Abstract
(−)-Epicatechin is a brain-permeable, natural product found at high concentrations in green tea and cocoa. Our previous research has shown that (−)-epicatechin treatment reduces hemorrhagic stroke injury via nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway in vivo. However, the mechanism of action of this compound in modulation of oxidant stress and in protection against hemoglobin-induced astrocyte injury is unclear. Therefore, we explored the cellular and molecular mechanisms that underlie these protective effects in vitro. Mouse primary astrocytes isolated from wild-type mice and Nrf2 knockout (KO) mice were preconditioned with hemoglobin to simulate intracerebral hemorrhage (ICH) in vitro. Effects of (−)-epicatechin were measured by Western blotting, immunostaining, MTT assay, and reactive oxidant stress (ROS) assay. (−)-Epicatechin increased Nrf2 nuclear accumulation and cytoplasmic levels of superoxide dismutase 1 (SOD1) in wild-type astrocytes but did not increase SOD1 expression in Nrf2 knockout (KO) astrocytes. Furthermore, (−)-epicatechin treatment did not alter heme oxygenase 1 (HO1) expression in wild-type astrocytes after hemoglobin exposure, but it did decrease HO1 expression in similarly treated Nrf2 KO astrocytes. In both wild-type and Nrf2 KO astrocytes, (−)-epicatechin suppressed phosphorylated JNK and nuclear expression of JNK, c-jun, and c-fos, indicating that inhibition of activator protein-1 (AP-1) activity by (−)-epicatechin is Nrf2-independent. These novel findings indicate that (−)-epicatechin protects astrocytes against hemoglobin toxicity through upregulation of Nrf2 and inhibition of AP-1 activity. These cellular and molecular effects may partially explain the cerebroprotection as we previously observed for (−)-epicatechin in animal models of ICH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracerebral hemorrhage (ICH) is a severe subtype of stroke that affects more than 1 million people worldwide annually and accounts for 10–30% of all strokes [1]. Although it is associated with high morbidity and mortality throughout the world, effective therapies are limited. The primary injury after ICH is caused by bleeding. In particular, hemoglobin released from damaged erythrocytes in the hematoma triggers oxidant stress, neuroinflammation, and neurotoxicity that contribute to secondary brain damage [2–4].
Oxidative stress caused by reactive oxygen species (ROS) plays an important role in ICH progression. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that regulates the expression of antioxidant response element (ARE)-related genes and mitigates secondary brain injury after ICH [3–5]. Using Nrf2 knockout (KO) mice and Nrf2 inducers, we and others have demonstrated that Nrf2 has neuroprotective effects in animal models of ICH [6–9]. Moreover, astrocytic activation of Nrf2 and the genes it regulates downstream, including heme oxygenase 1 (HO1), superoxide dismutase 1 (SOD1), and NAD(P)H oxidoreductase 1 (NQO1), can protect neurons from cytotoxicity both in vitro and in vivo [10–13]. These findings suggest that astrocytic Nrf2 activation is important after ICH.
The activator protein-1 (AP-1) is another transcription factor shown to be involved in regulation of inflammation, apoptosis, cell proliferation/differentiation, and oxidative stress [14]. The AP-1 complex is composed of proteins from the Jun (c-Jun, JunB, and JunD) and Fos (c-Fos, FosB, Fra-1, and Fra-2) families [15]. Studies have shown that AP-1 DNA binding activity is elevated in in vitro and in vivo models of cerebral ischemia [16–18]; thus, it might contribute to secondary brain injury [14, 19]. One study showed that the Jun amino-terminal kinase (JNK)/c-jun/AP-1 pathway is activated in astrocytes under conditions of oxygen-glucose deprivation [18]. However, the role of AP-1 in ICH is unclear.
(−)-Epicatechin (EC) is a brain-permeable, natural flavanol compound extracted from green tea and cocoa [20, 21] that exerts protective effects on cognition [22], vascular function [23], and ischemic stroke injury [24] in animals and humans. EC has the ability to cross the blood-brain barrier (BBB) both in vivo [25] and in vitro [26]. Furthermore, we have shown that EC ameliorates histologic and functional deficits after ICH and traumatic brain injury. Its effects are associated with a decrease in HO1 expression and increases in SOD1 and NQO1 expression, possibly via activation of Nrf2-dependent and Nrf2-independent pathways [7, 27]. All of these results indicate that EC could provide a promising treatment for ICH. However, the cell-specific function of EC remains largely unknown, and whether EC regulates Nrf2/HO1 in astrocytes needs to be determined.
To further explore the cellular and molecular mechanisms by which EC protects against ICH injury, in this study, we cultured primary astrocytes isolated from wild-type (WT) and Nrf2 KO mice and exposed them to hemoglobin to simulate ICH in vitro. Our results indicate that after hemoglobin exposure, AP-1 could be another target of EC in astrocytes in addition to Nrf2. Activation of AP-1 and Nrf2 in astrocytes may partially explain the cerebroprotective effects of EC as we previously observed in animal models of ICH.
