Abstract
Cochlear supporting cells (SCs), which include the cochlear progenitor cells, have been shown to be a promising resource for hair cell (HC) regeneration, but the mechanisms underlying the initiation and regulation of postnatal cochlear SC proliferation are not yet fully understood. Bmi1 is a member of the Polycomb protein family and has been reported to regulate the proliferation of stem cells and progenitor cells in multiple organs. In this study, we investigated the role of Bmi1 in regulating SC and progenitor cell proliferation in neonatal mice cochleae. We first showed that knockout of Bmi1 significantly inhibited the proliferation of SCs and Lgr5-positive progenitor cells after neomycin injury in neonatal mice in vitro, and we then showed that Bmi1 deficiency significantly reduced the sphere-forming ability of the organ of Corti and Lgr5-positive progenitor cells in neonatal mice. These results suggested that Bmi1 is required for the initiation of SC and progenitor cell proliferation in neonatal mice. Next, we found that DKK1 expression was significantly upregulated, while beta-catenin and Lgr5 expression were significantly downregulated in neonatal Bmi1−/− mice compared to wild-type controls. The observation that Bmi1 knockout downregulates Wnt signaling provides compelling evidence that Bmi1 is required for the Wnt signaling pathway. Furthermore, the exogenous Wnt agonist BIO overcame the downregulation of SC proliferation in Bmi1−/− mice, suggesting that Bmi1 knockout might inhibit the proliferation of SCs via downregulation of the canonical Wnt signaling pathway. Our findings demonstrate that Bmi1 plays an important role in regulating the proliferation of cochlear SCs and Lgr5-positive progenitor cells in neonatal mice through the Wnt signaling pathway, and this suggests that Bmi1 might be a new therapeutic target for HC regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sensory epithelium of the organ of Corti is made up of hair cells (HCs) and supporting cells (SCs). In mammals, loss of HCs causes permanent hearing loss because of the limited spontaneous HC regeneration in the cochlea. In non-mammalian vertebrates, however, new HCs can be regenerated after HC loss, and this leads to the functional recovery of hearing [1–3]. Recent studies reported that when SCs are isolated and placed in culture, they can be induced to proliferate and form spheres with the capacity to differentiate into multiple cell types in vitro [4, 5]. Neonatal mammalian cochleae also have limited HC regeneration capacity in vivo because they harbor stem cells and progenitor cells that can proliferate and regenerate new HCs [5–11], but no spontaneous HC regeneration has been observed in the mature mammalian cochlea [12–14].
In neonatal mouse cochleae, Wnt-responsive Lgr5-expressing cells have been identified as the progenitors that can regenerate HCs, and the upregulation of Wnt signaling can stimulate the proliferation of these Lgr5-positive progenitor cells [6, 8–11, 15]. The cochlear SCs, including Lgr5-positive progenitors, remain mitotically quiescent in the neonatal mouse cochlea, but the mechanisms underlying the initiation and regulation of postnatal cochlear SC proliferation remain poorly understood. Previous studies have shown that after HC damage, SCs can reenter the cell cycle and can be labeled with mitotic tracers [8, 16]. Promoting the proliferation of SCs first and then letting the proliferated SCs differentiate into HCs would preserve the SC number while regenerating HCs and would be a much better method for HC regeneration than relying on direct differentiation. Several genes have been reported to be involved in the regulation of SC proliferation, including p27 Kip1 and N-Myc. p27 Kip1 knockout leads to overgrowth of HCs and SCs in the cochlea [17], and conditional inactivation of N-Myc decreases the proliferation of cochlear prosensory cells [18].
B cell-specific Moloney murine leukemia virus integration site 1 (Bmi1) is a member of the polycomb protein family that is ubiquitously expressed in almost all tissues, and its expression levels are particularly high in the brain, esophagus, salivary gland, thymus, kidney, lungs, gonads, placenta, blood, and bone marrow [19–22]. Bmi1 has been reported to play important roles in regulating the proliferation activity of normal, stem, and progenitor cells in multiple organs [19–29]. Previous reports showed that transplanted fetal liver and bone marrow cells from Bmi1−/− mice are able to contribute only transiently to hematopoiesis, and this indicates a cell-autonomous defect in Bmi1−/− mice [22]. Bmi1-deficient leukemic stem and progenitor cells can be compromised because they eventually undergo proliferation arrest and show signs of differentiation and apoptosis [20]. Bmi1 has also been shown to regulate self-renewal activity in adult prostate stem cells, and loss of Bmi1 blocks the self-renewal activity induced by heightened beta-catenin signaling [21]. Our previous study in the mouse cochlea showed that Bmi1 is expressed in both HCs and SCs—in addition to the spiral ligament and spiral ganglion cells—and that Bmi1 can regulate redox homeostasis and reactive oxygen species (ROS) levels and thus plays an important role in the survival of auditory HCs [30]. However, the role of Bmi1 in regulating cochlear SC proliferation in the inner ear is unknown.
In neonatal mouse cochleae, Wnt signaling has been reported to play important roles in regulating the proliferation of SCs and inner ear progenitor cells [6, 8–11, 15]. A recent study showed that Bmi1 acts as an activator of the Wnt signaling pathway by repressing the Dickkopf (DKK) family of Wnt inhibitors. Bmi1 mediates the repression of DKK proteins, especially DKK1, which leads to upregulation of Wnt [31]. However, the mechanism involved in the function of Bmi1 in the inner ear remains unknown.
In the present study, we took advantage of Bmi1 knockout mice and found that the proliferation and sphere-forming abilities of cochlear SCs and Lgr5-positive progenitor cells were significantly decreased in neonatal Bmi1−/− mice. We further demonstrated that Bmi1 is required for Wnt signaling because Bmi1 knockout downregulated Wnt signaling in the mouse cochlea. Moreover, upregulation of Wnt signaling with the exogenous Wnt agonist BIO successfully restored the proliferative ability of SCs in Bmi1−/− mice. Taken together, these results suggest that Bmi1 regulates the proliferation of cochlear SCs and Lgr5-positive progenitor cells through the canonical Wnt signaling pathway.
