Abstract
Brain inflammation has a critical role in the pathophysiology of brain diseases. Microglia, the resident immune cells in the brain, play an important role in brain inflammation, while brain mast cells are the “first responder” in the injury rather than microglia. Functional aspects of mast cell-microglia interactions remain poorly understood. Our results demonstrated that site-directed injection of the “mast cell degranulator” compound 48/80 (C48/80) in the hypothalamus induced mast cell degranulation, microglial activation, and inflammatory factor production, which initiated the acute brain inflammatory response. “Mast cell stabilizer” disodium cromoglycate (cromolyn) inhibited this effect, including decrease of inflammatory cytokines, reduced microglial activation, inhibition of MAPK and AKT pathways, and repression of protein expression of histamine receptor 1 (H1R), histamine receptor 4 (H4R), protease-activated receptor 2 (PAR2), and toll-like receptor 4 (TLR4) in microglia. We also demonstrated that C48/80 had no effect on microglial activation in mast cell-deficient KitW-sh/W-sh mice. These results implicate that activated brain mast cells trigger microglial activation and stabilization of mast cell inhibits microglial activation-induced central nervous system (CNS) inflammation. Interactions between mast cells and microglia could constitute a new and unique therapeutic target for CNS immune inflammation-related diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The initiation and propagation of neuroinflammation appear to rely very much on the interaction between glia, immune cells, and neurons, although the glia-immune cell connection remained to be fully explored. Microglia, the resident immune cells in the brain, play a pivotal role in immune surveillance of the central nervous system (CNS). Consequently, these cells are likely to play an important role in either development of protective immune responses or progression of damaging inflammation during CNS disease states. Pathological states within the nervous system, including injury, ischemic stroke [1], and infection [2], can lead to microglial activation and production of a host of factors, including tumor necrosis factor-a (TNF-α), prostaglandin E2 (PGE2), interleukin-6 (IL-6), nitric oxide (NO), and reactive oxygen species (ROS). Accumulation of these proinflammatory and cytotoxic factors is deleterious directly to neurons and subsequently induces further activation of microglia, resulting in a vicious cycle [3, 4]. Thus, inhibition of microglial activation and subsequent inflammatory process may identify novel therapeutic strategies to eliminate microglia deleterious effects [5].
Besides releasing proinflammatory mediators, microglia also respond to proinflammatory signals released from other nonneuronal cells of immune origin. Mast cells represent a potentially important and underappreciated peripheral immune signaling link to the brain in an inflammatory setting [6]. Mast cells, best known for their role in allergic inflammation, are distributed in a variety of anatomical sites, including the CNS, where they are often found adjacent to blood vessels and nerves [7]. Mast cells are located perivascularly close to neurons and microglia and may contribute to brain inflammation through different mechanisms [8]. Mast cells can move through the blood–brain barrier of normal brain [9] but may also traverse the blood–spinal cord barrier and blood–brain barrier when compromised by disease. Theoharides et al. have suggested that perinatal mast cell activation by infectious, stress-related, environmental or allergic triggers can lead to release of proinflammatory and neurotoxic molecules, therefore contributing to brain inflammation and autism spectrum disorder pathogenesis, at least in a subgroup of these patients [10]. These reactions are mediated by degranulation, whereby a number of preformed or newly synthesized mediators and cytokines are secreted by activated mast cells, such as GnRH [11], monoamines [12], specific proteases, cytokines, and histamine [13]. These neurotransmitters and neuromodulators can initiate and modulate several inflammatory pathways implicated in neuroinflammatory diseases affected by stress [14]. Notably, mast cell mediators can recruit and activate T cells as well as permit them to enter the brain by disrupting the BBB [15].
Several molecular mechanisms for potential interactions between mast cells and microglia have been determined in vitro [16]. However, the effect of mast cell on microglia is still unclear. Yuan reported that mast cell activation can induce microglia to release neurotrophin [17]. We have previously reported that tryptase and histamine can induce microglial activation and inflammatory mediator release [18, 19], which also suggests the importance of mast cells in induction of inflammation in the CNS. However, the direct effect of mast cell on microglial activation has not been reported. In this study, mast cell degranulator and stabilizer, mast cell-deficient KitW-sh/W-sh mice were used to investigate the effect of mast cells on microglial activation and CNS immune inflammation.
