Abstract
Adenosine is an endogenous, autacoid purine nucleoside which performs many important biological roles, particularly during stressful events. Adenosine can signal through four adenosine receptor (AR) subtypes: A1, A2A, A2B, and A3. Of these, adenosine A1 receptor (A1AR) has a broad, wide distribution throughout different vertebrate cell types and the highest affinity to adenosine. The A1AR-dependent action of adenosine is well documented in reports from numerous studies that have used different selective A1AR agonists and antagonists as well as in animals that have a genetically manipulated A1AR gene. Despite its wide distribution and function, A1AR homo/hetero-oligomerization with other adenosine and non-adenosine receptors extends its biological role during developmental, physiological, and pathological situations. In this review, we initially discuss the A1AR structure and most important signaling pathway triggered by its activation. Next, we summarize some of the most well-known biological effects of A1AR in the central nervous system (CNS) during development and adulthood, in addition to its role in nervous system regeneration and repair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adenosine is an endogenous, ubiquitous purine nucleoside which not only acts as a primary structural compound for a genetic material of the cells or a reserve source to provide energy in the form of ATP but also is an important signaling molecule that mediates a broad range of physiological effects. Under certain pathological conditions such as ischemia or hypoxia, adenosine produces a variety of responses known as the “signal of life” [1] or “body’s natural defense” [2]. Primarily, adenosine is a metabolic by-product of ATP or its derivative (ADP, AMP, cAMP) in intra- or extracellular spaces. In addition, de novo adenosine production occurs via hydrolysis of S-adenosylhomocysteine [3, 4]. In addition, two main classes of nucleoside transporters mediate the influx or efflux of adenosine across the cell membrane [4].
Different physiological actions of adenosine are mediated by P1 receptors (or P1 purinoceptors) which are also known as adenosine receptors (ARs) (reviewed in [5]). The term adenosine receptor has been accepted by the International Union of Basic and Clinical Pharmacology (IUPHAR) [6]. Until now, there are four subtypes of AR cloned and described among vertebrates—the adenosine A1, A2A, A2B, and A3 receptors [6]. Although adenosine is the full agonist of all four receptor subtypes [6], the adenosine A1 receptor (A1AR) has the highest affinity for adenosine [7]. Despite many similarities between ARs, they have distinct distribution, signal transduction pathways, physiological effects, pharmacological properties, and therapeutic applications. Among these receptors, A1AR has attracted a substantial interest because of its conserved expression in vertebrates, wide distribution throughout different cells, and higher affinity to the endogenous ligand adenosine. The concepts addressed in this paper include A1AR structure, gene expression, and the most important findings that pertain to signaling through A1AR function in central nervous system (CNS) development, regeneration, and repair.
A1AR Structure and Its Expression Control
A1AR is a 35–36-kDa glycoprotein [8, 9]; similar to other ARs, this glycoprotein belongs to class A of the G protein-coupled receptor (GPCR) superfamily [10]. In general, GPCRs share seven putative transmembrane α-helix structures connected by three extracellular and three intracellular loops. The extracellular domain, in particular the N-terminus half of these receptors, is involved in ligand binding. The cytoplasmic region that includes the C-terminus is responsible for the interaction with GTP-binding proteins in order to modulate other downstream effectors [10, 11]. A1AR has a short N-terminus that lacks N-glycosylation sites. The A1AR N-glycosylation acceptor site is located in the second extracellular loop [12].
Chimeric A1/A2a receptor experiments show that transmembrane 1–4 (TM1–4) domains of human A1AR are involved in ligand binding. Although some authors believe that TM5–7 domains are important for ligand binding in humans and other species [13, 14], it has been reported that certain amino acid residues in these domains show a high degree of similarity between A1AR and A2aAR. Hence, they cannot distinguish between A1AR- and A2aAR-selective ligands. TM1–4 domains are also responsible for specificity of A1-selective agonist and antagonist interactions [15].
The A1AR gene is located on chromosome 22q11.2 [16]. A comparison study in rodents, bovines, dogs, and humans has shown an approximately 90% similarity in the coding region of the A1AR gene [8, 17]. The results of genomic cloning of human A1AR suggest that this gene contains six exons and five introns. The coding region encodes 326 amino acid residues of an integral protein. Expression of the A1AR gene is complicated in which exons 1 and 2 are never expressed and an alternative splicing mechanism causes transcription of exon 3 or 4 along with exons 5 and 6 [8]. There are two different transcripts of the A1AR gene: one that contains exons 3, 5, and 6 and the other includes exons 4, 5, and 6. Evidence has shown that different tissues contain both transcripts; however, A1AR transcripts that contain exons 3, 5, and 6 are more prominent in tissues with high expression of this gene [8, 18]. Precise molecular studies show that human A1AR gene expression is controlled by two distinct promoters, A and B. The distribution of transcriptional expression of these promoters is tissue specific. Expression of promoter A-mediated transcripts is limited to certain tissues, whereas the expression of transcripts mediated by promoter B is seen in all tissues that express A1AR. The transcriptional activity of promoter A is much higher than that of promoter B [19]. The activity of these putative promoters is regulated by different factors; for example, dexamethasone drives promoter B expression in humans. This effect is highlighted more in tissues where promoter A is inactive [20]. The promoter region of A1AR also includes consensus sequences for activator protein 1 (AP-1) and nuclear factor-κB (NF-κB) transcriptional factors. AP-1 appears to mediate the basal level of A1AR expression, but NF-κB promotes A1AR expression following oxidative stress [21]. The expression levels of A1AR in different tissues may change developmentally.
Signaling Through A1AR Activation
Traditionally, A1AR has been shown to couple downstream effectors via the Gi/Go group of G proteins and negatively regulate adenylyl cyclase activity. Of note, A1AR is one of the GPCRs that can evoke other G proteins such as Gs and Gi, depending on the agonist structure. The level of [35S]GTPγS binding to G proteins is immunoprecipitated with subtype-specific antibodies, and the level of effector activation has shown that small changes in agonist structures can alter the ability of A1AR to activate multiple G proteins with diverse potency and efficacy due to different receptor conformations [22]. This is a clear form of allosteric modulation which is named “functional selectivity” or “biased signaling.” Some authors also describe this as “agonist-dependent receptor signaling” [22].