Methods
Materials
Compound (−)-epicatechin, hemoglobin, MTT, and 2′,7′-dichlorofluorescein diacetate (DCF-DA) were purchased from Sigma Aldrich (Allentown, PA, USA). D-Hanks and 0.25% trypsin were purchased from Quality Biological (Gaithersburg, MD, USA). DMEM/F12, FBS, and Alexa Fluor 488 goat anti-rabbit secondary antibody were obtained from Life Technologies (Carlsbad, CA, USA). Antibodies of Nrf2 (H-300), Lamin a/c, and β-actin were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). SOD1 antibody was purchased from Abcam (Cambridge, UK). HO1 antibody was purchased from Stressgen, (San Diego, CA, USA). Antibodies of phospho-SAPK/JNK, SAPK/JNK, c-jun, c-fos, and peroxidase-coupled goat anti-rabbit or anti-mouse secondary antibody were purchased from Cell Signaling Technology (Danvers, MA, USA).
Mouse Primary Astrocyte Culture
All the experimental procedures were conducted with accordance with guidelines of the National Institutes for Health and approved by the institutional Animal Care and Use committee at Johns Hopkins University School of Medicine. Nrf2 KO mice (on a C57BL/6 background) were originally generated by Dr. Masayuki Yamamoto (Tohoku University, Japan). Primary astrocytes were cultured from newborn WT and Nrf2 KO mice (postnatal day 1) as described previously [28–30]. In brief, cortices were dissected from brain and cut into 1–3 mm2 pieces. Cells were dissociated in D-Hanks’ solution containing 0.25% trypsin and plated onto 75 cm2 cell culture flasks at 1 × 107 cells/mL in Dulbecco’s modified Eagle’s medium/Ham’s F-12 (DMEM/F12) with 10% fetal bovine serum (FBS). After 2 weeks, microglia and oligodendrocytes were removed from the mixed glial cells by shaking at 200 rpm overnight at 37 °C, and astrocytes were cultured in complete DMEM/F12 medium. Astrocytes that were >95% positive for GFAP staining (supplementary Fig. 1) and within five passages were used in our experiments.
(−)-Epicatechin Treatment
Cells were stimulated with 10 μM hemoglobin, and doses of EC were dissolved in 0.1% DMSO. For evaluation of cell signaling and transcription factors (Nrf2, phosphorylated JNK, c-jun, and c-fos), we pretreated cells with EC 1 h before hemoglobin injury and collected samples after 2 h of hemoglobin exposure. For other experiments, cells were preconditioned with hemoglobin beginning 1 h before the addition of EC, and cell supernatant or proteins were collected 24 h later. Astrocytes from WT mice (WT astrocytes) and Nrf2−/− mice (KO astrocytes) were grouped as follows: (1) control: cells were incubated in 0.1% DMSO, which did not cause cell death compared with untreated cells (supplementary Fig. 2); (2) Hb: cells were treated with 10 μM hemoglobin; (3) EC: cells were treated with EC before or after 10 μM hemoglobin stimulation.
MTT Assay
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, thiazolyl blue (MTT), was used to measure astrocyte cytotoxicity and viability. Cells were incubated with MTT (0.5 mg/mL) at 37 °C for 4 h. After the medium was removed, DMSO was added to each well. Absorbance at 550 nm was read on a SpectraMax M2 microplate reader (Molecular Devices, Downingtown, PA, USA).
ROS Measurement
The peroxide-sensitive fluorescent probe DCF-DA was used to detect the production of intracellular ROS [31]. Fluorescence intensity was read on a SpectraMax M2 microplate reader.
Western Blotting
For Western blotting [32], we used antibodies to the following proteins: Nrf2 (1:200), SOD1 (1:2000), HO1 (1:2000), phospho-SAPK/JNK (1:1000), SAPK/JNK (1:1000), Lamin a/c (1:1000), and β-actin (1:4000). Briefly, 30 μg of protein was separated by 4–20% SDS-PAGE gel and transferred onto polyvinylidene fluoride membranes. The membranes were blocked with 5% milk or 5% bovine serum albumin, incubated with primary antibodies at 4 °C overnight, and then incubated with peroxidase-coupled goat anti-rabbit or anti-mouse secondary antibody (1:5000). Membranes were immersed in enhanced chemiluminescence (ECL) solution and exposed under an ImageQuant ECL Imager (GE Healthcare, Little Chalfont, UK).
Immunostaining
Mouse primary astrocytes were fixed with 4% paraformaldehyde, blocked with 5% bovine serum albumin, and incubated with primary antibody to c-jun (1:400) or c-fos (1:200) at 4 °C overnight. Cells were then incubated at room temperature with appropriate secondary antibody (Alexa Fluor 488 goat anti-rabbit, 1:1000). Cell nuclei were stained with DAPI. Cell immunofluorescence intensity was calculated with ImageJ software [33], and the measurements followed published protocol [34].