Materials and Methods
Animals
Male Sprague Dawley (about 250 g) rats, mast cell-deficient KitW-sh/W-sh mice (STOCK KitW-sh/HNihrJaeBsmJNju, 6 months old), and littlermate controls were purchased from Mode Animal Research Center of Nanjing University (Nanjing, China). All animals were housed in groups of five animals per cage under standard laboratory conditions with free access to food and water, constant room temperature of 22 °C, 50–60 % humidity, a 12:12-day–night cycle, etc. All experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication no. 85-23, revised 1985) and the Guidelines for the Care and Use of Animals in Neuroscience Research by the Society for Neuroscience and approved by Institutional Animal Care and Use Committee of Nanjing Medical University (IACUC).
Reagents
Compound 48/80 (C48/80), disodium cromoglycate (cromolyn), and toluidine blue were purchased from Sigma (St. Louis, MO, USA). Anti-Mast Cell Tryptase antibody (AA1) and fluoroshield mounting medium with 4,6-diamidino-2-phenylindole (DAPI) were purchased from Abcam (Hong Kong, China). Specific mouse anti-Iba1 antibody was purchased from AbD Serotec (Raleigh, NC, USA). Specific rabbit moloclonal antibodies against p38, Phospho-p38, Jun N-terminal kinase (JNK), Phospho-JNK, extracellular signal-regulated kinases (ERK), Phospho-ERK, AKT, Phospho-AKT, and goat anti-rabbit secondary antibody were obtained from Cell Signaling (Beverly, MA, USA). Specific rabbit monoclonal anti-H3 receptor antibody was purchased from Abcam (Hong Kong, China). Specific rabbit polyclonal anti-H1 receptor and rabbit polyclonal anti-H2 receptor antibodies were purchased from Alomone Labs Ltd. (Israel), and rabbit polyclonal anti-H4 receptor was purchased from Santa Cruz (CA, USA). Phycoerythrin (PE)-conjugated mouse anti-rat OX-42 monoclonal antibody and isotype control and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit secondary antibody were purchased from BD (BD Biosciences, USA). Rat IL-6 Immunoassay Kit and Rat TNF-α Immunoassay Kit were obtained from R&D Systems, Inc. (Minneapolis, MN, USA).
Surgery and Drug Administration
The rats were randomly allocated to six groups (group A–F) with 12 rats in each group, and investigators were blinded to the experimental treatment. Rats of groups C–F were pretreated with mast cell stabilizer cromolyn (100 or 200 μg/μl) for 30 min, simultaneously rats of groups A and B pretreated with sterile saline. After 30 min, rats of groups B–D were injected with C48/80 (1 μg/μl) in the hypothalamus and rats of groups A (control rats), E, and F were injected with sterile saline for another 30 min.
Following anesthesia, rats were placed in the stereotaxic apparatus (Stoelting Instruments, USA). Guide cannulas (Plastic One) were inserted into the right hypothalamus as follows: 1.80 mm lateral and 1.90 mm posterior from Bregma, at a 10° angle and 8 mm deep [20]. The animals were allowed to recover in the animal facility for 14 days before use. Animals were handled daily to check the guide cannula and to familiarize them with the investigators. To determine whether mast cell degranulator C48/80 can activate microglia, we administered C48/80 centrally by an ipsilateral site injection in the hypothalamus. We chose to inject the hypothalamus because mast cells are plentiful in this area. Animals with implanted guide cannulas received 2.5 of 1 μg/μl C48/80 (2.5 μg) or 2.5 μl 0.9 % NaCl directly in the hypothalamus. Animals remained in their cages for 30 min or 24 h and were not restrained. To stabilize mast cells, the same site was pretreated with 1 μl of cromolyn (100 or 200 μg/μl) 30 min before C48/80 administration. Control animals were pretreated with 1 μl 0.9 % NaCl. Rats were killed after drug administration, and their brains, followed by the separation of meninges from which, were used for morphological (n = 6) and biochemical (n = 6) analyses.
The mice were divided into four treatment groups. Of the wild-type (WT) mice groups, one group received C48/80 administration (n = 5) and a control group received saline (n = 5). Similarly with the KitW-sh/W-sh mice, one group received C48/80 administration (n = 5) while the other group received saline (n = 5).
Following anesthesia, mice were mounted in the stereotaxic frame and kept at 37 °C using a heating pad. A burrhole was made to inject into the right hypothalamus as follows: 0.96 mm lateral and 0.82 mm posterior from Bregma, at a 10° angle and 4.82 mm deep [21]. A 33-gauge needle connected to a 10-μl syringe was then lowered to 4.82 mm, and either C48/80 (1 μg/μl, 0.8 μg) or saline (0.8 μl) was injected. The needle was then left in place for 5 min before being removed to suture the skin. Mice were killed after drug administration, and their brains, followed by the separation of meninges from which, were used for morphological (n = 5) analyses.