Gi protein-dependent adenylyl cyclase inhibition is the most prominent signaling pathway which couples A1AR with downstream effectors. However, A1AR can also modify intracellular calcium [Ca2+]i through coupling with Gi proteins. According to research, A1AR activation in a hamster vas deferens smooth muscle-derived DDT1MF-2 cell line is concentration dependent for both adenosine and its agonists, per se, or in synergism with ATP receptors. This can be coupled with phospholipase C (PLC) and can mobilize calcium from intracellular stores via enhancement of inositol 1,4,5-triphosphate (IP3) formation [23]. Possibly, these interactions of A1AR with ATP receptors can significantly increase and prolong the calcium response. The calcium response is pertussis toxin (PTX) sensitive, which can occur in the absence of extracellular calcium. A similar increase in [Ca2+]i after agonist stimulation has been observed in CHO-K1 cells transfected with the human brain A1AR sequence. The coupling of the receptor with phosphoinositide C directly or via βγ subunits was suggested [24]. However, significant evidence indicates that a verity of plasma membrane Ca2+ influx channels can be activated in response to A1AR stimulation and, in turn, lead to Ca2+ entry from the extracellular space [25]. In this situation, A1AR activation often induces Ca2+ influx via a G protein-coupled PLC and DAG/protein kinase C (PKC) as downstream effectors [25]. These effectors are known as activators of voltage-dependent Ca2+ channels (VDCCs) that include L-type Ca2+ channels and voltage-independent Ca2+ channels such as receptor-operated channels (ROCs) in different cells [25, 26]. In contrast, A1AR activation inhibits Ca2+ influx via VDCCs in neurons [27, 28]. Therefore, modulation of [Ca2+]i can be an important mechanism that triggers downstream signaling pathway following A1AR activation. A1AR stimulation is thought to contribute to mitogen-activated protein kinase (MAPK) activity in Chinese ovary (CHO) cells independent of PKC [29]. This indicates that A1AR has the capability to couple to more than one signal transducing pathway and, therefore, can influence different aspects of cell life.
Interaction Between A1AR and Other Proteins
Interestingly, other protein interactions may bias A1AR signal transduction and localization in cell membranes. Adenosine deaminase (ADA) is an intra/extracellular enzyme which catabolizes adenosine to inosine. Coexpression and functional interaction between A1AR and ADA in the pig brain cortical membrane and DDT1MF-2 cells has been reported [30, 31]. ADA increases the affinity of A1AR to agonists and significantly augments the signaling via A1AR [31]. There is concrete evidence that ADA increases A1AR desensitization accompanied by significant disappearance of A1AR from the cell surface [32, 33]. It has been considered that ADA expression on the cell surface not only acts as an ectoenzyme but also modulates A1AR signaling and trafficking.
Heat-shock cognate (hsc) proteins, such as hsc73, act as chaperones, assist with correct folding of misfolding proteins, and play a central role during stressful conditions [34]. The physical association between the intracellular domain of A1AR and that of hsc73 has been described in the cell body of rat cortical neurons, the rat cerebellum, and DDT1MF-2 cells [35]. A1AR interaction with ADA takes place outside of the cell membrane; however, hsc73 binding occurs inside the cell membrane. In contrast to ADA, significantly reduced ligand binding and agonist-mediated G protein coupling have been observed following cross-talk between A1AR and hsc73. It was postulated that ADA brought A1AR to a conformational structure more suitable for ligand binding. On the other hand, hsc73 might act in the opposite manner. However, in the presence of both proteins, the effect of ADA was shown to be stronger and could mask the effect of hsc73 [35].
The interaction between A1AR and 4.1G, a member of erythrocyte membrane cytoskeleton, is another example. Information obtained from the yeast two-hybrid method has shown that the third intracellular loop of A1AR binds to 4.1G. This interaction alters the localization of A1AR and attenuates the influence of A1AR on cAMP reduction. Also, this interaction mainly reduces the A1AR-mediated increase in [Ca2+]i [36].
Homo/Hetero-Oligomerization of A1AR with Other Receptors
The ability to form complexes with other membrane or cytosolic proteins provides an opportunity for cells to expand the complexity of signal transduction from certain receptors such as A1AR. For numerous years, researchers have believed that GPCRs exist and mediate their relevant physiological effects as monomer units. However, extensive evidence from pharmacological and biological experiments show that GPCRs have the capability to assemble and form dimers or oligomers of identical receptors (homo-oligomerization) or different receptors (hetero-oligomerization). It has been suggested that oligomer formation in GPCRs might influence cell surface expression, affinity, coupling properties, or a downstream signaling pathway and offer a type of allosteric regulation of these receptors (for review, see [37–39]). This novel view, therefore, established another level of complexity in A1AR signaling, which was similar to other GPCRs. Table 1 summarizes the background literature on the existence and biological significance of homo/hetero-oligomer formation between A1AR and other receptors. Taken together, these data show that cross-talk between A1AR and other receptors by altering signal transduction probably results in important physiological consequences. These data also support its wide-range developmental role and pivotal effects on neural diseases and regeneration.
Numerous lines of evidence indicate that the exact molecular mechanism which underlies A1AR signaling may differ according to cell type and effector system, the chemical properties of agonists and antagonists, presence of other cell membrane-binding proteins or receptors, and acute versus chronic stimulation. We present additional detailed evidence about A1AR-dependent signal transduction pathway and downstream effectors in the CNS.
A1AR in the CNS
This section briefly reviews information that pertains to the current knowledge of the roles of A1AR in the CNS. Because of genetically manipulated experimental animals as well as selective agonists and antagonists, the roles of this receptor in the CNS have attracted much interest and are the focus of different investigations.
Distribution of A1AR in the CNS
The distribution pattern of A1AR in the CNS shows that A1AR is widely expressed throughout the CNS, particularly the brain, where it controls a variety of functions. The distribution and density of A1AR based on positron emission tomography (PET) has been studied by the use of different radioligands in humans. These studies clearly localize a high density of this receptor in the putamen and thalamus, intermediate density in most cortical regions, and low density in the midbrain, brain stem, and cerebellum [56–58]. Studies show dissimilar distribution of A1AR among different cortical regions [57–59]. The presence of A1AR has been studied in the human hippocampus, too [59]. In situ and in vitro cellular localization studies show that neurons, astrocytes, oligodendrocytes, and microglia are equipped with A1AR. However, its distribution is not homogenous in different types of neurons and/or other cell types of the CNS. The subcellular localization of A1AR on neurons has been defined by radioligand autoradiography studies or receptor binding and Western blot analysis followed by cell fractioning experiments, particularly in the rat hippocampus. Research shows that A1ARs are expressed on axons [60]; however, most direct observations clearly demonstrate high concentrations of this receptor in the plasma membrane of nerve terminals, mainly in the presynaptic components of the active zone and postsynaptic density. A1AR is also found in the extrasynaptic part of nerve terminals [61, 62].