Results
Bmi1 Knockout Leads to a Reduction in SC Proliferation After Neomycin-Induced HC Damage
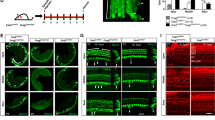
Cochlear SCs in the neonatal mouse maintain mitotic quiescence, but they begin to proliferate when HCs are damaged with aminoglycoside antibiotics. Our previous study in the mouse cochlea showed that Bmi1 is expressed in both HCs and SCs (Supp. Fig. 1), and Bmi1 is known to play an important role in the survival of auditory HCs [30]. However, the role of Bmi1 in regulating cochlear SC proliferation in the inner ear remains unknown. To investigate the role of Bmi1 in regulating SC proliferation, we isolated and cultured the cochleae from postnatal day (P)1 Bmi1−/−, Bmi1+/−, and wild-type (WT) mice. After 12 h of recovery, the cultured tissues were treated with 0.5 mM neomycin for 6 h to damage the HCs, and the tissues were allowed to recover for another 3 days without neomycin. At the end of the 3 days, the cochleae were treated with 10 μM 5-ethynyl-2ʹ-deoxyuridine (EdU) to label the mitotic cells. To quantify the proliferative SCs, we stained the tissues with the SC marker Sox2. After neomycin treatment, progressive HC loss was observed from the apical to the basal turns (apex = 7.8 ± 2.0 % HC loss compared to undamaged control cochleae; middle = 47.9 ± 16.3 % HC loss; Base = 70.6 ± 7.8 % HC loss; n = 4) (Fig. 1a, b1), and quantitative polymerase chain reaction (qPCR) data suggested that the expression of Bmi1 was upregulated after neomycin injury in P1 WT mice cochlea (Fig. 1b2). After neomycin treatment, the proliferative SCs were mostly distributed in the apical turns, and very few Sox2/EdU double-positive SCs were observed in the middle and basal turns (apex = 13.6 ± 1.7 Sox2/EdU double-positive SCs in the organ of Corti per 100-μm cochlear length; middle = 2.3 ± 0.6 Sox2/EdU double-positive SCs; Base = 1.0 ± 0.1 Sox2/EdU double-positive SCs; n = 4) (Fig. 1c). The proliferation of SCs in the apical and middle turns of the organ of Corti in Bmi1−/− mice was significantly decreased when compared to the WT and Bmi1+/− littermates with the same neomycin treatment (p < 0.05, n = 4) (Fig. 1d, e1–3ʹ; Table 1). These results showed that Bmi1 deficiency significantly inhibited the proliferation of SCs after neomycin injury.
Bmi1 knockout inhibited the proliferation of SCs. a After neomycin insult, HC death increased from the apical to basal turns of the cochlea. b The percentage of dead HCs was 7.8 ± 2.0 % in the apex, 47.9 ± 16.3 % in the middle, and 70.6 ± 7.8 % in the base of the cochlea, n = 4. b2 After neomycin insult for 6 h, the mRNA level of Bmi1 was unregulated in P1 WT mice. c The proliferative SCs were mostly distributed in the apical turns, n = 4. d In Bmi1−/− mice, the percentage of Sox2/EdU double-positive SCs was significantly decreased compared to WT mice, (n = 4). EdU/Sox2 double-positive cells in neomycin-damaged cochleae of Bmi1−/− (e1), Bmi1+/− (e2), and WT (e3) mice. e3ʹ The partial enlargement of E3. Compared to WT mice, the percentage of Sox2/EdU double-positive SCs was significantly decreased in Bmi1−/− mice. Data shown as mean ± S.D. *p < 0.05, **p < 0.01. Scale bars = 20 μm
Proliferation of Lgr5-Positive Progenitor Cells is Decreased in Bmi1 Knockout Mice After Neomycin-Induced HC Damage
Recent studies reported that Bmi1 plays an important role in regulating the proliferation of stem and progenitor cells in multiple organs [19–25, 27–29]. However, the role of Bmi1 in regulating the proliferation of inner ear progenitor cells remains unknown. Previous studies reported that Lgr5-positive cells are Wnt-responsive progenitor cells in the inner ear that are capable of proliferating to form clonal colonies and are capable of regenerating HCs [6–8, 11]. We investigated the role of Bmi1 in regulating the proliferation of Lgr5-positive progenitor cells by generating Lgr5EGFP-Ires-CreERT2/+; Rosa26R-tdTomato/+; Bmi1−/− transgenic mice in which tamoxifen exposure results in tdTomato expression in Lgr5-positive progenitor cells in Bmi1−/− mouse cochleae.
Tamoxifen was administered to P1 mice to trace the Lgr5-positive progenitor cells. At P3, the cochleae were dissected out and cultured. After a 12-h recovery period, the cultured tissues were treated with 0.5 mM neomycin for 6 h to damage the HCs, then recovered for another 3 days without neomycin, and finally treated with EdU (10 μM) to label the proliferating cells (Fig. 2a). Lgr5EGFP-Ires-CreERT2/+ and Rosa26R-tdTomato/+ littermates were used as controls. In undamaged cochleae, Lgr5 was expressed in Deiters’ cells, pillar cells, and inner phalangeal/border cells (Supp. Fig. 2). In the cochleae from control mice, we observed significant amounts of tdTomato/EdU double-positive cells in the apical turns, and the proliferation of Lgr5-positive progenitor cells in the cochleae of Bmi1−/− mice was significantly decreased in the apical and middle turns when compared to the control littermates with the same neomycin treatment (n = 4, p < 0.01) (Fig. 2b, c1–1ʹ, c2–3; Table 2). These results demonstrated that Bmi1 deficiency significantly inhibits the proliferation of Lgr5-positive progenitor cells after neomycin injury.
Bmi1 knockout inhibited the proliferation of Lgr5-positive progenitor cells. a Lgr5EGFP-Ires-CreERT2/+; Rosa26R-tdTomato/+; Bmi1−/− transgenic mice with tamoxifen administration at P1 were used to trace Lgr5-positive progenitor cells. Neomycin was used to damage the HCs in vitro, and EdU was used to trace proliferating cells. b When compared to the Lgr5EGFP-Ires-CreERT2/+; Rosa26R-tdTomato/+ littermates with the same neomycin treatment, the proliferation of Lgr5-positive progenitor cells in Bmi1−/− mice was significantly decreased, n = 4. After neomycin damage, the proliferation of Lgr5-originated cells was observed in WT (c1), Bmi1+/− (c2), and Bmi1−/− (c3) mice. c1ʹ Partial enlargement of c1. The proliferation of Lgr5-positive progenitor cells in Bmi1−/− mice was significantly decreased compared to the Lgr5EGFP-Ires-CreERT2/+; Rosa26R-tdTomato/+ littermates. Data shown as mean ± S.D. *p < 0.05, **p < 0.01, Scale bars = 20 μm
Bmi1 Knockout Reduces the Sphere-Forming Ability of the Organ of Corti
We performed a sphere-forming assay to further elucidate the role of Bmi1 in regulating the proliferation of organ of Corti cells. P1 cochleae from Bmi1−/−, Bmi1+/−, and WT mice were dissected out and dissociated into single cells. Comparison of the total number of cells isolated from each organ of Corti showed no difference between the different mice (100,166 ± 15,002.8; 100,817 ± 7637.6; and 101,667 ± 12,583.0 isolated cells from each organ of Corti in Bmi1−/−, Bmi1+/−, and WT mice, respectively, n = 9). A total of 50,000 isolated cells were plated onto non-adhesive 12-well culture plates at a density of 20 cells/μl and cultured for 5 days. In the first generation, the sphere number in the Bmi1−/− group was significantly lower than in the Bmi1+/− and WT groups, and there was no significant difference between the Bmi1+/− and WT group (65.5 ± 25.9, 145.5 ± 20.3, and 142.8 ± 21.0 spheres in the Bmi1−/−, Bmi1+/−, and WT groups, respectively, n = 4, p < 0.01) (Fig. 3a). The average sphere size in the Bmi1−/− group was slightly smaller than in the Bmi1+/− and WT groups (50.3 ± 1.8, 58.5 ± 1.4, and 62.3 ± 1.0 μm in the Bmi1−/−, Bmi1+/−, and WT groups, respectively, n = 10, p < 0.05) (Fig. 3b). After passage from the third to the fifth generations, the Bmi1−/− group formed significantly fewer spheres than the Bmi1+/− and WT groups, suggesting that the spheres from the Bmi1−/− group expanded significantly more slowly than those from the Bmi1+/− and WT groups (Fig. 3c and Table 3). From the sixth generation, there were almost no spheres observed in the Bmi1−/− group (Fig. 3c and Table 3). These results demonstrated that Bmi1 deficiency significantly reduces the sphere-forming ability of organ of Corti cells.