Statistical Analysis
All data are presented as means ± SD of five independent experiments. Comparisons of two groups were analyzed by unpaired, two-tailed t test. Comparisons among multiple groups were calculated by one-way ANOVA or two-way ANOVA multiple comparison with Bonferroni post hoc test. A p < 0.05 was considered significant. Nonlinear logistic regression was used to fit concentration-response curves and to calculate IC50.
Results
EC Reduces ROS Production in Hemoglobin-Stimulated Astrocytes via an Nrf2-Independent Pathway
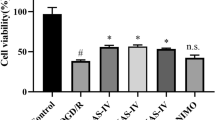
First, we used WT and Nrf2 KO astrocytes to study the effects of EC on ROS. EC cytotoxicity testing showed that concentrations as high as 500 μM EC were not toxic to cells (Fig. 1a) after a 24-h incubation. To study the effects of EC on hemoglobin-exposed astrocytes, we preconditioned the cells with hemoglobin (20 or 10 μM) for 1 h and then added various concentrations of EC for 24 h. Hemoglobin at 20 μM caused 23.4 ± 7.16% cell death in WT astrocytes and 36.3 ± 6.71% cell death in Nrf2 KO astrocytes. However, 20 μM EC significantly increased the viability of both WT and Nrf2 KO astrocytes exposed to 20 μM hemoglobin (Fig. 1b). To avoid the influence of hemoglobin-induced cytotoxicity, we tested lower concentrations of hemoglobin. When astrocytes were exposed to a lower, 10 μM concentration of hemoglobin, which produced little toxicity (Fig. 1c), ROS production in Nrf2 KO astrocytes was almost twice that in WT astrocytes (Fig. 1d). This finding implies that Nrf2 has a critical antioxidant effect in astrocytes. However, 20–500 μM EC decreased ROS production to a similar extent in WT and Nrf2 KO astrocytes (Fig. 1d). The IC50 of EC for ROS inhibition was 54.5 μM in WT astrocytes and 44.1 μM in Nrf2 KO astrocytes (Fig. 1e, 75% inhibition of ROS production, IC75 = 95.02 μM of WT, and 100.25 μM of KO). These results indicate that EC reduces hemoglobin-induced ROS elevation via an Nrf2-independent pathway. Based on these data, we selected 100 μM EC for most of the remaining experiments.
(−)-Epicatechin reduces reactive oxygen species (ROS) production in hemoglobin-exposed WT and Nrf2 KO astrocytes. a WT and Nrf2 KO astrocytes were treated with EC at concentrations from 0.03 to 1000 μM for 48 h. Cytotoxicity was observed only at 1000 μM. b Hemoglobin (Hb) at 20 μM caused approximately 23% cell death in WT astrocytes and 36% cell death in Nrf2 KO astrocytes. EC significantly increased cell viability of astrocytes exposed to hemoglobin (20 μM) for 24 h. c At a concentration of 10 μM, hemoglobin did not cause cell death in primary astrocytes from WT and Nrf2 KO mice. d Hemoglobin-induced ROS production in Nrf2 KO astrocytes was almost twice that in WT astrocytes. EC at concentrations from 0.8 to 500 μM reduced ROS production in astrocytes from Nrf2 KO and WT mice exposed to 10 μM hemoglobin. e The IC50 of EC for ROS inhibition was 54.5 μM in WT astrocytes and 44.1 μM in Nrf2 KO astrocytes. All data are presented as mean ± SD of five independent experiments. ###p < 0.001 vs. control group; +++p < 0.001 vs. WT Hb group; *p < 0.05, **p < 0.01, ***p < 0.001 vs. Hb alone
EC Increases Nrf2 Expression in Hemoglobin-Stimulated WT Astrocytes
Next, we examined the way EC regulates Nrf2 in astrocytes. For the time point of 2 h, EC was pretreated 1 h prior hemoglobin (10 μM) stimulation. After 2 h, cytoplasmic and nuclear proteins were extracted, and our results showed that nuclear Nrf2 was significantly increased compared with that in the control cells (Fig. 2b). EC (100 μM) further promoted Nrf2 expression in both cytoplasmic (Fig. 2a) and nuclear fractions (Fig. 2b). For the time point of 24 h, EC was added 1 h after hemoglobin exposure, and proteins were extracted 24 h later. Western blotting results showed that cytoplasmic Nrf2 (Fig. 2c) and nuclear Nrf2 (Fig. 2d) remained elevated in EC-treated astrocytes.