Neuromodulatory and Neuroprotective Effects of A1AR Signaling
Previously, researchers have stated that adenosine had critical neuromodulatory and neuroprotective effects. However, these effects are mainly mediated by A1AR occupancy and activation of downstream intracellular pathways in the CNS with possible involvement by A2AAR and other ARs [42]. Data suggest that A1AR signaling plays potent inhibitory roles in synaptic transmission. Evidence to confirm this phenomenon has been obtained from pharmacological assays that used selective A1AR agonists and antagonists along with genetically manipulated animal approaches. Evidence exists about how adenosine, through A1AR stimulation, regulates a variety of different effector systems to play a role as a modulator. (i) A1AR activation leads to the opening of different K+ channels in the presynaptic or postsynaptic plasma membrane, outward potassium current, and hyperpolarization of different neurons in various brain regions. In this way, A1AR reduces neurotransmitter release or decreases neuronal excitability. The coupling of A1AR to ATP-sensitive K+ (KATP) channels [63], G protein-dependent inwardly rectifying potassium (GIRK) channels [64, 65], and small-conductance Ca2+-activated K+ (SK) channels [65] has been demonstrated in hippocampus neurons [63], stellate neurons of the entorhinal cortex [28], and retinal ganglion cells [65]. (ii) Ca2+ influx from the extracellular space into the presynaptic nerve terminals presumably plays an important role in spontaneous quantal release or action potential-dependent release of various neurotransmitters. A1AR activation may inhibit Ca2+ influx through VDCCs in the presynaptic membrane [27, 28] and cause suppression of neurotransmitter release. (iii) Modulation of the synaptic secretory apparatus is another probable mechanism; however, no evidence currently exists. The release of different neurotransmitters such as glutamate [28, 66], GABA [27, 28, 67], serotonin [68], histamine [69], and dopamine [70, 71] may be inhibited by one or combination of these possible mechanisms. As previously mentioned, the analysis gives evidence about the coexistence of heteromeric complexes of A1AR with different proteins or other receptors. In these conditions, it seems likely that the existence of an A1AR partner can modify the functional consequences of A1AR activation including effects on neurotransmitter release [42].
The Effects of A1AR Signaling on Sleep
Adenosine levels increase during wakefulness as a result of ATP metabolism; hence, adenosine is considered a hypnotic factor [72]. Evidence suggests that adenosine acts through A1AR on different groups of neurons in various parts of the brain to regulate homeostatic sleep. In vivo studies have shown that deletion of A1AR messenger RNA (mRNA) by an A1AR antisense oligonucleotide in a rat cholinergic zone of the basal forebrain resulted in reduced non-rapid eye movement sleep and increased wakefulness [73]. A PET study showed that A1AR levels in humans exposed to 24 h of sleep deprivation increased in the whole brain [74]. A1AR signaling led to inhibition of discharge activity and generation of action potential or attenuated excitatory synaptic transmission in a subset of neurons that promote wakefulness. This mechanism has been found in cholinergic and some non-cholinergic neurons in the forebrain [75] considered responsible for cortical activity and in arousal, for promotion of hypocretin/orexin neurons from the lateral hypothalamus [76]. A1AR stimulation also inhibits histaminergic neurons in the tuberomammillary nucleus and promotes non-REM sleep [69]. Histaminergic neurons are involved in the arousal effects of histamine through H1 receptors [77]. Possibly the abovementioned mechanisms contribute to inhibition of these neurons via A1AR activation. On the other hand, it has been shown that administration of an A1AR agonist into the lateral preoptic area induces waking [78]. Therefore A1AR fulfills the criteria as a mediator of hypnogenic effects of adenosine in the brain. However, some authors state that the exact effects of A1AR may be region dependent [69].
The Effects of A1AR Signaling on Glial Cells
Glial cells are other CNS-resident cells that express A1AR [79, 80]. However, there are few reports about the role of A1AR signaling in regulating different functions of these cells in normal physiological or pathophysiological conditions. It has been shown that activation of A1AR in cortical astrocytes downregulates sustained [Ca2+]i response elicited by stimulation of P2 purinoceptors, apparently through inhibition of adenylyl cyclase [80]. A1AR activation also mediates reduction in Ca2+ mobilization induced by ATP into microglial cells [79]. This phenomenon may have significant physiological meaning considering the importance of Ca2+ signaling in different cellular functions. Inhibition of the hyperactive state of microglia under pathological conditions may explain AR-mediated neuroprotection. Oligodendrocytes display a high level of A1AR expression, but the exact biological role of this receptor in oligodendrocytes remains to be described [81]. The role of this receptor in oligodendrocytes and other members of oligodendrocyte lineage cell development has been studied to a certain extent.
The Biological Roles of A1AR Activation During Nervous System Development
An expanding body of data shows that adenosine through A1AR exerts a potent biological role during prenatal development. These data have attracted profound interest because coffee, an ARs antagonist, is globally the most widely consumed beverage, even among pregnant women. Average caffeine consumption of more than 100 mg/day is associated with an increased risk of fetal growth restriction and reduction in birth weight [82]. On the other hand, A1AR, among other known GPCRs, expresses early during embryogenesis and is involved in the development of the CNS and heart. The brain and heart are two main sites of A1AR expression during embryogenesis. Here, we provide insights into the role of A1AR expression and its activity during nervous system development.
Additional information about A1AR expression has been obtained from studies of the fetal life of rats [83]. This study reported the presence of A 1 AR mRNA in neural tissue as early as gestation day 11 according to in situ hybridization and a radioligand receptor assay. However, receptor-labeling autoradiography detected A1AR protein levels in neural tissue no sooner than gestation day 14; after which, A1AR expression and concentration increased in the CNS with further development [83]. Several researchers believe that A1ARs which exist in the brains of immature animals are not able to fully couple to G proteins. The [35S]GTPγS binding assay indicated a developmental delay in A1AR signaling in the brains of neonate rats. This delay might be due to poor A1AR expression or coupling to G proteins in younger animals [84]. Therefore, another pronounced concern about A1AR activity is the developmental changes in its expression and signaling.
It is important to note that functional A1ARs also express on different neural lineage cells. RT-PCR analysis of oligodendrocyte progenitor cells (OPCs) shows that both embryonic and postnatal OPCs of mice express A1AR [85]. Functional protein expression of A1AR at different developmental stages of oligodendrocytes, from OPCs to mature oligodendrocytes, has been detected by immunocytochemistry and radioreceptor assays. Mature oligodendrocytes reportedly express higher levels of A1AR compared to OPCs [81]. Expression of A1AR in human embryonic stem cell-derived OPCs has been shown in the authors’ laboratory (in press).
A1AR expression is simultaneous with the time in which neural formation, migration, and differentiation are very active and local adenosine changes with metabolic states of cells or environmental conditions. A potential role is played by A1AR signaling during the development of the nervous system. A1AR signaling appears to inhibit neurite outgrowth by activation of Rho A kinase in PC12 cells that express A1AR and a primary culture of cortical and hippocampal neurons [86]. Previously, it has been reported that small GTPase such as Rho A is involved in reorganization of the cytoskeleton and induces axon retraction through activation of Rho kinases [87, 88]. Converging lines of evidence therefore provide support for the concept that A1AR activity may influence neuronal differentiation by modulating neurite outgrowth.