Mast Cell Staining and Counting
Slides were stained in 0.05 % toluidine blue for thalamic sections. A 1 % stock solution in 70 % ethanol was dissolved in 0.5 % NaCl solution (pH 2.2–2.3), and the slides were immersed for 30 min in the above concentration. They were then washed twice in distilled water, dehydrated in a series of increasing concentrations of ethanol, followed by placement in butyl acetate ester. Samples were coverslipped using Eukitt® mounting medium and were allowed to dry overnight.
Mast cell counting was always performed in a blinded fashion by an experimenter that was unaware of the sample identity. Criteria for degranulation included loss of purple staining, fuzzy appearance, distorted shape, or multiple granules visible in the vicinity of the cell. For thalamic sections, the entire surface area of the ipsilateral and contralateral thalamus was scanned manually using a light microscope at ×200 magnification and mast cells were calculated with the help of the Cell D software (Olympus).
Immunochemistry
Rats and mice were anesthetized by chloral hydrate and perfused first with 0.9 % saline and then with cold 4 % paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The brains were dissected out and maintained in 4 % paraformaldehyde overnight. Brains were cryopreserved in 30 % sucrose in phosphate-buffered saline (PBS) and then stored at −70 °C until used. Free-floating sections encompassing the entire brain were prepared using a cryostat. Sections were processed for Iba1 and mast cell tryptase immunohistochemistry as follows. After incubation for 1 h in 10 % bovine serum albumin with 0.3 % Triton X-100 in 0.01 M PBS, tissue sections (30 μm) were incubated with primary antibodies overnight at 4 °C. Primary antibodies used in this study were as follows: rabbit anti-Iba1 (1:200; Wako Chemicals USA, Inc.) and mouse anti-Mast Cell Tryptase (1:100; abcam, USA). Immunostaining was visualized by using 3,3′-diaminobenzidine. Sections were then counterstained with hematoxylin.
Flow Cytometry Analysis
To determine the receptor expression affected by C48/80 in microglia, the ipsilateral hypothalamus tissues were minced with sterile scissors and digested with 0.25 % Trypsin–EDTA solution for 5 min at 37 °C. Trypsinization was stopped by adding an equal volume of culture medium. The dissociated cells were passed through a 100-μm-pore mesh, pelleted at 200 g for 10 min at 4 °C, and resuspended and fixed in 4 % paraformaldehyde for 30 min. After washing, the cells were incubated with rabbit anti-H1R, anti-H2R, anti-H3R, anti-H4R, anti-PAR2, and anti-toll-like receptor 4 (TLR4) antibodies or normal rabbit IgG, respectively, overnight at 4 °C, followed by 1 μg/ml of FITC-conjugated goat anti-rabbit secondary antibody alone with PE-conjugated mouse anti-rat OX-42 monoclonal antibody or isotype control (1:200) at 37 °C for 1 h. Cells were finally resuspended in PBS and analyzed on a FACSCalibur flow cytometer with CellQuest software (BD Biosciences, USA).
TNF-α and IL-6 Assay
The content of TNF-α and IL-6 in rat brain tissue extracts was measured with a commercial ELISA kit from R&D Systems.
Western Blotting
Ipsilateral hypothalamus tissue extracts were collected and homogenized in 200 ml of lysis buffer. After incubation for 20 min on ice, cell lysate was centrifuged and protein concentration in the extracts was determined by the Bradford assay. Proteins (50 μg) in cell extracts were denatured with sodium dodecyl sulfate (SDS) sample buffer and separated by 10 % SDS–polyacrylamide gel electrophoresis. Proteins were transferred to PVDF membranes (Millipore) by using a Bio-Rad miniprotein-III wet transfer unit. The membranes were incubated with 5 % BSA dissolved in Tris-buffered saline with Tween 20 (TBST) (pH 7.5, 10 mM Tris–HCl, 150 mM NaCl, and 0.1 % Tween 20) at room temperature for 1 h. This was followed by incubating the membranes with different antibodies overnight at 4 °C. The following primary antibodies were used: rabbit monoclonal anti-c-Jun N-terminal kinase (JNK), anti-phospho-JNK, anti-p38, anti-phospho-p38, anti-ERK, phospho-ERK, anti-AKT, and anti-phospho-AKT (1:1000). After adding the goat-anti-rabbit secondary antibody (1:1000) for 1 h, the protein bands on the membranes were detected with an enhanced chemiluminescence kit.