Bmi1 knockout reduced the sphere-forming ability in the organ of Corti. a The primary sphere number in the Bmi1−/− group was significantly lower than in the Bmi1+/− and WT groups, n = 4. b The average diameter of the spheres in Bmi1−/− mice was slightly smaller than in Bmi1+/− and WT mice, n = 10. c The Bmi1−/− group formed significantly fewer spheres than the Bmi1+/− and WT groups from the third to fifth generations, and from the sixth generation onward the number of spheres in the Bmi1−/− group fell rapidly. d Compared to the Bmi1+/− and WT groups, the primary sphere number was significantly lower in the Bmi1−/− group. Data shown as mean ± S.D. *p < 0.05, **p < 0.01. Scale bars = 50 μm
Bmi1 Knockout Decreases the Sphere-Forming Ability of Lgr5-Positive Progenitor Cells
To determine the role of Bmi1 in regulating the proliferation of Lgr5-positive progenitor cells, we investigated the sphere-forming ability of Lgr5-positive progenitor cells by generating Lgr5EGFP-Ires-CreERT2/+; Bmi1−/− transgenic mice. In this experiment, we dissected out the P1 cochleae from Lgr5EGFP-Ires-CreERT2/+; Bmi1−/− mice and purified the Lgr5-positive progenitor cells by flow cytometry. We found that around 2.3, 2.7, and 2.8 % of the cells were Lgr5-EGFP-positive in Bmi1−/−, Bmi1+/−, and WT mice, respectively (n = 4) (Fig. 4a1–3). Immunohistochemical staining showed that 96.7 ± 1.2 % of the Lgr5-positive cells sorted by flow cytometry were also enhanced green fluorescent protein (EGFP)-positive (n = 4). Isolated Lgr5-EGFP-positive cells showed no staining for the HC marker myosin7a (n = 4) (Fig. 4b1), but almost all cells were positive for the SC marker Sox2 (94.3 ± 1.5 %, n = 4) (Fig. 4b2). A total of 200 isolated cells were plated onto a 96-well ultra-low attachment plate at a density of 2 cells/μl for 5 days. Cells from Lgr5EGFP-Ires-CreERT2/+ mice were used as the control. In the first generation, the number of spheres in the Bmi1−/− group was significantly lower than the Bmi1+/− and WT group (10.3 ± 2.4, 26.3 ± 2.7, and 28.3 ± 2.0 spheres in the Bmi1−/−, Bmi1+/−, and WT groups, respectively, n = 4, p < 0.01) (Fig. 4c). The average diameter of the spheres in the Bmi1−/− group was also slightly smaller than in the control groups (49.0 ± 2.1, 58.3 ± 1.7, and 59.3 ± 2.7 μm in the Bmi1−/−, Bmi1+/−, and WT groups, respectively, n = 10, p < 0.05) (Fig. 4d). After multiple passages, the Bmi1−/− group formed significantly fewer spheres than the control group (p < 0.01) suggesting that spheres from the Bmi1−/− group expanded significantly more slowly than the control group (Fig. 4e and Table 4). These results demonstrated that Bmi1 deficiency significantly reduces the sphere-forming ability of Lgr5-positive progenitor cells.
Bmi1 knockout reduced the sphere-forming ability of Lgr5-positive progenitor cells. By flow cytometry, the percentages of Lgr5-EGFP-positive cells sorted were around 2.3, 2.7, and 2.8 % in Bmi1−/− (a1), Bmi1+/− (a2), and WT (a3) mice, n = 4. Immunohistochemical staining of Myosin7a (b1) and Sox2 (b2) showed that 96.7 ± 1.2 % of the Lgr5-positive cells sorted by flow cytometry were also EGFP positive, n = 4. Isolated Lgr5-EGFP-positive cells showed no staining for the HC marker myosin7a, n = 4, and 94.3 ± 1.5 % of cells were positive for the SC marker Sox2, n = 4. c The sphere number in the Bmi1−/− group in the first generation was significantly lower than the control group, n = 4. d The average diameter of the spheres in Bmi1−/− mice was slightly smaller than Bmi1+/− and WT mice, n = 10. e The Bmi1−/− group formed significantly fewer spheres than the Bmi1+/− and WT group in the first and second generations, and from the third generation, the sphere number of the Bmi1−/− group fell rapidly. f Compared to the control groups, the primary sphere number was significantly lower in the Bmi1−/− group. Data shown as mean ± S.D. *p < 0.05, **p < 0.01, Scale bars = 50 μm
Bmi1 Knockout Inhibits Wnt Signaling in the Organ of Corti of Neonatal Mice
Previous experiments showed that Bmi1 activates the Wnt signaling pathway by repressing the Wnt inhibitor DKK1 [31]. To further understand the mechanism for how Bmi1 regulates cell proliferation in the inner ear, we investigated Wnt signaling activity and the expression of the Wnt inhibitor DKK1 in Bmi1−/− mice. In neonatal mice, qPCR results showed that the messenger RNA (mRNA) levels of the key Wnt signaling factor beta-catenin and the Wnt downstream targets Lgr5, Axin2, and Sp5 were significantly reduced in P1 Bmi1−/− mice compared to P1 WT controls (Fig. 5a). In contrast, the expression of the Wnt inhibitor DKK1 was significantly increased, and the expression of the HC marker Brn3.1 and the SC marker Sox2 remained unchanged (Fig. 5a). We also found that the expression of p27Kip1, a cell cycling inhibitor expressed in SCs [5], was significantly increased in Bmi1−/− mice. Furthermore, Western blot results demonstrated that DKK1 and p27Kip1 were significantly increased, while beta-catenin was significantly decreased in neonatal Bmi1−/− mice (Fig. 5b, c). Immunohistochemistry further confirmed that compared with neonatal WT mice, the expression level of beta-catenin was decreased and p27Kip1 was increased in Bmi1−/− mice (Fig. 5d1–2, e1–2). The Bmi1+/− mice showed no difference compared to WT mice (Supp. Fig. 3a, b). These results demonstrated that Bmi1 deficiency significantly downregulates the Wnt signaling pathway in the mouse cochlea.