Axon navigation to appropriate targets is also a critical stage in nervous system development in which a precise topographic pattern of neuron connections is formed. In this process, navigating neurons send out axons to reach the correct target. Axon guidance responds to a balance of molecular cues which attract them to a special target and repel them from inappropriate targets [89]. Engrailed homeoproteins, which are transcriptional and translational regulators, participate in axonal guidance [90, 91]. According to research, these proteins sensitize temporal growth cones of retinal ganglionic cells (RGCs) to a low concentration of ephrin A5 and induce temporal RGC collapse in the anterior portion of the optic tectum [92]. More importantly, sensitization has been reported to occur through A1AR signaling during chick development. According to this observation, engrailed induces ATP synthesis and release from growth cones. Released ATP is subsequently degraded to adenosine which, in turn, stimulates A1AR and enhances ephrin A5 activity. Temporal RGCs have higher concentrations of A1AR than nasal ganglionic cells; hence, it is not surprising that engrailed is differentially involved in axon guidance activity of RGCs [92].
A study of other aspects of nervous system formation shows that A1AR stimulation can effectively increase OPC migration, with no effect on OPC viability, proliferation, or differentiation. This study suggests that A1AR signaling reduces cAMP accumulation in oligodendrocytes [81]; however, there is no data about the relationship between cAMP reduction and OPC migration. OPC migration is more prominent during white matter formation and after neural tissue injury [93]. A1AR agonists may have a promising role in nervous system regeneration. However, in vivo evidence to ascertain the contribution of A1AR on OPCs remains to be elucidated.
A literature survey revealed that exaggerated A1AR activation exerted adverse effects on the developing brain rather than the protective roles observed in adults. A1AR may have a role in the pathogenesis of some developmental brain injuries even in postnatal life. This is the time when the brain is yet vulnerable to hypoxia-ischemia conditions and brain development is not completed. According to research, A1AR stimulation could not prevent ischemic brain damage in 7-day-old rats. It was suggested that A1AR, compared to periphery, is poorly developed in the brain at this stage and this phenomenon hampers the ability of A1AR to exert functional responses [84]. On the other hand, an A1AR-mediated ventriculomegaly (expansion of the brain ventricle) has been shown in early postnatal life of mice exposed to hypoxia from P3 to P14. Mice with loss of A1AR expression attenuated ventriculomegaly and retained myelin basic protein (MBP) expression and white matter although they were kept in hypoxic conditions [94]. Ventricle enlargement can be caused by overproduction of cerebrospinal fluid (CSF). In the choroid plexus, the main site of CSF production, Na+-K+ ATPase pump activity is closely associated with CSF secretion [95]. Increased expression of Na+-K+ ATPase has been observed in A1AR overexpression in transgenic mice [96] and in the choroid plexus of rats treated by an A1AR agonist for 2 weeks or long-term exposure to caffeine [97]. Therefore, it is thought that increased expression of Na+-K+ ATPase and overproduction of CSF are a possible underlying mechanism on the effect of A1AR stimulation on ventriculomegaly [97]. The effects of A1AR signaling on Na+-K+ ATPase expression during earlier developmental time points remain to be clarified. Instead, in perinatal mice exposed to hypoxia, ventriculomegaly is secondary to a reduction in the preoligodendrocyte process arborization and development, which, in turn, affects white matter formation. It is suggested that abnormal oligodendrocyte development may have been a consequence of A1AR activation since caffeine can prevent ventriculomegaly and hypomyelination [98]. Such studies have also confirmed the potential role of A1AR action to mediate the immediate adverse effects of hypoxia-ischemia on the developing brain. Additional, direct evidence by using selective A1AR agonists is needed. Chronic administration of caffeine to pregnant rats has been reported to cause a significant decrease in the total number of A1ARs in the fetus as well as the maternal brain [87].
In the past decade, in vitro and in vivo studies on rodent models have shown that the developing nervous system is also sensitive to the harmful effects of metabolic disorders such as hypoglycemia. Administration of A1AR antagonists or A1AR ablation leads to protection against hypoglycemia [99]. This data provides a link between the prominent roles of A1AR in signal transduction of the consequences of environmental changes such as metabolic disorders to nervous system development.
In summary, it seems signaling through A1AR has a potentially dual feature during nervous system development. Although we know more about the long-lasting trophic effects of A1AR activation during this process, data about the effects of A1AR signaling on correct formation of the nervous system is limited. Considering today’s knowledge, pharmacological inhibition of the central A1AR may have beneficial effects against some environmental disturbances that occur during nervous system development [98].
A1AR Signaling Might Be Effective in Adult Nervous System Repair
It has been clearly shown that adenosine through A1AR stimulation can attenuate spontaneous or evoked release of neurotransmitter release [28, 66] and/or fine-tune regulation of neuronal activity, in basal physiological conditions [28, 65, 100]. These neuromodulatory effects may play a role in neuroprotection and have a physiological implication which is critically highlighted in stressful situations.
A1AR Activation Effects on Trauma and Seizure
Synaptic transmission or neuronal firing rate is suppressed by A1AR during ischemia and trauma [101] or metabolic disorders [63], thereby preventing excessive neurotoxicity [27]. These conditions are accompanied by elevated concentrations of extracellular adenosine [102]. Central activation of A1AR suppresses seizures and exerts antiepileptic effects. Many findings support the observation that this finding is related to the neuroprotective effects of adenosine acting at A1AR [103–106]. It has been reported that the presence of some special single nucleotide polymorphism (SNP) in the A1AR human gene is accompanied by more severe posttraumatic seizures after a traumatic brain injury (TBI) [107]. This report provides more evidence for the importance of A1AR signaling in reducing the adverse effects of TBI.
A1AR Activation Effects on Multiple Sclerosis
Of note, A1AR signaling plays a prominent neuroprotective role in chronic neurodegenerative disorders such as multiple sclerosis (MS). A1AR-induced neuroprotective responses in this situation, however, are supported by several in vivo studies on A1AR knockdown animals or pharmacological drugs [108, 109]. Lack of A1AR in a mice model of MS has resulted in increased demyelination and axonal injury, whereas A1AR activation maintained myelin sheath integrity and improved neurobehavioral responses in A1AR+/+ animals [108]. Reduced demyelination and significantly enhanced remyelination after treatment with an A1AR agonist were reported in another lysophosphatidylcholine-induced demyelination rat model [109]. Researchers observed downregulation of A1AR expression on blood-derived monocytic cells and microglia in the brains of MS patients [110, 111] as well as in the spinal cords of an experimental autoimmune encephalomyelitis (EAE) model of MS in mice [108]. Interestingly, double application of an A1AR agonist with a common AR antagonist such as caffeine has potentiated the ability of A1AR to ameliorate the severity of MS symptoms, which was most likely due to enhanced A1AR gene expression [108]. Upregulation of A1AR transcription has been observed in a guinea pig spinal cord homogenate-induced EAE model in rats after chronic caffeine application, which was accompanied with significant reduction in EAE symptoms [112]. In agreement with these results, a case-control study on the relationship between the type of routine daily diet and MS risk in 75 case and 75 matched control women indicated a reverse association between coffee consumption and the risk of MS [113]. Recently, a large-scale retrospective case-control study of 2779 cases and 3960 matched controls further strengthened the advantage of drinking coffee in MS. These results indicated consumption of high amounts of coffee significantly reduced the risk of MS [114]. However, in these studies, there was no data about the exact factor in coffee that determined the benefits of this beverage or probable mechanisms. Based on previous studies on experimental animals, the authors in the aforementioned study postulated that caffeine-dependent A1AR mRNA upregulation and/or consequence effects of A1AR activation on the immune system were the most probable mechanisms for these observations [114]. Glucocorticoids such as dexamethasone, which is used for immunomodulatory therapy in MS patients, upregulate A1AR gene expression in the brain tissue [20, 115]. These observations may raise the usefulness of caffeine consumption or some other routine medications to treat MS symptoms through alterations in A1AR signaling.