Statistical Analysis
All values are means ± SEM. The significance of the difference between control and samples treated with various drugs was determined by one-way ANOVA followed by the post hoc least significant difference test. Differences were considered significant at P < 0.05.
Results
Cromolyn Repressed C48/80-Induced Mast Cell Activation in Hypothalamus
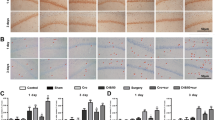
At first, we investigated the activated brain mast cells induced by C48/80. Brain mast cells were quantified in toluidine blue (TB) and mast cell tryptase-stained sections (Fig. 1a, b). As shown in Fig. 1c, directed site injection of 2.5 μl of 1 μg/μl C48/80 (2.5 μg), which is a mast cell degranulator, in the right hypothalamus for 30 min significantly induced activated mast cell number which increased in both the ipsilateral and contralateral hypothalamus and which was significantly greater than those of the control group. Mast cell stabilizer cromolyn (100 or 200 μg) repressed the mast cell activation induced by C48/80.
Cromolyn repressed C48/80-induced mast cell activation in both the ipsilateral and contralateral hypothalamus. a Brain mast cells were stained with toluidine blue (TB). Scale bar: 100 μm. b Immunostaining was used to detect mast cell tryptase. Scale bar: 100 μm. c Quantification of activated mast cells in the ipsilateral and contralateral hypothalamus. * P < 0.05, ** P < 0.01 versus control group. # P < 0.05, ## P < 0.01 versus C48/80 group. Data are presented as the mean ± SEM (n = 6)
Stabilization of Mast Cell Inhibited Microglial Activation in Hypothalamus
In order to explore the effects of activated mast cells on microglial activation, immunostaining was used to detect Iba1, marker for microglia. C48/80 could induce notable microglial activation in both the ipsilateral and contralateral hypothalamus, indicated by a large number of Iba1-ir cells, which had larger cell body and poorly ramified short and thick processes. Cromolyn (100 or 200 μg) pretreatment resulted significantly in suppression of microglial activation in both the ipsilateral and contralateral hypothalamus, indicated by few Iba1-ir cell numbers, small cell body, and ramified and thin processes. Moreover, site injection of cromolyn (100 or 200 μg) alone had no effect on microglial activation in the brain (Fig. 2). However, site injection of C48/80 in the right hypothalamus had no significant effect on the mast cell and microglial activation in both ipsilateral and contralateral cerebral cortices (data not shown). These results suggest that stabilization of mast cells can inhibit microglial activation in the hypothalamus which does not propagate to the cerebral cortex within the analyzed time period.
Stabilization of mast cell inhibited microglial activation in hypothalamus. a Immunostaining was used to detect Iba1, markers for microglia. Rats treated with vehicle showed minimal microglial activation in both the ipsilateral and contralateral hypothalamus (a, g) indicated by few Iba1-ir cell number and small cell body with ramified and thin processes. In C48/80 (2.5 μg)-treated rats, there were numerous Iba1-ir cells in both the ipsilateral and contralateral hypothalamus (b, h), which had larger cell body and poorly ramified short and thick processes. Rats pretreated with cromolyn (100 or 200 μg) reduced the number of C48/80-induced Iba1-positive cells. Scale bar: 100 μm. b Quantification of Iba1-positive cells in the ipsilateral and contralateral hypothalamus. * P < 0.05, ** P < 0.01 versus control group. # P < 0.05, ## P < 0.01 versus C48/80 group. Data are presented as the mean ± SEM (n = 6)
C48/80 Had No Effect on Microglial Activation in Hypothalamus of KitW-sh/W-sh Mice
To further confirm whether activated mast cells can induce microglial activation, we used the mast cell-deficient KitW-sh/W-sh mice. In Fig. 3a, TB- and mast cell tryptase-stained cells in hypothalamus were found in WT mice, but not in KitW-sh/W-sh mice, indicating that TB and tryptase staining were specific to mast cells. As shown in Fig. 3b, c, directed site injection of 0.8 μl of 1 μg/μl C48/80 (0.8 μg) in the hypothalamus for 30 min induced notable microglial activation in both the ipsilateral and contralateral hypothalamus of the WT mice but had no effect on the microglial activation in KitW-sh/W-sh mice. These results suggest that C48/80 induces microglial activation through activating mast cells.