Bmi1 knockout inhibited Wnt signaling in the organ of Corti of neonatal mice. a Compared with P1 WT controls, the mRNA level of the Wnt downstream targets Lgr5, beta-catenin, Axin2, and Sp5 were significantly reduced in P1 Bmi1−/− mice. On the contrary, the expression of DKK1 and p27 Kip1 was significantly increased, and the expression of the HC marker Brn3.1 and the SC marker Sox2 remained unchanged (n = 3). b Compared with P1 WT controls, the protein level of DKK1 and p27Kip1 were increased, and beta-catenin was decreased, in Bmi1−/− mice. c Relative protein expression levels from B. Compared to P1 WT mice (d1), the expression level of beta-catenin was significantly decreased in P1 Bmi1−/− mice (d2). Meanwhile, compared to P1 WT mice (e1), the expression level of p27Kip1 was significantly increased in P1 Bmi1−/− mice (e2). Data shown as mean ± S.D. *p < 0.05, **p < 0.01, Scale bars = 50 μm
Bmi1 Knockout Inhibits Lgr5 Expression in the Organ of Corti of Neonatal and Adult Mice
To investigate the expression of Lgr5 in Bmi1−/− mice, we generated Lgr5EGFP-Ires-CreERT2/+ Bmi1−/− transgenic mice and used the Lgr5EGFP-Ires-CreERT2/+ mice as controls. Lgr5 was expressed in Deiters’ cells, pillar cells, and inner phalangeal/border cells in P1 control mice as previously reported [7]. Interestingly, a mosaic Lgr5 expression pattern was observed in P1 Bmi1−/− mice (Fig. 6a1–2) and the expression of Lgr5 was significantly reduced in Bmi1−/−mice compared to WT controls (p < 0.01) (Fig. 6c1). Compared with the P1 control, there were significantly more third-row Deiters’ cells and inner pillar cells lacking Lgr5-EGFP expression in P1 Bmi1−/− mice in all three turns of the cochlea (Deiters’ cells = 34.8 ± 12.2 and 195.0 ± 33.7 cells lacking Lgr5-EGFP expression in P1 control and Bmi1−/− mice, respectively, p < 0.01; inner pillar cells = 26.5 ± 13.9 and 222.3 ± 37.3 cells lacking Lgr5-GFP expression per cochlea in the control and Bmi1−/− mice, respectively, p < 0.01, n = 4) (Fig. 6b1–2, d1–2). No significant difference was detected between the WT control and Bmi1+/− mice (Fig. 6d1, 2; Supp. Fig. 4a). The distribution of third-row Deiters’ cells and inner pillar cells lacking Lgr5-EGFP expression in P1 Bmi1−/− mice showed no significant differences from the apical to the basal turns (Fig. 6d3–4; Tables 5 and 6).
Bmi1 knockout inhibited the expression of Lgr5 in the organ of Corti of neonatal and P30 mice. Lgr5EGFP expression in whole organ of Corti from P3 WT (a1), P3 Bmi1−/− (a2), P30 WT (a3), and P30 Bmi1−/− (a4) mice. Detailed enlargements of Lgr5EGFP expression in P3 WT (b1), P3 Bmi1−/− (b2), P30 WT (b3), and P30 Bmi1−/− (b4) mice. c1 The expression of Lgr5 was significantly reduced in P1 Bmi1−/− mice compared to WT controls. c2 In P30 adult mice, the mRNA level of Lgr5, Brn3.1, and Prestin were significantly reduced in Bmi1−/− mice compared to WT controls. Compared with the P1 WT control, there were significantly more inner pillar cells (IPC) (d1) and third-row Deiters’ cells (TDC) (d2) lacking Lgr5-EGFP expression in Bmi1−/− mice in all three turns, n = 4. The distribution of cells lacking Lgr5-EGFP expression in the IPC (d3) and TDC (d4) in P1 Bmi1−/− mice showed no significant differences from the apical to the basal turns, n = 4. Compared with P30 WT controls, there were significantly more third-row Deiters’ cells lacking Lgr5-EGFP expression in P30 Bmi1−/− mice (d5), and the distribution showed no differences from the apical to basal turns, n = 4 (d6). Data shown as mean ± S.D. *p < 0.05, **p < 0.01. Scale bars = 20 μm
In P30 adult mice, qPCR results showed that the mRNA level of Lgr5 was also significantly reduced in Bmi1−/−mice compared to WT controls (p < 0.01), that the expression of HC markers Brn3.1 and Prestin were reduced due to the HC loss in Bmi1−/− mice (p < 0.05) [30], and that the SC markers Sox2 and P 27Kip1 remained unchanged (Fig. 6C2). In P30 adult mice, Lgr5 was only expressed in the third-row Deiters’ cells [7]. Similar to the neonatal mice, a mosaic pattern of Lgr5 expression was also observed in the adult Bmi1−/− mice (Fig. 6a3–4). Compared with the P30 controls, there were significantly more third-row Deiters’ cells lacking Lgr5-EGFP expression in P30 Bmi1−/− mice in all three turns (56.3 ± 10.1 and 367.3 ± 22.6 cells lacking Lgr5-EGFP expression per cochlea in the control and Bmi1−/− mice, respectively, p < 0.01, n = 4) (Fig. 6b3–4, d5–6; Table 7). No significant difference was detected between the WT control and Bmi1+/− mice (Fig. 6d5–6; Supp. Fig. 4b). Compared with the neonatal mice, significantly less Lgr5-EGFP expression was observed in adult Bmi1−/− mice (28.1 ± 4.3 and 48.6 ± 7.6 % of third-row Deiters’ cells were lacking Lgr5-EGFP expression at P3 and P30, respectively, p < 0.01, n = 4) (Fig. 6d1, 2, 5). These results demonstrated that Bmi1 deficiency inhibits Wnt signaling in the cochleae of both neonatal and adult mice.