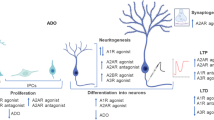
A1AR activation may exert beneficial effects on MS through triggering the following mechanisms. (i) It has been suggested that A1AR inhibition is accompanied by increased macrophage/microglia activation which enhances expression and release of pro-inflammatory cytokine including interleukin-1β (IL-1β) and matrix metalloproteinase-12 (MMP-12). Such cytokines induce demyelination and axonal injury upon oligodendrocyte degeneration. A1AR signaling activation attenuates pro-inflammatory responses and maintains myelin sheath integrity by augmenting anti-inflammatory responses [108]. An obvious shift from Th1 to Th2 immune response has been shown in a model of rat EAE which is possibly related to A1AR activation [112]. (ii) Neural stem cells (NSCs) also express A1AR. Activation of this receptor induces proliferation of NSCs through direct activation of ERK/MEK and Akt signaling pathways [116]. NSCs constitute a well-defined self-renewal multipotent cell population in neurogenic niches that have the capability to differentiate to all types of CNS cells. These cells participate in normal CNS development and are responsible for neurogenesis during adulthood or improved recovery after diseases or insults (reviewed in [117, 118]). They can also produce oligodendrocyte progenitors and participate in remyelination. Therefore, it has been suggested that an enhanced level of adenosine during CNS injury stimulates neuroregeneration through A1AR-induced NSC proliferation in a paracrine/autocrine manner, although there is no direct evidence for this assumption [116]. (iii) A1AR agonists stimulate OPC migration [81]. OPC recruitment to damaged white matter area as well as their differentiation is frequently severely inhibited in MS disease that leads to reduced remyelination [119]. (iv) It has been suggested that A1AR activation may promote OPC differentiation. Recently, it was reported that sustained, outward rectifier Ik current increased after in vitro A1AR stimulation in OPCs (unpublished data in [120]). On the other hand, it was demonstrated that modulation of the Ik current had a direct association with oligodendrocyte development, in which inhibition of Ik prevented OPC differentiation and vice versa [120–122]. Direct evidence is needed for clarification. Figure 1 summarizes the most important proposed mechanisms of the beneficial effects of A1AR stimulation on MS.
A1AR Activation Effects on Glioblastoma
Recently, researchers reported that A1AR signaling was implicated in cancer stem cells (CSCs) derived from glioblastoma multiforme, the most malignant brain tumor. A1AR stimulation significantly inhibited CSC proliferation. The A1AR agonist initially affected CSC fate because of reduction in their stemness as well as increased differentiation. Continued treatment with an A1AR agonist could promote cell apoptosis in differentiated cells. A1AR stimulation also has been shown to sensitize CSCs to chemotherapy. According these observations, it was suggested that sequential application of an A1AR agonist with chemotherapy might more efficiently inhibit tumor growth [123].
A1AR Activation Effects on Pain
A1AR stimulation reduces pain, another interesting finding which highlights the potential role of this receptor at both the central and peripheral nervous system levels. Several studies that have researched selective A1AR agonists and antagonists report that activation of A1AR is responsible for the antinociceptive effects of adenosine in neuropathic [124, 125] or inflammatory [126–128] models of pain. Increased hyperalgesia has been also reported in mice that lack A1AR [129, 130]. However, the exact molecular mechanisms triggered by A1AR activation have not been fully elucidated. The analgesic effects of A1AR stimulation reportedly depend on prevention of nociceptive neuron sensitization. This conclusion has been drawn from the following observations. (i) A1AR activation led to activation of the nitric oxide/cGMP/protein kinase G/KATP channel signaling pathway [131]. It was demonstrated that activation of this signaling pathway by enhancing K+ currents from KATP channels led to hyperpolarization of nociceptive neurons [132], which directly reduced neuronal sensitization. (ii) The analgesic effect of A1AR activation, at least on inflammatory pain, has been shown to depend on inhibition of the hyperactive state of microglia by suppressing Ca2+ entry into these cells. Non-active microglia has a decreased ability to facilitate nociceptive neuron firing [79, 125]. Thereby, A1AR activation can also indirectly block nociceptive neurons. Attempts to produce antinociceptive drugs based on this physiological effect of A1AR are underway [133].
Conclusion
A1AR is one of the four subtypes of the AR. It represents the common features of G protein-coupled receptors and usually directs signal transduction through the Gi/Go protein to different downstream effectors. Numerous evidences show that signaling through A1AR plays a prominent role in mediating the potent effects of adenosine in neuromodulation. Because A1AR has the highest affinity to adenosine, it is fully stimulated by a slight increase in adenosine levels. A1AR particularly exerts its critical neuroprotective roles in stressful events that are more emphasized in adults. A1AR is the earliest, dominant AR subtype expressed in embryos that has a significant involvement in nervous system development. Partial agonists and allosteric ligands of A1AR will be promising to increase beneficial effects and simultaneously avoid undesirable effects of adenosine [133, 134]. The modulatory effect of A1AR on neuroinflammation and neuroprotection, neural development and axogenesis, oligodendrocyte progenitor migration, as well as NSC-mediated repair are possible targets of this group of AR for therapeutic purposes.
