neuroinflammation and its clinical severity during experimental autoimmune encephalomyelitis in mice. PLoS One 6(11):e27875. doi:10.1371/journal.pone.0027875
Brambilla R, Morton PD, Ashbaugh JJ, Karmally S, Lambertsen KL, Bethea JR (2014) Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination. Glia 62(3):452–467. doi:10.1002/glia.22616
Hedegaard CJ, Krakauer M, Bendtzen K, Lund H, Sellebjerg F, Nielsen CH (2008) T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology 125(2):161–169. doi:10.1111/j.1365-2567.2008.02837.x
Tsutsui S, Schnermann J, Noorbakhsh F, Henry S, Yong VW, Winston BW, Warren K, Power C (2004) A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J Neurosci 24(6):1521–1529. doi:10.1523/JNEUROSCI.4271-03.2004
Vincenzi F, Corciulo C, Targa M, Merighi S, Gessi S, Casetta I, Gentile M, Granieri E et al (2013) Multiple sclerosis lymphocytes upregulate A2A adenosine receptors that are antiinflammatory when stimulated. Eur J Immunol 43(8):2206–2216. doi:10.1002/eji.201343314
Shin T, Ahn M, Jung K, Heo S, Kim D, Jee Y, Lim YK, Yeo EJ (2003) Activation of mitogen-activated protein kinases in experimental autoimmune encephalomyelitis. J Neuroimmunol 140(1–2):118–125
Hauser SL GD (2008) Multiple sclerosis and other demyelinating diseases. In: Harrison’s Principles of Internal Medicine, vol II. McGraw-Hill Medical, New York, pp 2611–2621
Acharjee S, Nayani N, Tsutsui M, Hill MN, Ousman SS, Pittman QJ (2013) Altered cognitive-emotional behavior in early experimental autoimmune encephalitis—cytokine and hormonal correlates. Brain Behav Immun 33:164–172. doi:10.1016/j.bbi.2013.07.003
Peruga I, Hartwig S, Thone J, Hovemann B, Gold R, Juckel G, Linker RA (2011) Inflammation modulates anxiety in an animal model of multiple sclerosis. Behav Brain Res 220(1):20–29. doi:10.1016/j.bbr.2011.01.018
Bermel RA, You X, Foulds P, Hyde R, Simon JH, Fisher E, Rudick RA (2013) Predictors of long-term outcome in multiple sclerosis patients treated with interferon beta. Ann Neurol 73(1):95–103. doi:10.1002/ana.23758
Rissanen E, Virta JR, Paavilainen T, Tuisku J, Helin S, Luoto P, Parkkola R, Rinne JO et al (2013) Adenosine A2A receptors in secondary progressive multiple sclerosis: a [(11)C]TMSX brain PET study. J Cereb Blood Flow Metab 33(9):1394–1401. doi:10.1038/jcbfm.2013.85
Tsutsui S, Vergote D, Shariat N, Warren K, Ferguson SS, Power C (2008) Glucocorticoids regulate innate immunity in a model of multiple sclerosis: reciprocal interactions between the A1 adenosine receptor and beta-arrestin-1 in monocytoid cells. FASEB J 22(3):786–796. doi:10.1096/fj.07-9002com
Kean RB, Spitsin SV, Mikheeva T, Scott GS, Hooper DC (2000) The peroxynitrite scavenger uric acid prevents inflammatory cell invasion into the central nervous system in experimental allergic encephalomyelitis through maintenance of blood-central nervous system barrier integrity. J Immunol 165(11):6511–6518
Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N et al (2007) Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nat Med 13(10):1173–1175. doi:10.1038/nm1651
Zhang GX, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A (2003) Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol 170(4):2153–2160
Pitt D, Werner P, Raine CS (2000) Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med 6(1):67–70. doi:10.1038/71555
Carlson T, Kroenke M, Rao P, Lane TE, Segal B (2008) The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp Med 205(4):811–823. doi:10.1084/jem.20072404
Brereton CF, Sutton CE, Lalor SJ, Lavelle EC, Mills KH (2009) Inhibition of ERK MAPK suppresses IL-23- and IL-1-driven IL-17 production and attenuates autoimmune disease. J Immunol 183(3):1715–1723. doi:10.4049/jimmunol.0803851
Osterberg A, Boivie J (2010) Central pain in multiple sclerosis—sensory abnormalities. Eur J Pain 14(1):104–110. doi:10.1016/j.ejpain.2009.03.003
Day YJ, Liou JT, Lee CM, Lin YC, Mao CC, Chou AH, Liao CC, Lee HC (2014) Lack of interleukin-17 leads to a modulated micro-environment and amelioration of mechanical hypersensitivity after peripheral nerve injury in mice. Pain 155(7):1293–1302. doi:10.1016/j.pain.2014.04.004
Muto J, Lee H, Lee H, Uwaya A, Park J, Nakajima S, Nagata K, Ohno M et al (2014) Oral administration of inosine produces antidepressant-like effects in mice. Sci Rep 4:4199. doi:10.1038/srep04199
Hughes RN, Hancock NJ, Henwood GA, Rapley SA (2014) Evidence for anxiolytic effects of acute caffeine on anxiety-related behavior in male and female rats tested with and without bright light. Behav Brain Res 271:7–15. doi:10.1016/j.bbr.2014.05.038
Kulkarni SK, Singh K, Bishnoi M (2007) Involvement of adenosinergic receptors in anxiety related behaviours. Indian J Exp Biol 45(5):439–443
Kroencke DC, Lynch SG, Denney DR (2000) Fatigue in multiple sclerosis: relationship to depression, disability, and disease pattern. Mult Scler 6(2):131–136
Flachenecker P, Bihler I, Weber F, Gottschalk M, Toyka KV, Rieckmann P (2004) Cytokine mRNA expression in patients with multiple sclerosis and fatigue. Mult Scler 10(2):165–169
Acknowledgments
We thank Gabriela Segat, Thaís Barbosa Alberti, Rodrigo Marcon and Allisson Freire Bento for technical assistance. Grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Apoio a Pesquisa do Estado de Santa Catarina (FAPESC), and Programa de Pós-Graduação em Neurociências (PGN), all from Brazil, supported this work. S.C.J., I.S.C and V.L. are PhD students in neuroscience receiving grants from CAPES and FAPESC. M.P.C. holds a postdoctoral fellowship from CAPES.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts of interest.
Additional information
Highlights
• Inosine displays pronounced anti-inflammatory and antinociceptive properties.
• Inosine inhibits demyelinating process and astroglial activation.
• Inosine modulates adenosinergic receptors, especially A2AR.
Rights and permissions
About this article
Cite this article
Junqueira, S.C., dos Santos Coelho, I., Lieberknecht, V. et al. Inosine, an Endogenous Purine Nucleoside, Suppresses Immune Responses and Protects Mice from Experimental Autoimmune Encephalomyelitis: a Role for A2A Adenosine Receptor. Mol Neurobiol 54, 3271–3285 (2017). https://doi.org/10.1007/s12035-016-9893-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9893-3