(−)-Epicatechin increases Nrf2 nuclear translocation in hemoglobin-exposed astrocytes. a, b EC was added to the medium 1 h before hemoglobin (Hb, 10 μM) treatment, and cytoplasmic (a) and nuclear (b) protein was extracted 2 h after hemoglobin stimulation. c, d EC (1, 10, or 100 μM) was added to the medium 1 h after hemoglobin, and cytoplasmic (c) and nuclear (d) protein was collected 24 h later. All data are presented as mean ± SD of five independent experiments. #p < 0.05, ##p < 0.01 vs. control group; *p < 0.05, **p < 0.01, ***p < 0.001 vs. Hb alone
Effects of EC on Hemoglobin-Induced HO1 and SOD1 Expression
HO1 and SOD1 are both downstream proteins of Nrf2 translocation and are triggered under oxidant stress. After 24 h of hemoglobin exposure, HO1 expression, but not SOD1 expression, was significantly increased. EC (1, 10, and 100 μM) significantly increased SOD1 expression in WT astrocytes (Fig. 3a) but did not further increase HO1 protein level (Fig. 3a). In contrast, under the same conditions, EC (100 μM) decreased HO1 level in Nrf2 KO astrocytes (Fig. 3b) but had no effect on SOD1 (Fig. 3b). We then measured HO1 and SOD1 levels at 2 h post-hemoglobin. EC (100 μM) did not alter HO1 level in WT astrocytes (Fig. 3c) or Nrf2 KO astrocytes (Fig. 3d). Moreover, EC (100 μM) led to SOD1 upregulation in WT astrocytes but had no effect in Nrf2 KO astrocytes. These data indicate that in hemoglobin-stimulated astrocytes, EC increases SOD1 expression in an Nrf2-dependent manner but regulates HO1 expression in an Nrf2-independent manner.
Effects of (−)-epicatechin on HO1 and SOD1 expression in hemoglobin-exposed astrocytes. a, b EC was added to the medium 1 h after hemoglobin (Hb, 10 μM). a After 24 h, SOD1 expression was significantly increased in WT astrocytes, but HO1 expression was unchanged. b At the same time point, 100 μM EC decreased HO1 expression in Nrf2 KO astrocytes without changing SOD1 expression. c, d EC was added to the medium 1 h before hemoglobin, and protein was collected 2 h after the addition of hemoglobin. c EC significantly elevated SOD1 expression in WT astrocytes exposed to hemoglobin for 2 h. d EC did not change HO1 or SOD1 expression in Nrf2 KO astrocytes exposed to hemoglobin for 2 h. All data are presented as mean ± SD of five independent experiments. ##p < 0.01, ###p < 0.001 vs. control group; *p < 0.05, **p < 0.01, ***p < 0.001 vs. Hb alone
EC Suppresses the AP-1 Signaling Pathway in both WT and Nrf2 KO Astrocytes
To continue exploring the mechanism of EC in modulation of HO1 and identify the Nrf2-independent targets of EC, we further evaluated AP-1 activation in WT and Nrf2 KO astrocytes. Astrocytes were pretreated with EC (100 μM) for 1 h before being exposed to hemoglobin (10 μM) for 2 h. Then, samples were collected for analysis of JNK/c-jun/AP-1 and c-fos/AP-1. Western blotting showed that in hemoglobin-stimulated WT and KO astrocytes, EC markedly decreased the phosphorylation of JNK (Fig. 4a, c). Immunostaining showed that 100 μM EC prevented JNK nuclear translocation (Fig. 4b, d) and inhibited c-jun nuclear expression (Fig. 4e p < 0.05). The nuclear expression of another important AP-1 family protein, c-fos, was also decreased in WT and KO astrocytes by EC treatment (p < 0.05, Fig. 4f). These results suggest that AP-1 is another major target of EC for rescuing hemoglobin-damaged astrocytes.