References
Engler RL (1991) Adenosine. The signal of life? Circulation 84(2):951–954
Mullane K, Bullough D (1995) Harnessing an endogenous cardioprotective mechanism: cellular sources and sites of action of adenosine. J Mol Cell Cardiol 27(4):1041–1054
Park J, Gupta RS (2013) Adenosine metabolism, adenosine kinase, and evolution. In: Adenosine. Springer. pp. 23–54
Latini S, Pedata F (2001) Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem 79(3):463–484
Burnstock G et al (2010) The birth and postnatal development of purinergic signalling. Acta Physiol 199(2):93–147
Fredholm BB et al (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev 63(1):1–34
Pasini FL, Capecchi P, Di Perri T (2000) Adenosine and chronic ischemia of the lower limbs. Vasc Med 5(4):243–250
Ren H, Stiles GL (1994) Characterization of the human A1 adenosine receptor gene. Evidence for alternative splicing. J Biol Chem 269(4):3104–3110
Linden J, et al. (1987) Agonist and antagonist radioligands and photoaffinity labels for the adenosine A1 receptor. In: Topics and perspectives in adenosine research. Springer. pp. 3–14
Latek D et al (2012) G protein-coupled receptors—recent advances. Acta Biochim Pol 59(4):515–529
Gilman AG (1987) G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56(1):615–649
Libert F et al (1991) The orphan receptor cDNA RDC7 encodes an A1 adenosine receptor. EMBO J 10(7):1677
Townsend-Nicholson A, Schofield PR (1994) A threonine residue in the seventh transmembrane domain of the human A1 adenosine receptor mediates specific agonist binding. J Biol Chem 269(4):2373–2376
Olah ME et al (1992) Cloning, expression, and characterization of the unique bovine A1 adenosine receptor. Studies on the ligand binding site by site-directed mutagenesis. J Biol Chem 267(15):10764–10770
Rivkees SA, Lasbury ME, Barbhaiya H (1995) Identification of domains of the human A1 adenosine receptor that are important for binding receptor subtype-selective ligands using chimeric A1/A2a adenosine receptors. J Biol Chem 270(35):20485–20490
Libert F et al (1991) Chromosomal mapping of A1 and A2 adenosine receptors, VIP receptor, and a new subtype of serotonin receptor. Genomics 11(1):225–227
Mahan LC et al (1991) Cloning and expression of an A1 adenosine receptor from rat brain. Mol Pharmacol 40(1):1–7
Ren H, Stiles GL (1994) Posttranscriptional mRNA processing as a mechanism for regulation of human A1 adenosine receptor expression. Proc Natl Acad Sci 91(11):4864–4866
Ren H, Stiles GL (1995) Separate promoters in the human A1 adenosine receptor gene direct the synthesis of distinct messenger RNAs that regulate receptor abundance. Mol Pharmacol 48(6):975–980
Ren H, Stiles GL (1999) Dexamethasone stimulates human A1 adenosine receptor (A1AR) gene expression through multiple regulatory sites in promoter B. Mol Pharmacol 55(2):309–316
Nie Z et al (1998) Oxidative stress increases A1 adenosine receptor expression by activating nuclear factor κB. Mol Pharmacol 53(4):663–669
Cordeaux Y, IJzerman AP, Hill SJ (2004) Coupling of the human A1 adenosine receptor to different heterotrimeric G proteins: evidence for agonist-specific G protein activation. Br J Pharmacol 143(6):705–714
Gerwins P, Fredholm B (1992) ATP and its metabolite adenosine act synergistically to mobilize intracellular calcium via the formation of inositol 1,4,5-trisphosphate in a smooth muscle cell line. J Biol Chem 267(23):16081–16087
Iredale PA, Alexander SP, Hill SJ (1994) Coupling of a transfected human brain A1 adenosine receptor in CHO-K1 cells to calcium mobilisation via a pertussis toxin-sensitive mechanism. Br J Pharmacol 111(4):1252–1256
Sabourin J et al (2012) Activation of transient receptor potential canonical 3 (TRPC3)-mediated Ca2+ entry by A1 adenosine receptor in cardiomyocytes disturbs atrioventricular conduction. J Biol Chem 287(32):26688–26701
Robin E et al (2011) Adenosine A1 receptor activation is arrhythmogenic in the developing heart through NADPH oxidase/ERK-and PLC/PKC-dependent mechanisms. J Mol Cell Cardiol 51(6):945–954
Jeong H-J et al (2003) Adenosine A1 receptor-mediated presynaptic inhibition of GABAergic transmission in immature rat hippocampal CA1 neurons. J Neurophysiol 89(3):1214–1222
Li Y et al (2011) Adenosine modulates the excitability of layer II stellate neurons in entorhinal cortex through A1 receptors. Hippocampus 21(3):265–280
Dickenson JM, Blank JL, Hill SJ (1998) Human adenosine A1 receptor and P2Y2-purinoceptor-mediated activation of the mitogen-activated protein kinase cascade in transfected CHO cells. Br J Pharmacol 124(7):1491–1499
Saura C et al (1996) Adenosine deaminase interacts with A1 adenosine receptors in pig brain cortical membranes. J Neurochem 66(4):1675–1682
Ciruela F et al (1996) Adenosine deaminase affects ligand-induced signalling by interacting with cell surface adenosine receptors. FEBS Lett 380(3):219–223
Saura CA et al (1998) Adenosine deaminase and A1 adenosine receptors internalize together following agonist-induced receptor desensitization. J Biol Chem 273(28):17610–17617
Escriche M et al (2003) Ligand-induced caveolae-mediated internalization of A 1 adenosine receptors: morphological evidence of endosomal sorting and receptor recycling. Exp Cell Res 285(1):72–90
Garrido C et al (2012) The small heat shock proteins family: the long forgotten chaperones. Int J Biochem Cell Biol 44(10):1588–1592
Sarrió S et al (2000) The heat shock cognate protein hsc73 assembles with A1 adenosine receptors to form functional modules in the cell membrane. Mol Cell Biol 20(14):5164–5174
Dongcheng L et al (2004) Cytoskeletal protein 4.1 G binds to the third intracellular loop of the A1 adenosine receptor and inhibits receptor action. Biochem J 377(1):51–59
Prinster SC, Hague C, Hall RA (2005) Heterodimerization of G protein-coupled receptors: specificity and functional significance. Pharmacol Rev 57(3):289–298
Milligan G (2009) G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br J Pharmacol 158(1):5–14
Kamal M, Jockers R (2011) Biological significance of GPCR heteromerization in the neuro-endocrine system. Frontiers Endocrinol 2
Ciruela F et al (1995) Immunological identification of A1 adenosine receptors in brain cortex. J Neurosci Res 42(6):818–828
Gracia E et al (2013) Homodimerization of adenosine A 1 receptors in brain cortex explains the biphasic effects of caffeine. Neuropharmacology 71:56–69
Ciruela F et al (2006) Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1–A2A receptor heteromers. J Neurosci 26(7):2080–2087
Yoshioka K, Saitoh O, Nakata H (2001) Heteromeric association creates a P2Y-like adenosine receptor. Proc Natl Acad Sci 98(13):7617–7622
Yoshioka K et al (2002) Hetero-oligomerization of adenosine A 1 receptors with P2Y 1 receptors in rat brains. FEBS Lett 531(2):299–303
Yoshioka K, Saitoh O, Nakata H (2002) Agonist-promoted heteromeric oligomerization between adenosine A 1 and P2Y 1 receptors in living cells. FEBS Lett 523(1):147–151