C48/80 had no effect on microglial activation in hypothalamus of KitW-sh/W-sh mice. a Toluidine blue and mast cell tryptase staining of mast cells in the hypothalamus of WT and KitW-sh/W-sh mice. b Immunostaining was used to detect Iba1, markers for microglia. c Quantification of Iba1-positive cells in the ipsilateral and contralateral hypothalamus. Scale bar: 50 μm. * P < 0.05, ** P < 0.01. Data are presented as the mean ± SEM (n = 5)
Stabilization of Mast Cell Inhibited C48/80-Induced TNF-α and IL-6 Production
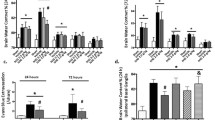
Since microglia-mediated neuroinflammation is mainly due to the excessive proinflammatory factors from activated microglia and their downstream signaling cascades, the levels of proinflammatory factors TNF-α and IL-6 were detected. The results showed that injection of C48/80 (2.5 μg) in hypothalamus for 30 min or 24 h significantly increased TNF-α content in ipsilateral hypothalamus (756 and 847 pg/ml) and contralateral hypothalamus (372 and 554 pg/ml). However, injection of C48/80 in hypothalamus had no effect on the TNF-α content in the ipsilateral and contralateral cerebral cortices. Furthermore, cromolyn (100 or 200 μg) could attenuate the content of TNF-α in the ipsilateral and contralateral hypothalamus (Fig. 4a). The same as the TNF-α, cromolyn also inhibited C48/80-induced IL-6 production in the ipsilateral and contralateral hypothalamus but had no effect on the IL-6 content in the ipsilateral and contralateral cerebral cortices (Fig. 4b). However, site injection of cromolyn (100 or 200 μg) in hypothalamus for 30 min or 24 h alone had no significant effect on the content of TNF-α or IL-6 in the hypothalamus and cerebral cortex. These results suggest that stabilization of mast cells can inhibit the production of proinflammatory factors.
Stabilization of mast cell inhibited C48/80-induced TNF-α and IL-6 production. Site injection of C48/80 (2.5 μg) in hypothalamus for 30 min or 24 h significantly increased TNF-α (a) and IL-6 (b) content in ipsilateral and contralateral hypothalamus. Furthermore, cromolyn (100 or 200 μg) could partially inhibit the effect of C48/80. * P < 0.05, ** P < 0.01 versus control group. ## P < 0.01 versus C48/80 group. Data are presented as the mean ± SEM (n = 6)
Stabilization of Mast Cell Depressed MAPK and PI3K/AKT Activation in Hypothalamus
Mitogen-activated protein kinases (MAPK) are the predominant signaling transduction pathways responsible for the synthesis and production of proinflammatory mediators from microglia [22, 23]. Phosphorylation of AKT is a downstream target of PI3K activation and therefore is a proxy for activation of the PI3K pathway [24]. So, we investigated whether C48/80 could affect MAPK and AKT phosphorylation, which might in turn regulate the production of proinflammatory mediators. As shown in Fig. 5, site injection of C48/80 (2.5 μg) in hypothalamus for 30 min evoked the phosphorylation of p38, JNK, ERK, and AKT, indicative of p38, JNK, ERK, and AKT activation. However, pretreatment of cromolyn (100 or 200 μg) for 30 min before C48/80 administration partially suppressed the effect of C48/80 on the phosphorylation of MAPK and AKT. These results suggest that cromolyn can inhibit mast cell degranulator C48/80-induced MAPK and AKT signaling pathway activation.
Cromolyn Inhibited Activated Mast Cells Which Induced Receptor Change on Microglia in Hypothalamus
We have previously reported that tryptase and histamine, which are the mediators released from degranulated mast cells, were able to induce microglia activation and inflammatory mediator release [18, 19]. In order to determine if degranulated mast cells can modulate expression of some receptor proteins in microglia, flow cytometry analysis was employed in the present study. The results showed that site injection of C48/80 (2.5 μg) in hypothalamus for 24 h selectively provoked upregulation of expressions of H1R, H4R, PAR2, and TLR4 (to 141, 142, 136, and 144 % of control, respectively), but deregulation of H2R and H3R expressions in the ipsilateral hypothalamus microglia. Site injection of cromolyn (100 or 200 μg) in hypothalamus for 24 h alone had no significant effect on the expression of these receptor proteins. However, pretreatment of cromolyn (100 or 200 μg) for 30 min before C48/80 administration could partially inhibit the effect of C48/80 on receptor protein expression in microglia (Fig. 6). These results suggest that activated mast cells can modulate receptor protein expression in microglia, which can be inhibited by a mast cell stabilizer.