Wnt Signaling is Involved in the Inhibition of SC Proliferation in Bmi1−/− Mice
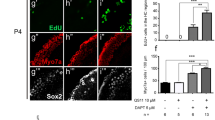
To determine whether Wnt signaling is involved in the inhibition of SC proliferation in the inner ear of Bmi1-deficient mice, we tested the effects of a Wnt agonist on Bmi1−/− cochlear explants. In this experiment, we isolated and cultured the cochleae from P1 WT or Bmi1−/− mice. After 12 h of recovery, the cultured tissues were treated with 0.5 mM neomycin for 6 h. The neomycin was washed away, and the cells were treated with the Wnt agonist BIO (5 μM) for 3 days, which specifically represses glycogen synthase kinase-3 beta (GSK-3β) and promotes β-catenin-mediated transcriptional activity. EdU (10 μM) was administered during the last 2 days of culture to label the proliferating cells (Fig. 7a). We found that Bmi1 knockout significantly reduced the expression of Wnt downstream target genes and inhibited the proliferation of SCs compared to WT control (p < 0.01, n = 4). Exposure to BIO completely restored SC proliferation in Bmi1−/− mice in the apical and middle turns (p < 0.01, n = 4) (Fig. 7b, d1–1ʹ, d2–4; Table 8). qPCR data showed that exogenous BIO treatment significantly increased the expression of the Wnt signaling target genes Lgr5, Axin2, and Sp5 in Bmi1−/− mice (p < 0.05) (Fig. 7c). These results demonstrated that Wnt activation could bypass the requirement of Bmi1 in regulating SC proliferation, and this suggested that Bmi1 deficiency might inhibit the proliferation of SCs by downregulating the canonical Wnt signaling pathway in the inner ear. Furthermore, when we compared the WT and Bmi1−/− groups both treated with BIO, we found that the WT group treated with BIO still had higher SC proliferation ability than the Bmi1−/− group treated with BIO (p < 0.05) (Fig. 7d1–2). This result also suggested that Bmi1 is involved in Wnt activation-induced SC proliferation.
Cell proliferation in P1 Bmi1−/− mice could be rescued by a Wnt agonist in vitro. a The Wnt agonist BIO was used to promote β-catenin-mediated transcriptional activity, and EdU was used to label the proliferating cells. b After BIO treatment, the number of proliferative cells in Bmi1−/− mice was completely rescued, n = 4. c The expression level of the Wnt signaling target genes Lgr5, Axin2, and Sp5 in Bmi1−/− mice was increased after exogenous BIO treatment, n = 3. After BIO treatment, the number of proliferating cells was counted in WT (d1) and Bmi1−/− (d2) mice. d1ʹ is the partial enlargement of D1. Without BIO treatment, the number of proliferating cells was also counted in WT (d3) and Bmi1−/− mice (d4) after neomycin insult. Data shown as mean ± S.D. *p < 0.05, **p < 0.01. Scale bars = 20 μm
Discussion
In mammals, numerous studies have shown that the SC population in the neonatal cochlea contains stem cells and progenitor cells that can proliferate and regenerate new HCs; thus, SCs can serve as a reliable resource to regenerate HCs [5–11]. Recent studies have also shown that a subpopulation of SCs, the Lgr5-positive cells, can proliferate and regenerate HCs and are an enriched population of HC progenitor cells compared to the whole SC population [6, 8–11, 15]. Thus, it is important to understand the mechanism regulating the proliferation of SCs and Lgr5-positive progenitor cells. Bmi1 is known to regulate the proliferation of stem and progenitor cells in multiple organs. However, the role of Bmi1 in regulating the proliferation of SCs and Lgr5-positive progenitor cells in the mouse inner ear remains unknown. Wnt activation promotes the proliferation of SCs and Lgr5-positive progenitor cells, but the interaction between Bmi1 and the Wnt signaling pathway in cell proliferation still needs to be determined in the postnatal mouse cochlea. In this study, we present a new role for Bmi1 in regulating the proliferation and sphere-forming ability of SCs and Lgr5-positive progenitor cells in the postnatal mouse cochlea. We further demonstrate that Bmi1 regulates the proliferation of SCs and Lgr5-positive progenitor cells through the Wnt signaling pathway.
The polycomb gene Bmi1 has been reported to regulate the proliferation of stem cells and rapidly dividing cells, including cancer cells and lymphocytes [19–29]. For example, Bmi1-deficient leukemic stem and progenitor cells can be compromised because they eventually undergo proliferation arrest and show signs of differentiation and apoptosis [20]. Bmi1 regulates the self-renewal activity of adult prostate stem cells, and loss of Bmi1 blocks the self-renewal activity induced by heightened beta-catenin signaling [21]. Our own previous research reported the expression pattern of Bmi1 in the mouse inner ear and demonstrated that Bmi1 is required for the survival of auditory HCs and that Bmi1 deficiency leads to HC death through increased redox activity and ROS accumulation in the mouse cochlea [30]. However, the role of Bmi1 in regulating the proliferation and sphere-forming ability of SCs and Lgr5-positive progenitor cells in the inner ear remains uninvestigated. It has been reported that several genes are involved in the proliferation of SCs and that the loss of these genes leads to inhibited proliferation in SCs. For example, in p27Kip1 knockout mice supernumerary HCs and SCs have been observed [17], and decreased proliferation in the cochlear prosensory region has been reported in N-Myc conditional knockout mice [18]. In this study, we have provided evidence that Bmi1 deficiency inhibits the proliferation and sphere-forming ability of SCs and Lgr5-positive progenitor cells. We showed that the numbers of EdU-labeled SCs and Lgr5-positive progenitor cells were significantly reduced in the cochlear sensory epithelia of Bmi1 knockout mice after neomycin injury and Lgr5-positive progenitor cells in the organ of Corti of Bmi1 knockout mice formed significantly fewer spheres.
In the mouse inner ear, Wnt/β-catenin signaling plays important roles in regulating cell proliferation, cochlear development, and HC regeneration. Activating Wnt signaling in the Lgr5-positive progenitor cells leads to proliferation and mitotic generation of HCs in the postnatal mouse cochlea in vivo [6, 11]. In other organs, the interaction of Bmi1 with the Wnt signaling pathway plays a pivotal role in regulating the proliferation of stem and progenitor cells in different cellular and biological contexts. It has been reported that KLF4 directly inhibits the expression of Bmi1 in colon cancer cells [32], and Bmi1 also inhibits the Wnt inhibitor DKK1 thus upregulating the Wnt signaling pathway [31]. In this study, we have provided direct evidence that loss of Bmi1 significantly upregulates DKK1 and inhibits Wnt signaling in the mouse cochlea, and we showed that Bmi1 deficiency significantly reduces the mRNA expression level of Wnt downstream target genes in both neonatal and adult mice. In Bmi1 knockout mice, the expression of Lgr5-EGFP decreased around twice as much in adult Bmi1 knockout mice compared to neonatal mice.