Tonazzini I et al (2007) Co-localization and functional cross-talk between A1 and P2Y1 purine receptors in rat hippocampus. Eur J Neurosci 26(4):890–902
Suzuki T et al (2006) Regulation of pharmacology by hetero-oligomerization between A 1 adenosine receptor and P2Y 2 receptor. Biochem Biophys Res Commun 351(2):559–565
Chandrasekera PC et al (2013) Adenosine A 1 receptors heterodimerize with β 1-and β 2-adrenergic receptors creating novel receptor complexes with altered G protein coupling and signaling. Cell Signal 25(4):736–742
Ciruela F et al (2001) Metabotropic glutamate 1α and adenosine A1 receptors assemble into functionally interacting complexes. J Biol Chem 276(21):18345–18351
Kamikubo Y et al (2013) Functional cooperation of metabotropic adenosine and glutamate receptors regulates postsynaptic plasticity in the cerebellum. J Neurosci 33(47):18661–18671
Kamikubo Y et al (2015) Complex formation and functional interaction between adenosine A1 receptor and type-1 metabotropic glutamate receptor. J Pharmacol Sci 128(3):125–130
Ferré S et al (1998) Adenosine A1 receptor-mediated modulation of dopamine D1 receptors in stably cotransfected fibroblast cells. J Biol Chem 273(8):4718–4724
Ginés S et al (2000) Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc Natl Acad Sci 97(15):8606–8611
Torvinen M et al (2002) Interactions among adenosine deaminase, adenosine A 1 receptors and dopamine D 1 receptors in stably cotransfected fibroblast cells and neurons. Neuroscience 113(3):709–719
Toda S, Alguacil LF, Kalivas PW (2003) Repeated cocaine administration changes the function and subcellular distribution of adenosine A1 receptor in the rat nucleus accumbens. J Neurochem 87(6):1478–1484
Heiss W-D, Herholz K (2006) Brain receptor imaging. J Nucl Med 47(2):302–312
Bauer A et al (2003) In vivo imaging of adenosine A 1 receptors in the human brain with [18 F] CPFPX and positron emission tomography. NeuroImage 19(4):1760–1769
Fukumitsu N et al (2005) Adenosine A1 receptor mapping of the human brain by PET with 8-dicyclopropylmethyl-1-11C-methyl-3-propylxanthine. J Nucl Med 46(1):32–37
Svenningsson P et al (1997) Distribution of adenosine receptors in the postmortem human brain: an extended autoradiographic study. Synapse 27(4):322–335
Swanson TH, Drazba JA, Rivkees SA (1995) Adenosine A1 receptors are located predominantly on axons in the rat hippocampal formation. J Comp Neurol 363(4):517–531
Tetzlaff W, Schubert P, Kreutzberg G (1987) Synaptic and extrasynaptic localization of adenosine binding sites in the rat hippocampus. Neuroscience 21(3):869–875
Rebola N et al (2003) Subcellular localization of adenosine A 1 receptors in nerve terminals and synapses of the rat hippocampus. Brain Res 987(1):49–58
Kawamura M, Ruskin DN, Masino SA (2010) Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J Neurosci 30(11):3886–3895
Trussell L, Jackson M (1987) Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci 7(10):3306–3316
Clark BD, Kurth-Nelson ZL, Newman EA (2009) Adenosine-evoked hyperpolarization of retinal ganglion cells is mediated by G-protein-coupled inwardly rectifying K+ and small conductance Ca2+-activated K+ channel activation. J Neurosci 29(36):11237–11245
Hawryluk JM et al (2012) Adenosine inhibits glutamatergic input to basal forebrain cholinergic neurons. J Neurophysiol 107(10):2769–2781
Floran B et al (2002) Adenosine A1 receptors control dopamine D1-dependent [3 H] GABA release in slices of substantia nigra pars reticulata and motor behavior in the rat. Neuroscience 115(3):743–751
Okada M et al (1997) Effects of adenosine receptor subtypes on hippocampal extracellular serotonin level and serotonin reuptake activity. J Neurochem 69(6):2581–2588
Oishi Y et al (2008) Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc Natl Acad Sci 105(50):19992–19997
Ross AE, Venton BJ (2015) Adenosine transiently modulates stimulated dopamine release in the caudate–putamen via A1 receptors. J Neurochem 132(1):51–60
O'Neill C et al (2007) Adenosine A1 receptor-mediated inhibition of dopamine release from rat striatal slices is modulated by D1 dopamine receptors. Eur J Neurosci 26(12):3421–3428
Porkka-Heiskanen T, Kalinchuk AV (2011) Adenosine as a sleep factor. Sleep and Biological Rhythms 9(s1):18–23
Thakkar MM, Winston S, McCarley RW (2003) A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J Neurosci 23(10):4278–4287
Elmenhorst D et al (2007) Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J Neurosci 27(9):2410–2415
Arrigoni E et al (2006) Adenosine inhibits basal forebrain cholinergic and noncholinergic neurons in vitro. Neuroscience 140(2):403–413
Liu Z-W, Gao X-B (2007) Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypothalamus: a possible sleep-promoting effect. J Neurophysiol 97(1):837–848
Thakkar MM (2011) Histamine in the regulation of wakefulness. Sleep Med Rev 15(1):65–74
Methippara MM et al (2005) Effects on sleep of microdialysis of adenosine A1 and A2a receptor analogs into the lateral preoptic area of rats. Am J Phys Regul Integr Comp Phys 289(6):R1715–R1723
Luongo L et al (2014) The A1 adenosine receptor as a new player in microglia physiology. Glia 62(1):122–132
Alloisio S et al (2004) Differential modulation of ATP-induced calcium signalling by A1 and A2 adenosine receptors in cultured cortical astrocytes. Br J Pharmacol 141(6):935–942
Othman T, Yan H, Rivkees SA (2003) Oligodendrocytes express functional A1 adenosine receptors that stimulate cellular migration. Glia 44(2):166–172
Group CS (2008) Maternal caffeine intake during pregnancy and risk of fetal growth restriction: a large prospective observational study. BMJ: Br Med J 337
Rivkees SA (1995) The ontogeny of cardiac and neural A1 adenosine receptor expression in rats. Dev Brain Res 89(2):202–213
Ådén U et al (2001) Adenosine A 1 receptor agonism in the immature rat brain and heart. Eur J Pharmacol 426(3):185–192
Stevens B et al (2002) Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36(5):855–868
Thevananther S, Rivera A, Rivkees SA (2001) A1 adenosine receptor activation inhibits neurite process formation by Rho kinase-mediated pathways. Neuroreport 12(14):3057–3063
León D et al (2002) Adenosine A1 receptor down-regulation in mothers and fetal brain after caffeine and theophylline treatments to pregnant rats. J Neurochem 82(3):625–634
Kranenburg O et al (1999) Activation of RhoA by lysophosphatidic acid and Gα12/13 subunits in neuronal cells: induction of neurite retraction. Mol Biol Cell 10(6):1851–1857
Bashaw GJ, Klein R (2010) Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol 2(5):a001941
Brunet I et al (2005) The transcription factor engrailed-2 guides retinal axons. Nature 438(7064):94–98
Wizenmann A et al (2009) Extracellular engrailed participates in the topographic guidance of retinal axons in vivo. Neuron 64(3):355–366
Stettler O et al (2012) Engrailed homeoprotein recruits the adenosine A1 receptor to potentiate ephrin A5 function in retinal growth cones. Development 139(1):215–224