Cromolyn inhibited activated mast cells which induced receptor change on microglia in hypothalamus. Flow cytometry-assisted expression of H1R, H2R, H3R, H4R, PAR2, and TLR4 in the ipsilateral hypothalamus microglia. Site injection of C48/80 (2.5 μg) in hypothalamus for 24 h selectively provoked upregulation of expressions of H1R, H4R, PAR2, and TLR4, but deregulation of H2R and H3R expressions in the ipsilateral hypothalamus microglia. Pretreatment of cromolyn (100 or 200 μg) for 30 min before C48/80 administration could partially inhibit the effect of C48/80 on receptor proteins expression in microglia. * P < 0.05, ** P < 0.01 versus control group. # P < 0.05 versus C48/80 group. Data are presented as the mean ± SEM (n = 6)
Discussion
Signaling mechanisms that regulate neuroinflammation and that target regulators of neuroinflammation may prove to be a useful therapeutic strategy to nervous system disorders. It also raises the question of whether we are missing important therapeutic avenues by studying glia and mast cells in isolation from each other [25]. Emerging evidence suggests the possibility of mast cell–microglia communication. However, the direct evidence that whether mast cells could affect microglial activation is deficiency. In this study, we found that activated brain mast cell was able to activate microglia and aggravate neuroinflammation.
Considerable effort has been directed to inhibiting the inflammatory cascade of blood-borne neutrophil and phagocyte infiltration in neuroinflammation, but few studies have focused on resident brain cell types capable of mounting immediate host responses in the brain and meninges, namely mast cells. Through release of their proinflammatory mediators, mast cells actively participate in the pathogenesis of inflammation [26, 27]. In the present study, we found that directed site injection of mast cell degranulator C48/80 in the right hypothalamus for 30 min induced mast cell and microglial activation in both the ipsilateral and contralateral hypothalamus but had no effect on microglial activation in mast cell-deficient KitW-sh/W-sh mice. Mast cell activation is the “first responder” in this injury and not microglia [28]. Although other resident cells in the CNS produce TNF-α, most notably microglia/macrophages [29, 30] and endothelial cells [31], the presence and release of TNF-α from mast cells preceded its detection in other cells. In our study, we found that injection of mast cell degranulator C48/80 in hypothalamus for 30 min or 24 h significantly increased TNF-α and IL-6 contents in ipsilateral and contralateral hypothalamus. These results further confirm that only the activated mast cells can induce microglial activation and proinflammatory factor release.
Neuroinflammatory disorders are conditions in which components of the nervous system are damaged by inflammatory effectors derived from the innate and adaptive immune systems, as well as glia within the CNS. Microglia, in particular, acts as sensor cells for disturbed brain tissue homeostasis and accumulates locally in response to neuron injury [32]. Microglial activation has been demonstrated to be an early sign that often precedes and triggers neuronal death in chronic neurodegenerative diseases [5, 33]. Few studies until now have focused on resident cell types capable of mounting immediate host responses in the brain. Mast cells are effector cells of the innate immune system and represent the “first responders” to injury rather than microglia [28]. If the immediate/early mast cell activation is necessary for the initiation of the inflammatory cascade and ultimate tissue damage, inhibition of this response should be neuroprotective. We found that inhibition of immediate mast cell activation by mast cell stabilizer cromolyn limited microglial activation, and C48/80 had no effect on microglial activation in KitW-sh/W-sh mice. Furthermore, pretreatment with cromolyn (100 or 200 μg) 30 min before C48/80 administration could attenuate the content of TNF-α and IL-6 increase induced by C48/80 in the ipsilateral and contralateral hypothalamus. These results indicate that stabilization of mast cell can inhibit microglial activation and production of proinflammatory factors.
The factors responsible for the overactivation of microglia are largely undefined. It is well established that the CNS contains mast cells that are located on the brain side of the blood–brain barrier [34]. They could mediate alterations in blood flow, neurotransmission, and local immune responses in the brain as well [35]. Mast cells are characterized by a large complement of secretory granules which store a wide variety of mediators, including biogenic amines, neuropeptides, cytokines, sulfated proteoglycans, and neutral proteases. Mast cell degranulation is a very rapid process [36], which results in the upregulation of numerous chemokines [37, 38]. When released in the CNS, mast cell secretary products can alter the function of both neural [39], T cell [15], and vascular elements [40]. These cytokines also induce a proinflammatory profile in microglia [41, 42]. We have previously reported that mast cell tryptase can induce microglial activation via the PAR2-MAPK-NF-kappa B signaling pathway [18] and histamine can evoke microglial activation via the H1R and H4R-MAPK and PI3K/AKT-NF-kappa B signaling pathways [19], which also suggests the importance of mast cells in induction of inflammation in the CNS. Here, we demonstrate that injection of mast cell degranulator C48/80 in the hypothalamus for 24 h significantly increased the expression of H1R, H4R, PAR2, and TLR4 in microglia. Hence, once mast cells degranulated, the released mediators combine with these receptors on microglia to induce its activation, following with MAPK and AKT signaling pathways, which contributes to neuroinflammation and the exacerbation of CNS immune inflammation-related disease [25].