(−)-Epicatechin suppresses AP-1 signaling in both WT and Nrf2 KO astrocytes. Astrocytes were pretreated with 100 μM EC for 1 h before being exposed to hemoglobin (Hb, 10 μM) for 2 h. a EC inhibited the phosphorylation of JNK in WT astrocytes exposed to hemoglobin. b Immunostaining showed that EC inhibited phosphorylated-JNK (pJNK, green) translocation (DAPI, blue) in WT astrocytes exposed to hemoglobin. c EC inhibited the phosphorylation of JNK in Nrf2 KO astrocytes exposed to hemoglobin. d Immunostaining showed that EC inhibited pJNK (green) translocation (DAPI, blue) in Nrf2 KO astrocytes exposed to hemoglobin. e Immunostaining showed that EC inhibited nuclear expression (DAPI, blue) of c-jun (green) in WT and Nrf2 KO astrocytes exposed to hemoglobin. f Immunostaining showed that EC reduced nuclear (DAPI, blue) expression of c-fos (green) in WT and Nrf2 KO astrocytes exposed to hemoglobin. All data are presented as mean ± SD of five independent experiments. Scale bar = 20 μm. ###p < 0.001 vs. control group; *p < 0.05, **p < 0.01 vs. Hb alone
Discussion
In our study, we used hemoglobin-stimulated astrocytes in vitro to investigate the effects of EC, a brain-permeable, natural flavonoid compound with potentially multiple targets. We have shown that EC treatment is protective in animal models of ICH [7] and traumatic brain injury [27]. Here, we further determined the effects of EC on cultured astrocytes exposed to hemoglobin in vitro. We demonstrated for the first time that (1) the IC50 of EC for ROS inhibition is 54.5 μM in WT astrocytes and 44.1 μM in Nrf2 KO astrocytes; (2) EC increases Nrf2 nuclear translocation and expression of its downstream target SOD1 while protecting astrocytes against hemoglobin-induced oxidative injury; (3) the EC-induced increase in SOD1 expression, but not HO1 expression, is lost in Nrf2 KO astrocytes; and (4) hemoglobin increases JNK phosphorylation and nuclear expression of JNK, c-jun, and c-fos in WT and Nrf2 KO astrocytes, and EC inhibits these effects in both genotypes. Together, these findings provide clear in vitro evidence that EC protects astrocytes against hemoglobin toxicity through upregulation of Nrf2 and inhibition of AP-1 activity. Indeed, our finding that EC did not alter HO1 expression in WT astrocytes after hemoglobin exposure may result from a balance between the signaling pathways of Nrf2 activation and AP-1 inhibition (Fig. 5).
(−)-Epicatechin protects astrocytes against hemoglobin toxicity via Nrf2 and AP-1 signaling pathways. In astrocytes exposed to hemoglobin (Hb), EC treatment increases expression of cytoplasmic and nuclear Nrf2, which upregulates downstream target proteins SOD1 and NQO1. In addition, EC treatment inhibits AP-1 activity by downregulating JNK phosphorylation and nuclear expression of JNK/c-jun/c-fos, which could be another target of EC. In hemoglobin-exposed astrocytes, EC may fail to alter HO1 expression because the opposing effects of Nrf2 activation and AP-1 inhibition may produce a net zero response. ARE antioxidant response element, HO1 heme oxygenase 1, NQO1 NAD(P)H oxidoreductase 1, ROS reactive oxygen species, SOD1 superoxide dismutase 1
Nrf2 could be a therapeutic target for ICH. We and others have shown that Nrf2 deletion exacerbates ICH-induced DNA damage and apoptosis in neurons [6], whereas Nrf2 activation mitigates ICH injury [7, 8, 35]. Nrf2 can be induced in microglia [36]. It also upregulates scavenger receptor CD36 expression and enhances the microglial phagocytosis of red blood cells [37]. After transient cerebral ischemia, Nrf2 immunoreactivity can be detected in astrocytes, microglia, and neurons in the penumbra [38]. In the central nervous system, investigators have confirmed astrocytes to be major antioxidant resources that protect neurons against oxidative damage [39–41]. Furthermore, in mixed neuron/astrocyte cultures subjected to oxygen-glucose deprivation, astrocytes are the sole locus for Nrf2 activation in response to oxidative stress [42, 43]. Interestingly, a new study has shown that astrocytes with mutant SOD1 increase P-glycoprotein in endothelial cells in vitro [44]. Moreover, astrocytic Nrf2 activation helps to prevent oligodendrocyte loss and demyelination in an animal model of multiple sclerosis [45]. These data suggest that induction of Nrf2 in astrocytes contributes to neuroprotection, BBB repair, and white matter recovery after various brain injuries. In contrast, Nrf2 expression in neurons is much lower than that in astrocytes [46], a fact that supports the important role of the astrocytic Nrf2 pathway in the brain. However, our knowledge about the role of astrocytic Nrf2 under hemorrhagic conditions is limited. Hemoglobin increases TNF-α and decreases TGF-β level by dose-dependently activating NF-κB in human astrocytes exposed to hypoxia [47]. To explore the role of astrocytic Nrf2 in ICH, we chose hemoglobin-injured primary astrocytes in vitro to simulate acute post-ICH conditions. In the present study, we assessed the effects of hemoglobin on astrocytes after 2 and 24 h of exposure and found that hemoglobin stimulated Nrf2 nuclear translocation in WT astrocytes and induced ROS production in Nrf2 KO astrocytes at a level almost twice that in WT astrocytes. This finding strongly implies a critical antioxidant role for astrocytic Nrf2 signaling after ICH. To further study Nrf2 activation and signaling in ICH, we will consider using a neuron-astrocyte coculture system to investigate Nrf2-mediated neuroprotection in our future studies.