Recent studies have shown Wnt activation in Sox2-positive SCs and Lgr5-positive progenitor cells in the cochlear epithelium results in increased SC proliferation and HC generation [6, 10]. Our work supports the idea that Bmi1 deficiency leads to downregulation of Wnt signaling in the cochlear epithelium, which results in decreased proliferation of Sox2-positive SCs and Lgr5-positive progenitor cells. Furthermore, when we used the Wnt agonist BIO to restore Wnt signaling in Bmi1 knockout mice, we found that the BIO treatment successfully upregulated Wnt signaling in the cochlear epithelium and completely bypassed the effects of Bmi1 knockout on SC proliferation. This indicated that Bmi1 deficiency might inhibit the proliferation of SCs by downregulating the Wnt signaling pathway. It is interesting to note that the mRNA expression levels of Wnt downstream target genes in Bmi1 knockout cochlea with BIO treatment were higher than the WT control cochlea without BIO treatment and that the proliferation of SCs in Bmi1 knockout mice with BIO treatment was also higher than the WT control mice without BIO treatment. This suggests that Wnt activation could completely bypass the effects of Bmi1 knockout on SC proliferation. Moreover, when we compared the WT and Bmi1 knockout groups both treated with BIO, we found that the WT groups still had higher SC proliferation ability than the Bmi1 knockout group, and this also suggested that Bmi1 is involved in the Wnt activation-induced SC proliferation. Taken together, all of our results indicate that there might be complex regulatory loops between Bmi1 and the Wnt signaling pathway, and these should be further investigated in future studies.
In summary, we report here that Bmi1 deficiency inhibits the proliferation and sphere-forming abilities of cochlear SCs and Lgr5-positive progenitor cells. We also show that Bmi1 deficiency leads to the downregulation of Wnt signaling in the cochlear epithelium. Finally, we show that knockout of Bmi1 might inhibit the proliferation of SCs by downregulating the Wnt signaling pathway in the neonatal mouse cochlea. Our findings might be useful in further dissecting the mechanisms regulating mammalian inner ear progenitor cell proliferation and might suggest a new therapeutic target for stimulating HC regeneration after injury to the cochlea.
Material and Methods
Animals
Bmi1+/− mice (129OIa/FVB/N hybrid background) were backcrossed 10–12 times into a C57BL/6J background and then mated to generate Bmi1−/−, Bmi1+/−, and wild-type (WT) mice [20]. Lgr5EGFP-Ires-CreERT2 [33] and Rosa26R-tdTomato [34] mice in the C57BL/6J background were purchased from the Jackson Laboratory (Cat. #8875 and #7908, respectively). For Cre activation, tamoxifen (Sigma-Aldrich, St. Louis, MO, USA) was given to postnatal day (P) 0 mice (2 mg/25 g, i.p.). All animal procedures were performed according to protocols approved by the Animal Care and Use Committee of Fudan University and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and to prevent their suffering.
Genotyping, RT-PCR and qPCR
Transgenic mice were genotyped using genomic DNA from tail tips by adding 70 μl 50 mM NaOH, incubating at 98 °C for 20–40 min, and adding 7 μl 1 M HCl. The genotyping primers were as follows. Bmi1: wild-type (F) CAG TTA GGC AGT ATG TAG TTT TC; (R) GTT GTG GTG GAG TGT AAG AGT GT; mutant (F) AAG ATG TTG GCG ACC TCG TAT TGG; (R) GCA AGA CCT GCC TGA AAC CGA ACT; Lgr5: (F) CTG CTC TCT GCT CCC AGT CT; wild-type (R) ATA CCC CAT CCC TTT TGA GC; mutant (R) GAA CTT CAG GGT CAG CTT GC; tdTomato: wild-type (F) AAG GGA GCT GCA GTG GAG T; (R) CCG AAA ATC TGT GGG AAG TC; mutant (F) GGC ATT AAA GCA GCG TAT C; (R) CTG TTC CTG TAC GGC ATG G.
For reverse transcriptase polymerase chain reaction (RT-PCR) and quantitative polymerase chain reaction (qPCR), total RNA was extracted from the cochlea using TRIzol (Invitrogen, Carlsbad, CA, USA) with the stria vascularis and modiolus micro-dissected away. This was followed by complementary DNA (cDNA) synthesis using the GoScriptTM Reverse Transcription System (Promega, Madison, WI, USA). qPCR reactions were performed with GoTaq® qPCR Master Mix (Promega) on a 7500HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Each PCR reaction was carried out in triplicate, and the relative quantification of gene expression was analyzed using the ΔΔCT method with β-actin as the endogenous reference.
Primer pairs were designed using the online Primer3 software. Lgr5 (F) CCT ACT CGA AGA CTT ACC CAG T; (R) GCA TTG GGG TGA ATG ATA GCA; Sox2 (F) GCG GAG TGG AAA CTT TTG TCC; (R) CGG GAA GCG TGT ACT TAT CCT T; Axin2 (F) TGA CTC TCC TTC CAG ATC CCA; (R) TGC CCA CAC TAG GCT GAC A; Sp5 (F) TGG GTT CAC CCT CCA GAC TTT; (R) CCG GCG AGA ACT CGT AAG G; Brn3.1 (F) CGA CGC CAC CTA CCA TAC C; (R) CCC TGA TGT ACC GCG TGA T; Prestin (F) GAA AGG CCC ATC TTC AGT CAT C; (R) GCC ACT TAG TGA TAG GCA GGA AC; p27Kip1 (F) TCA AAC GTG AGA GTG TCT AAC G; (R) CCG GGC CGA AGA GAT TTC TG; β-actin (F) GGC TGT ATT CCC CTC CAT CG; (R) CCA GTT GGT AAC AAT GCC ATG T; beta-catenin (F) ATG CGC TCC CCT CAG ATG GTG TC; (R) TCG CGG TGG TGA GAA AGG TTG TGC.
Western Blot Analysis
Total protein was isolated with RIPA buffer. Protein concentrations were measured using a bicinchoninic acid (BCA) protein kit (Thermo Fisher Scientific, Rockford, IL), and proteins were separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred onto polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore, Bedford, MA, USA). The membranes were blocked with 10 % nonfat dried milk in Tris-buffered saline with Tween 20 (TBST) (50 mM Tris–HCl (pH 7.4), 150 mM NaCl, and 0.1 % Tween-20) for 1 h at room temperature and then blotted overnight with primary antibodies at 4°w. The following antibodies were used as primary antibodies: anti-p27Kip1 (1:500 dilution), anti-DKK1 (1:500 dilution), anti-beta-catenin (1:800 dilution) (Santa Cruz Biotechnology, CA, USA), and anti-beta-actin (1:1000 dilution, Cell Signaling Technology, Danvers, MA, USA).