In summary, the present study identifies that site-directed injection of the “mast cell degranulator” C48/80 in the hypothalamus induces microglial activation, inflammatory factors production, phosphorylation of MAPK and AKT pathways, and increase of protein expressions of H1R, H4R, PAR2, and TLR4 receptors in microglia, which was inhibited by the “mast cell stabilizer” cromolyn. However, C48/80 has no effect on microglial activation in mast cell-deficient KitW-sh/W-sh mice. These results implicate that only activated brain mast cells can trigger microglial activation, and stabilization of mast cell inhibits microglial activation-induced CNS inflammation. Interactions between mast cells and microglia could constitute a new and unique therapeutic target for CNS immune inflammation-related diseases.
References
Nilupul Perera M, Ma HK, Arakawa S, Howells DW, Markus R, Rowe CC, Donnan GA (2006) Inflammation following stroke. J Clin Neurosci 13:1–8
De Chiara G, Marcocci ME, Sgarbanti R, Civitelli L, Ripoli C, Piacentini R, Garaci E, Grassi C et al (2012) Infectious agents and neurodegeneration. Mol Neurobiol 46:614–638
Block ML, Hong JS (2005) Microglial and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76:77–98
Herrera AJ, Tomas-Camardiel M, Venero JL, Cano J, Machado A (2005) Inflammatory process as a determinant factor for the degeneration of substantia nigra dopaminergic neurons. J Neural Transm 112:111–119
Gao HM, Liu B, Zhang W, Hong JS (2003) Novel anti-inflammatory therapy for Parkinson’s disease. Trends Pharmacol Sci 24:395–401
Skaper SD, Facci L, Giusti P (2014) Mast cells, glia and neuroinflammation: partners in crime? Immunology 141:314–327
Dropp JJ (1976) Mast cells in mammalian brain. Acta Anat 94:1–21
Theoharides TC, Stewart JM, Panagiotidou S, Melamed I. (2015). Mast cells, brain inflammation and autism. Eur J Pharmacol. doi: 10.1016/j.ejphar.2015.03.086
Silverman AJ, Sutherland AK, Wilhelm M, Silver R (2000) Mast cells migrate from blood to brain. J Neurosci 20:401–408
Theoharides TC, Angelidou A, Alysandratos KD, Zhang B, Asadi S, Francis K, Toniato E, Kalogeromitros D (2012) Mast cell activation and autism. Biochim Biophys Acta 1822:34–41
Khalil MH, Silverman AJ, Silver R (2003) Mast cells in the rat brain synthesize gonadotropin-releasing hormone. Neurobiol 56:113–124
Silver R, Silverman AJ, Vitkovic L, Lederhendler I (1996) Mast cells in the brain: evidence and functional significance. Trends Neurosci 19:25–31
Ikarashi Y, Yuzurihara M (2002) Experimental anxiety induced by histaminergics in mast cell-deficient and congenitally normal mice. Pharmacol Biochem Behav 72:437–441
Theoharides TC, Cochrane DE (2004) Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol 146:1–12
Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, Zak-Nejmark T, Koga R et al (2001) Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature 413:420–425
Skaper SD, Facci L (2012) Mast cell–glia axis in neuroinflammation and therapeutic potential of the anandamide congener palmitoylethanolamide. Philos Trans R Soc LondB Biol Sci 367:3312–3325
Yuan H, Zhu X, Zhou S, Chen Q, Zhu X, Ma X, He X, Tian M et al (2010) Role of mast cell activation in inducing microglial cells to release neurotrophin. J Neurosci Res 88:1348–1354
Zhang S, Zeng X, Yang H, Hu G, He S (2012) Mast cell tryptase induces microglia activation via protease-activated receptor 2 signaling. Cell Physiol Biochem 29:931–940
Dong H, Zhang W, Zeng X, Hu G, Zhang H, He S, Zhang S (2014) Histamine induces upregulated expression of histamine receptors and increases release of inflammatorymediators from microglia. Mol Neurobiol 49:1487–1500
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, Inc, Orlando, Florida, p 32887
Paxinos G, Franklin K (2001) The mouse brain in stereotatic coordinates. Acadmic Press. pp. Figs, London, pp 1–100