EC is a natural flavonoid compound that is enriched in green tea and cocoa. Recent studies have shown that EC has the capability of crossing the BBB in rats [25] and of being transported across BBB cells (RBE-4, immortalized cell line of rat capillary cerebral endothelial cells; hCMEC/D3, immortalized human cerebral microvessel endothelial cell line) at a concentration of 30 μM [26]. Moreover, EC enhanced ARE expression in Nrf2 overexpressed astrocytes [48]. However, the effects of EC on astrocytes stimulated with hemoglobin are unknown. Furthermore, our previous in vivo study showed that EC mitigates histologic and neurologic deficits after ICH in both WT and Nrf2 KO mice, suggesting that EC may act through Nrf2-dependent and Nrf2-independent pathways and could be a novel drug candidate for ICH treatment [7]. Here, we showed that EC increased cytoplasmic and nuclear Nrf2 expression and protected WT astrocytes after hemoglobin exposure. Interestingly, EC also protected Nrf2 KO astrocytes against hemoglobin toxicity. In combination, these data suggest that EC exerts protective effects via Nrf2-dependent and Nrf2-independent processes in animal models of ICH and in astrocytes exposed to hemoglobin.
One notable finding from this study is that although SOD1 and HO1 are both downstream of Nrf2, effects of EC on these two proteins are quite different. EC increased SOD1 expression in WT but not in Nrf2 KO astrocytes. This result indicates that upregulation of SOD1 expression by EC is Nrf2-dependent in astrocytes exposed to hemoglobin. In contrast, EC did not upregulate HO1 expression in hemoglobin-stimulated WT astrocytes but decreased HO1 expression in Nrf2 KO astrocytes. This finding indicates that the other targets of EC may regulate HO1 expression. Interestingly, the role of HO1 after ICH is controversial [5]. In ICH patients, HO1 expression in the hemorrhagic brain is associated with CD163 expression at 72 h after ICH [49], and serum HO1 level is increased in ICH patients [50]. In an animal model of ICH, systemic hemin therapy was reported to increase HO1 expression and attenuate blood-brain barrier disruption [51]. Additionally, porphyrin nonselective HO1 inhibitors have consistently been protective in animal models of ICH [52–54], and downregulation of HO1 by valproic acid protects against hemin toxicity [55]. Moreover, we have reported previously that HO1 deletion in mice reduces early ICH injury [56]. Therefore, the effect of EC on HO1 expression after ICH seems to be complicated and not entirely Nrf2-dependent.
AP-1 is a stress-activated transcription factor that has been implicated in ischemic stroke and traumatic brain injury [14]. In fibroblasts from Nrf2 KO mice, HO1 expression is increased by arsenite treatment, probably via AP-1 activation [57]. In astrocytes, high glucose-induced HO1 expression is regulated by AP-1 activation and contributes to neuronal apoptosis [58]. AP-1 is also involved in the regulation of matrix metalloproteinase-9 [59], which contributes to early ICH injury [60, 61]. Here, we showed that in astrocytes from WT and Nrf2 KO mice, EC treatment decreased the nuclear expressions of JNK, c-jun, and c-fos after hemoglobin exposure, which implies that EC strongly inhibits AP-1 transcription. Therefore, the failure of EC to alter HO1 expression in WT astrocytes after hemoglobin exposure may result from a balance between increased Nrf2 activity and decreased AP-1 activity. In contrast, our previous in vivo study showed that EC decreases HO1 expression level in WT mice after ICH [7]. Considering that our current study examined astrocytes alone in vitro, the effects of hemoglobin with and without EC treatment on other cell types such as neurons, microglia, and oligodendrocytes need to be explored in the future. The interactions between these different cell types will be important for explaining the overall action of EC on the ICH brain.
In conclusion, we identified two potential targets of EC in astrocytes exposed to hemoglobin. Nrf2-ARE/SOD1 is an important signaling pathway by which EC protects astrocytes against hemoglobin toxicity. Furthermore, hemoglobin can activate AP-1 signaling, and EC-induced inhibition of this pathway can provide complementary protection even in Nrf2 KO astrocytes. These cellular and molecular effects provide additional evidence to support EC’s cerebroprotective effects as we previously observed in animal models of ICH. As a transcription factor that regulates inflammation, cell death, and cell proliferation, AP-1 could be a new target for ICH treatment.
Abbreviations
- AP-1:
-
Activator protein-1
- DAPI:
-
4,6-diamidino-2-phenylindole
- EC:
-
(−)-Epicatechin
- HO1:
-
Heme oxygenase 1
- ICH:
-
Intracerebral hemorrhage
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- ROS:
-
Reactive oxygen species
- SOD1:
-
Superoxide dismutase 1
References
Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V (2009) Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 8(4):355–369. doi:10.1016/S1474-4422(09)70025-0
Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW (2014) Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol 115:25–44. doi:10.1016/j.pneurobio.2013.11.003
Wang J (2010) Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol 92(4):463–477
Zhou K, Zhong Q, Wang YC, Xiong XY, Meng ZY, Zhao T, Zhu WY, Liao MF et al (2016) Regulatory T cells ameliorate intracerebral hemorrhage-induced inflammatory injury by modulating microglia/macrophage polarization through the IL-10/GSK3beta/PTEN axis. J Cereb Blood Flow Metab. doi:10.1177/0271678X16648712
Zhang Z, Zhang Z, Lu H, Yang Q, Wu H, Wang J (2016) Microglial polarization and inflammatory mediators after intracerebral hemorrhage. Mol Neurobiol in press. doi: 10.1007/s12035-016-9785-6.