Sphere Culture
For each experiment, we dissected the organs of Corti from two or three mice. Each organ of Corti was individually inspected, dissected from the surrounding tissue, and carefully rinsed in Hank’s balanced salt solution. All tissues were collected in PBS at pH 7.4 on ice before they were subjected to digestion with 0.125 % trypsin in PBS for 8 min at 37 °C in a total volume of 100 μl. The enzymatic reaction was stopped by adding 50 μl of 20 mg/ml soybean trypsin inhibitor and 2 mg/ml DNAse I solution (Invitrogen) in Dulbecco’s modified Eagle’s medium (DMEM)/high glucose and F12 media (mixed 1:1). The cells were carefully triturated with plastic pipette tips (epTIPS Filter 20–300 μl; Eppendorf) and diluted with 2 ml DMEM/high glucose and F12 media (mixed 1:1) supplemented with N2 and B27 (Invitrogen), EGF (20 ng/ml), bFGF (10 ng/ml), IGF-1 (50 ng/ml) (Thermo Fisher Scientific, Rockford, IL, USA), and heparin sulfate (50 ng/ml, Sigma-Aldrich). Cell separation was ensured via microscopic inspection. The cell suspension was then passed through a 40-μm cell strainer directly into plastic Petri dishes (Greiner Bio-one, Monroe, NC, USA). A total of 50,000 isolated cells were plated into non-adhesive 12-well culture clusters (Corning, NY, USA) at a density of 20 cells/μl. The total numbers of primary spheres were assessed after 5 days in culture. For propagation, spheres were collected after 5 days in culture and dissociated with 0.25 % trypsin at 37 °C for 6 min followed by addition of 10 % serum/DMEM media to block the reaction. The cell suspensions were re-plated into non-adhesive 12-well culture clusters, and the propagation was repeated at 5-day intervals.
Flow Cytometry and Sphere Culture
Each cochlea from the Lgr5-EGFP-creERT2 Bmi1−/− mice was carefully dissected out from the surrounding tissue and carefully rinsed in Hank’s balanced salt solution. For each experiment, six cochleae were collected in PBS at pH 7.4 on ice before they were subjected to digestion with 0.125 % trypsin in PBS for 8 min at 37 °C in a total volume of 100 μl. The enzymatic reaction was blocked by adding 50 μl of 20 mg/ml soybean trypsin inhibitor and 2 mg/ml DNAse I solution (Invitrogen, Carlsbad, CA, USA) in DMEM/high glucose and F12 media (mixed 1:1). Following dissociation, cells were passed through a 40-μm cell strainer (Greiner Bio-one) to achieve a single-cell suspension. Propidium iodide (1 μg/mL) (Sigma-Aldrich) was used to label nonviable cells. The dissociated cells were sorted on a MoFlo® SX FACS cytometer (Beckman Coulter, CA, USA) using the channels for GFP, and positive and negative fractions were collected. The purity of the sorted cells was assessed by resorting and immunostaining.
Purified cells (2 cells/μl) from cell sorting were cultured in low-attachment 96-well plates (Corning) for 5 days to generate neurospheres in DMEM/F12 supplemented with N2, B27, EGF, IGF-1, bFGF, and heparin sulfate (same as earlier).
Tissue Cultures
Cochleae were isolated under sterile conditions with the anlage of the stria vascularis, modiolus, and tectorial membrane removed with fine forceps. Whole-mount cochleae were then placed onto 10-mm coverslips pre-coated with poly-l-lysine (Sigma-Aldrich). Whole organs were cultured in DMEM/F12 medium (mixed 1:1) supplemented with N2 and B27 in four-well Petri dishes (media and supplements were obtained from Invitrogen). Neomycin (0.5 mM, Sigma-Aldrich) was added for 6 h to kill the HCs. After neomycin was removed, the tissues were washed twice with PBS and cultured in serum-free medium with 10 μM 5-ethynyl-2ʹ-deoxyuridine (EdU) for an additional 72 h. For the Wnt signaling upregulation experiment, 6-Bromoindirubin-3'-oxime (Bio) (5 μM, Stemgent, Lexington, MA, USA) was added to the medium for 72 h after the neomycin was removed. Culture medium was replenished every 1–2 days.
Immunohistochemistry
Tissues were fixed for 10 min at room temperature with 4 % paraformaldehyde in 0.1 M phosphate buffer and then immersed in blocking solution consisting of 10 % donkey serum and 1 % Triton-X100 in PBS at pH 7.4 for 1 h at room temperature. Incubation with primary antibodies that were diluted in blocking solution was performed overnight at 4 °C in a humidified chamber. The following day, tissues were rinsed with PBS and then incubated with secondary antibodies diluted in 1 % Triton-X100 in PBS for 1 h at room temperature. After washing with PBS, tissues were mounted in antifade fluorescence mounting medium and cover-slipped. The primary antibodies were as follows: mouse anti-beta-catenin (1:500 dilution, Santa Cruz Biotechnology), mouse anti-p27Kip1 (1:500 dilution, Santa Cruz Biotechnology), rabbit anti-myosin7A (1:500 dilution; Proteus Biosciences, Ramona, CA, USA), and goat anti-Sox2 (1:500 dilution, Santa Cruz Biotechnology). The corresponding secondary antibodies were tetramethylrhodamine (TRITC), fluorescein isothiocyanate (FITC), and Cy5 (Jackson Immuno Research, West Grove, PA, USA). DAPI (Sigma-Aldrich) was used to show the cell nuclei. EdU detection was performed using an Alexa Fluor 555 and 647 Imaging Kit (Invitrogen).
Statistical Analysis
Statistical analyses were conducted using Microsoft Excel and GraphPad Prism software. For all experiments, n represents the number of animals examined. Two-tailed, unpaired Student’s t tests were used to determine statistical significance when comparing two groups, and one-way ANOVA followed by a Dunnett’s multiple comparisons test was used when comparing more than two groups. A value of p < 0.05 was considered to be statistically significant. Data are shown as the mean ± S.D.