Akundi RS, Candelario-Jalil E, Hess S, Hull M, Lieb K, Gebicke-Haerter PJ, Fiebich BL (2005) Signal transduction pathways regulating cyclooxygenase-2 in lipopolysaccharide-activated primary rat microglia. Glia 51:199–208
Ciallella JR, Saporito M, Lund S, Leist M, Hasseldam H, McGann N, Smith CS, Bozyczko-Coyne D et al (2005) CEP-11004, an inhibitor of the SAPK/JNK pathway, reduces TNF-alpha release from lipopolysaccharide-treated cells and mice. Eur J Pharmacol 515:179–187
Desai P, Thurmond RL (2011) Histamine H4 receptor activation enhances LPS-induced IL-6 production in mast cells via ERK and PI3K activation. Eur J Immunol 41:1764–1773
Skaper SD, Giusti P, Facci L (2012) Microglia and mast cells: two tracks on the road to neuroinflammation. Faseb J 26:3103–3017
Biran V, Cochois V, Karroubi A, Arrang JM, Charriaut-Marlangue C, Heron A (2008) Stroke induces histamine accumulation and mast cell degranulation in the neonatal rat brain. Brain Pathol 18:1–9
Lozada A, Maegele M, Stark H, Neugebauer EM, Panula P (2005) Traumatic brain injury results in mast cell increase and changes in regulation of central histamine receptors. Neuropathol Appl Neurobiol 31:150–162
Jin Y, Silverman AJ, Vannucci SJ (2009) Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke 40:3107–3112
Gregersen R, Lambertsen K, Finsen B (2000) Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab 20:53–65
Lambertsen KL, Meldgaard M, Ladeby R, Finsen B (2005) A quantitative study of microglial-macrophage synthesis of tumor necrosis factor during acute and late focal cerebral ischemia in mice. J Cereb Blood Flow Metab 25:119–135
Hallenbeck JM (2002) The many faces of tumor necrosis factor in stroke. Nat Med 8:1363–1368
David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12:388–399
Minghetti L (2005) Role of inflammation in neurodegenerative diseases. Curr Opin Neurol 18:315–321
Maurer M, Theoharides T, Granstein RD, Bischoff SC, Bienenstock J, Henz B, Kovanen P, Piliponsky AM et al (2003) What is the physiological function of mast cells? Exp Dermatol 12:886–910
Galli SJ, Nakae S, Tsai M (2005) Mast cells in the development of adaptive immune responses. Nat Immunol 6:135–142
Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M (2005) Mast cells as “Tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol 23:749–786
Feuser K, Thon KP, Bischoff SC, Lorentz A (2012) Human intestinal mast cells are a potent source of multiple chemokines. Cytokine 58:178–185
Bulanova E, Bulfone-Paus S (2010) P2 receptor-mediated signaling in mast cell biology. Purinergic Signal 6:3–17
Khalil MH, Silverman AJ, Silver R (2004) Mast cell mediators alter electrical activity of rat thalamic neurons. In: Keystone symposium on mast cells in physiology, host defense and disease. Beyond IgE, Taos, New Mexico, p 57
Esposito P, Gheorghe D, Kandere K, Pang X, Connolly R, Jacobson S, Theoharides TC (2001) Acute stress increases permeability of the blood–brain barrier through activation of brain mast cells. Brain Res 888:117–127
Skuljec J, Sun H, Pul R, Bénardais K, Ragancokova D, Moharregh-Khiabani D, Kotsiari A, Trebst C et al (2011) CCL5 induces a pro-inflammatory profile in microglia in vitro. Cell Immunol 270:164–171
Chakraborty S, Kaushik DK, Gupta M, Basu A (2010) Inflammasome signaling at the heart of central nervous system pathology. J Neurosci Res 88:1615–1631
Acknowledgments
This project was sponsored by the National Natural Science Foundation of China (nos. 81102422, 81373398, and 81471410), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Additional information
Hongquan Dong and Xiang Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Dong, H., Zhang, X., Wang, Y. et al. Suppression of Brain Mast Cells Degranulation Inhibits Microglial Activation and Central Nervous System Inflammation. Mol Neurobiol 54, 997–1007 (2017). https://doi.org/10.1007/s12035-016-9720-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9720-x