Wang J, Fields J, Zhao C, Langer J, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S et al (2007) Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med 43(3):408–414. doi:10.1016/j.freeradbiomed.2007.04.020
Chang CF, Cho S, Wang J (2014) (−)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Annals of clinical and translational neurology 1(4):258–271. doi:10.1002/acn3.54
Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, Grotta JC, Aronowski J (2007) Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke 38(12):3280–3286. doi:10.1161/STROKEAHA.107.486506
Iniaghe LO, Krafft PR, Klebe DW, Omogbai EK, Zhang JH, Tang J (2015) Dimethyl fumarate confers neuroprotection by casein kinase 2 phosphorylation of Nrf2 in murine intracerebral hemorrhage. Neurobiol Dis 82:349–358. doi:10.1016/j.nbd.2015.07.001
Pehar M, Vargas MR, Cassina P, Barbeito AG, Beckman JS, Barbeito L (2005) Complexity of astrocyte-motor neuron interactions in amyotrophic lateral sclerosis. Neurodegener Dis 2(3–4):139–146. doi:10.1159/000089619
Vargas MR, Pehar M, Cassina P, Beckman JS, Barbeito L (2006) Increased glutathione biosynthesis by Nrf2 activation in astrocytes prevents p75NTR-dependent motor neuron apoptosis. J Neurochem 97(3):687–696. doi:10.1111/j.1471-4159.2006.03742.x
Calkins MJ, Vargas MR, Johnson DA, Johnson JA (2010) Astrocyte-specific overexpression of Nrf2 protects striatal neurons from mitochondrial complex II inhibition. Toxicol Sci 115(2):557–568. doi:10.1093/toxsci/kfq072
Zhao X, Aronowski J (2013) Nrf2 to pre-condition the brain against injury caused by products of hemolysis after ICH. Translational stroke research 4(1):71–75. doi:10.1007/s12975-012-0245-y
Raivich G, Behrens A (2006) Role of the AP-1 transcription factor c-Jun in developing, adult and injured brain. Prog Neurobiol 78(6):347–363. doi:10.1016/j.pneurobio.2006.03.006
Hess J, Angel P, Schorpp-Kistner M (2004) AP-1 subunits: quarrel and harmony among siblings. J Cell Sci 117(Pt 25):5965–5973. doi:10.1242/jcs.01589
Akaji K, Suga S, Fujino T, Mayanagi K, Inamasu J, Horiguchi T, Sato S, Kawase T (2003) Effect of intra-ischemic hypothermia on the expression of c-Fos and c-Jun, and DNA binding activity of AP-1 after focal cerebral ischemia in rat brain. Brain Res 975(1–2):149–157
Tao X, Sun X, Yin L, Han X, Xu L, Qi Y, Xu Y, Li H et al (2015) Dioscin ameliorates cerebral ischemia/reperfusion injury through the downregulation of TLR4 signaling via HMGB-1 inhibition. Free Radic Biol Med 84:103–115. doi:10.1016/j.freeradbiomed.2015.03.003
Dong Y, Liu HD, Zhao R, Yang CZ, Chen XQ, Wang XH, Lau LT, Chen J et al (2009) Ischemia activates JNK/c-Jun/AP-1 pathway to up-regulate 14-3-3gamma in astrocyte. J Neurochem 109(Suppl 1):182–188. doi:10.1111/j.1471-4159.2009.05974.x
Nijboer CH, Heijnen CJ, Groenendaal F, van Bel F, Kavelaars A (2009) Alternate pathways preserve tumor necrosis factor-alpha production after nuclear factor-kappaB inhibition in neonatal cerebral hypoxia-ischemia. Stroke 40(10):3362–3368. doi:10.1161/STROKEAHA.109.560250
El-Salamouny S, Ranwala D, Shapiro M, Shepard BM, Farrar RR Jr (2009) Tea, coffee, and cocoa as ultraviolet radiation protectants for the beet armyworm nucleopolyhedrovirus. J Econ Entomol 102(5):1767–1773
Aree T, Jongrungruangchok S (2016) Crystallographic evidence for beta-cyclodextrin inclusion complexation facilitating the improvement of antioxidant activity of tea (+)-catechin and (−)-epicatechin. Carbohydr Polym 140:362–373. doi:10.1016/j.carbpol.2015.12.066
Knezevic B, Komatsuzaki Y, de Freitas E, Lukowiak K (2016) A flavonoid component of chocolate quickly reverses an imposed memory deficit. J Exp Biol. doi:10.1242/jeb.130765
Dower JI, Geleijnse JM, Gijsbers L, Zock PL, Kromhout D, Hollman PC (2015) Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: a randomized, double-blind, placebo-controlled, crossover trial. Am J Clin Nutr 101(5):914–921. doi:10.3945/ajcn.114.098590