References
Balak KJ, Corwin JT, Jones JE (1990) Regenerated hair cells can originate from supporting cell progeny: evidence from phototoxicity and laser ablation experiments in the lateral line system. J Neurosci 10:2502–2512
Corwin JT, Cotanche DA (1988) Regeneration of sensory hair cells after acoustic trauma. Science 240:1772–1774
Ryals BM, Rubel EW (1988) Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science 240:1774–1776
Li H, Liu H, Heller S (2003) Pluripotent stem cells from the adult mouse inner ear. Nat Med 9:1293–1299
White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N (2006) Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature 441:984–987
Chai R, Kuo B, Wang T, Liaw EJ, Xia A, Jan TA, Liu Z, Taketo MM et al (2012) Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A 109:8167–8172
Chai R, Xia A, Wang T, Jan TA, Hayashi T, Bermingham-McDonogh O, Cheng AG (2011) Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J Assoc Res Otolaryngol 12:455–469
Cox BC, Chai R, Lenoir A, Liu Z, Zhang L, Nguyen D, Chalasani K, Steigelman KA et al (2014) Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development 141:816–829
Jan TA, Chai R, Sayyid ZN, van Amerongen R, Xia A, Wang T, Sinkkonen ST, Zeng YA et al (2013) Tympanic border cells are Wnt-responsive and can act as progenitors for postnatal mouse cochlear cells. Development 140:1196–1206
Shi F, Hu L, Edge AS (2013) Generation of hair cells in neonatal mice by beta-catenin overexpression in Lgr5-positive cochlear progenitors. Proc Natl Acad Sci U S A 110:13851–13856
Shi F, Kempfle JS, Edge AS (2012) Wnt-responsive lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci 32:9639–9648
Bohne BA, Ward PH, Fernandez C (1976) Irreversible inner ear damage from rock music. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol 82:ORL50–ORL59
Hawkins JE Jr, Johnsson LG, Stebbins WC, Moody DB, Coombs SL (1976) Hearing loss and cochlear pathology in monkeys after noise exposure. Acta Otolaryngol 81:337–343
Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR (2008) Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol 9:65–89
Bramhall NF, Shi F, Arnold K, Hochedlinger K, Edge AS (2014) Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Rep 2:311–322
Li W, Wu J, Yang J, Sun S, Chai R, Chen ZY, Li H (2015) Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc Natl Acad Sci U S A 112(1):166–71. doi:10.1073/pnas.1415901112
Chen P, Segil N (1999) p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development 126:1581–1590
Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B (2011) Conditional deletion of N-Myc disrupts neurosensory and non-sensory development of the ear. Dev Dyn 240:1373–1390
Fasano CA, Dimos JT, Ivanova NB, Lowry N, Lemischka IR, Temple S (2007) shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell 1:87–99
Lessard J, Sauvageau G (2003) Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 423:255–260
Lukacs RU, Memarzadeh S, Wu H, Witte ON (2010) Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell 7:682–693
Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF (2003) Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423:302–305
Biehs B, Hu JK, Strauli NB, Sangiorgi E, Jung H, Heber RP, Ho S, Goodwin AF et al (2013) BMI1 represses Ink4a/Arf and Hox genes to regulate stem cells in the rodent incisor. Nat Cell Biol 15:846–852
Bruggeman SW, Hulsman D, Tanger E, Buckle T, Blom M, Zevenhoven J, van Tellingen O, van Lohuizen M (2007) Bmi1 controls tumor development in an Ink4a/Arf-independent manner in a mouse model for glioma. Cancer Cell 12:328–341
Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M (1999) The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397:164–168
Lopez-Arribillaga E, Rodilla V, Pellegrinet L, Guiu J, Iglesias M, Roman AC, Gutarra S, Gonzalez S et al (2015) Bmi1 regulates murine intestinal stem cell proliferation and self-renewal downstream of Notch. Development 142:41–50
Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ (2003) Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 425:962–967
Park IK, Morrison SJ, Clarke MF (2004) Bmi1, stem cells, and senescence regulation. J Clin Invest 113:175–179
Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, Huang CH, Kao SY et al (2010) Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol 12:982–992
Chen Y, Li L, Ni W, Zhang Y, Sun S, Miao D, Chai R, Li H (2015) Bmi1 regulates auditory hair cell survival by maintaining redox balance. Cell death & disease 6, e1605
Cho JH, Dimri M, Dimri GP (2013) A positive feedback loop regulates the expression of polycomb group protein BMI1 via WNT signaling pathway. J Biol Chem 288:3406–3418
Yu T, Chen X, Zhang W, Colon D, Shi J, Napier D, Rychahou P, Lu W et al (2012) Regulation of the potential marker for intestinal cells, Bmi1, by beta-catenin and the zinc finger protein KLF4: implications for colon cancer. J Biol Chem 287:3760–3768
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J et al (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449:1003–1007
Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD et al (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13:133–140
Acknowledgments
This work was supported by grants from the Major State Basic Research Development Program of China (973 Program) (2011CB504500, 2015CB965000), the National Natural Science Foundation of China (Nos. 81570913, 81470692, 81371094, 81230019, 81500790, 81570921, 31500852, 31501194), the Jiangsu Province Natural Science Foundation (BK20150022, BK20140620, BK20150598), the Project from China Scholarship Council (201406105052), the Program of Leading Medical Personnel in Shanghai, the Fundamental Research Funds for the Central Universities (2242014R30022, 021414380037), the Construction Program of Shanghai Committee of Science and Technology (12DZ2251700), and the Major Program of Shanghai Committee of Science and Technology (11441901000).
Author Contributions
HL and RC conceived and designed the experiments. XL, SS, QJ, ZS, WL, LL, and YZ performed the experiments. XL, SS, RC, and HL analyzed the data. DM provided the Bmi1 KO mice. XL, RC, and HL wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Xiaoling Lu and Shan Sun contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supp Fig 1
Bmi1 is expressed in both HCs and SCs in the apical turn of the organ of Corti. Scale bars = 20 μm (DOCX 870 kb)
Supp Fig 2
Without damage, Lgr5 was expressed in Deiters’ cells, pillar cells, and inner phalangeal/border cells in neonatal WT mice. Scale bars = 20 μm (DOCX 717 kb)
Supp Fig 3
a Compared with P0 WT mice, the expression level of beta-catenin showed no difference in P0 Bmi1+/− mice. b The expression level of p27Kip1 in heterozygous mice showed no difference compared to P0 control. Scale bars = 20 μm (DOCX 809 kb)
Supp Fig 4
a The expression level of Lgr5 between P0 WT and Bmi1+/− mice showed no difference. b The expression level of Lgr5 between P30 WT and Bmi1+/− mice showed no difference. Scale bars = 20 μm. (DOCX 836 kb)
Rights and permissions
About this article
Cite this article
Lu, X., Sun, S., Qi, J. et al. Bmi1 Regulates the Proliferation of Cochlear Supporting Cells Via the Canonical Wnt Signaling Pathway. Mol Neurobiol 54, 1326–1339 (2017). https://doi.org/10.1007/s12035-016-9686-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9686-8