Baumann N, Pham-Dinh D (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81(2):871–927
Turner CP et al (2003) A1 adenosine receptors mediate hypoxia-induced ventriculomegaly. Proc Natl Acad Sci 100(20):11718–11722
Damkier HH, Brown PD, Praetorius J (2013) Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev 93(4):1847–1892
Lankford AR et al (2002) Gene expression profile of mouse myocardium with transgenic overexpression of A1 adenosine receptors. Physiol Genomics 11(2):81–89
Han M-E et al (2009) Regulation of cerebrospinal fluid production by caffeine consumption. BMC Neurosci 10(1):110
Back SA et al (2006) Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann Neurol 60(6):696–705
Kim M et al (2005) Susceptibility of the developing brain to acute hypoglycemia involving A1 adenosine receptor activation. American Journal of Physiology-Endocrinology and Metabolism 289(4):E562–E569
Brown P, Dale N (2000) Adenosine A1 receptors modulate high voltage-activated Ca2+ currents and motor pattern generation in the Xenopus embryo. J Physiol 525(3):655–667
Kochanek PM et al (2006) Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J Cereb Blood Flow Metab 26(4):565–575
Fredholm B (2007) Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death & Differentiation 14(7):1315–1323
Güttinger M et al (2005) Seizure suppression and lack of adenosine A 1 receptor desensitization after focal long-term delivery of adenosine by encapsulated myoblasts. Exp Neurol 193(1):53–64
Fedele DE et al (2006) Adenosine A 1 receptors are crucial in keeping an epileptic focus localized. Exp Neurol 200(1):184–190
Palomero-Gallagher N et al (2012) Multireceptor analysis in human neocortex reveals complex alterations of receptor ligand binding in focal epilepsies. Epilepsia 53(11):1987–1997
Akula KK, Kulkarni S (2014) Effect of curcumin against pentylenetetrazol-induced seizure threshold in mice: possible involvement of adenosine A1 receptors. Phytother Res 28(5):714–721
Wagner AK et al (2010) Adenosine A1 receptor gene variants associated with post-traumatic seizures after severe TBI. Epilepsy Res 90(3):259–272
Tsutsui S et al (2004) A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J Neurosci 24(6):1521–1529
Asghari AA, Mirnajafi-Zadeh SJ, Javan M (2012) Effect of the adenosine A1 receptor agonist on demyelination and remyelination processes in lysolecithin induced demyelination in rat optic chiasm. KAUMS Journal (FEYZ) 16(1):1–8
Mayne M et al (1999) Dysregulation of adenosine A1 receptor-mediated cytokine expression in peripheral blood mononuclear cells from multiple sclerosis patients. Ann Neurol 45(5):633–639
Johnston JB et al (2001) Diminished adenosine A1 receptor expression on macrophages in brain and blood of patients with multiple sclerosis. Ann Neurol 49(5):650–658
Chen GQ et al (2010) Chronic caffeine treatment attenuates experimental autoimmune encephalomyelitis induced by guinea pig spinal cord homogenates in Wistar rats. Brain Res 1309:116–125
Jahromi SR et al (2012) Dietary pattern and risk of multiple sclerosis. Iranian journal of neurology 11(2):47
Hedström A et al (2016) High consumption of coffee is associated with decreased multiple sclerosis risk; results from two independent studies. J Neurol Neurosurg Psychiatry. doi:10.1136/jnnp-2015-312176
Svenningsson P, Fredholm BB (1997) Glucocorticoids regulate the expression of adenosine A1 but not A2A receptors in rat brain. J Pharmacol Exp Ther 280(2):1094–1101
Migita H et al (2008) Activation of adenosine A1 receptor-induced neural stem cell proliferation via MEK/ERK and Akt signaling pathways. J Neurosci Res 86(13):2820–2828
Alvarez-Buylla A, García-Verdugo JM, Tramontin AD (2001) A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 2(4):287–293
Götz M, Huttner WB (2005) The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6(10):777–788
Sim FJ et al (2002) The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci 22(7):2451–2459
Coppi E et al (2013) Adenosine A 2A receptors inhibit delayed rectifier potassium currents and cell differentiation in primary purified oligodendrocyte cultures. Neuropharmacology 73:301–310
Chittajallu R et al (2002) Regulation of Kv1 subunit expression in oligodendrocyte progenitor cells and their role in G1/S phase progression of the cell cycle. Proc Natl Acad Sci 99(4):2350–2355
Vautier F et al (2004) Shaker-type potassium channel subunits differentially control oligodendrocyte progenitor proliferation. Glia 48(4):337–345
Daniele S et al (2014) Modulation of A1 and A2B adenosine receptor activity: a new strategy to sensitise glioblastoma stem cells to chemotherapy. Cell Death Dis 5(11):e1539
Schaddelee MP et al (2005) Pharmacokinetic/pharmacodynamic modelling of the anti-hyperalgesic and anti-nociceptive effect of adenosine A 1 receptor partial agonists in neuropathic pain. Eur J Pharmacol 514(2):131–140
Luongo L et al (2012) 5′-Chloro-5′-deoxy-(±)-ENBA, a potent and selective adenosine A1 receptor agonist, alleviates neuropathic pain in mice through functional glial and microglial changes without affecting motor or cardiovascular functions. Molecules 17(12):13712–13726
Poon A, Sawynok J (1998) Antinociception by adenosine analogs and inhibitors of adenosine metabolism in an inflammatory thermal hyperalgesia model in the rat. Pain 74(2):235–245
Nascimento FP et al (2014) Adenosine A1 receptor-dependent antinociception induced by inosine in mice: pharmacological, genetic and biochemical aspects. Mol Neurobiol 51(3):1368–1378
Gao X et al (2014) Norisoboldine attenuates inflammatory pain via the adenosine A1 receptor. Eur J Pain 18(7):939–948
Wu W-P et al (2005) Increased nociceptive response in mice lacking the adenosine A 1 receptor. Pain 113(3):395–404
Johansson B et al (2001) Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci 98(16):9407–9412
Lima FO et al (2010) Direct blockade of inflammatory hypernociception by peripheral A1 adenosine receptors: involvement of the NO/cGMP/PKG/KATP signaling pathway. Pain 151(2):506–515
Cunha TM et al (2010) Morphine peripheral analgesia depends on activation of the PI3Kγ/AKT/nNOS/NO/KATP signaling pathway. Proc Natl Acad Sci 107(9):4442–4447
Korboukh I et al (2012) Orally active adenosine A1 receptor agonists with antinociceptive effects in mice. J Med Chem 55(14):6467–6477
Franchetti P et al (2009) N 6-Cycloalkyl-and N 6-bicycloalkyl-C 5′(C 2′)-modified adenosine derivatives as high-affinity and selective agonists at the human A1 adenosine receptor with antinociceptive effects in mice. J Med Chem 52(8):2393–2406
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kashfi, S., Ghaedi, K., Baharvand, H. et al. A1 Adenosine Receptor Activation Modulates Central Nervous System Development and Repair. Mol Neurobiol 54, 8128–8139 (2017). https://doi.org/10.1007/s12035-016-0292-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0292-6