Shah ZA, Li RC, Ahmad AS, Kensler TW, Yamamoto M, Biswal S, Dore S (2010) The flavanol (−)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 30(12):1951–1961
Wu L, Zhang QL, Zhang XY, Lv C, Li J, Yuan Y, Yin FX (2012) Pharmacokinetics and blood-brain barrier penetration of (+)-catechin and (−)-epicatechin in rats by microdialysis sampling coupled to high-performance liquid chromatography with chemiluminescence detection. J Agric Food Chem 60(37):9377–9383. doi:10.1021/jf301787f
Faria A, Pestana D, Teixeira D, Couraud PO, Romero I, Weksler B, de Freitas V, Mateus N et al (2011) Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food Funct 2(1):39–44. doi:10.1039/c0fo00100g
Cheng T, Wang W, Li Q, Han X, Xing J, Qi C, Lan X, Wan J et al (2016) Cerebroprotection of flavanol (−)-epicatechin after traumatic brain injury via Nrf2-dependent and -independent pathways. Free Radic Biol Med 92:15–28. doi:10.1016/j.freeradbiomed.2015.12.027
Bal-Price A, Brown GC (2001) Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci Off J Soc Neurosci 21(17):6480–6491
Lan X, Liu R, Sun L, Zhang T, Du G (2011) Methyl salicylate 2-O-beta-D-lactoside, a novel salicylic acid analogue, acts as an anti-inflammatory agent on microglia and astrocytes. J Neuroinflammation 8:98. doi:10.1186/1742-2094-8-98
Pan LN, Zhu W, Li Y, Xu XL, Guo LJ, Lu Q, Wang J (2014) Astrocytic toll-like receptor 3 is associated with ischemic preconditioning-induced protection against brain ischemia in rodents. PLoS One 9(6):e99526. doi:10.1371/journal.pone.0099526
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5(2):227–231
Zhao X, Wu T, Chang CF, Wu H, Han X, Li Q, Gao Y, Li Q et al (2015a) Toxic role of prostaglandin E2 receptor EP1 after intracerebral hemorrhage in mice. Brain Behav Immun 46:293–310. doi:10.1016/j.bbi.2015.02.011
Han X, Lan X, Li Q, Gao Y, Zhu W, Cheng T, Maruyama T, Wang J (2016) Inhibition of prostaglandin E2 receptor EP3 mitigates thrombin-induced brain injury. J Cereb Blood Flow Metab 36(6):1059–1074. doi:10.1177/0271678X15606462
Gavet O, Pines J (2010) Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell 18(4):533–543. doi:10.1016/j.devcel.2010.02.013
Yin XP, Chen ZY, Zhou J, Wu D, Bao B (2015a) Mechanisms underlying the perifocal neuroprotective effect of the Nrf2-ARE signaling pathway after intracranial hemorrhage. Drug Des Devel Ther 9:5973–5986. doi:10.2147/DDDT.S79399
Yin XP, Zhou J, Wu D, Chen ZY, Bao B (2015b) Effects of that ATRA inhibits Nrf2-ARE pathway on glial cells activation after intracerebral hemorrhage. Int J Clin Exp Pathol 8(9):10436–10443
Zhao X, Sun G, Ting SM, Song S, Zhang J, Edwards NJ, Aronowski J (2015b) Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance. J Neurochem 133(1):144–152. doi:10.1111/jnc.12974
Dang J, Brandenburg LO, Rosen C, Fragoulis A, Kipp M, Pufe T, Beyer C, Wruck CJ (2012) Nrf2 expression by neurons, astroglia, and microglia in the cerebral cortical penumbra of ischemic rats. J Mol Neurosci 46(3):578–584. doi:10.1007/s12031-011-9645-9
Trendelenburg G, Dirnagl U (2005) Neuroprotective role of astrocytes in cerebral ischemia: focus on ischemic preconditioning. Glia 50(4):307–320. doi:10.1002/glia.20204
Park JS, Jung JS, Jeong YH, Hyun JW, Le TK, Kim DH, Choi EC, Kim HS (2011) Antioxidant mechanism of isoflavone metabolites in hydrogen peroxide-stimulated rat primary astrocytes: critical role of hemeoxygenase-1 and NQO1 expression. J Neurochem 119(5):909–919. doi:10.1111/j.1471-4159.2011.07395.x
Park JS, Lee YY, Kim J, Seo H, Kim HS (2016) Beta-Lapachone increases phase II antioxidant enzyme expression via NQO1-AMPK/PI3K-Nrf2/ARE signaling in rat primary astrocytes. Free Radic Biol Med 97:168–178. doi:10.1016/j.freeradbiomed.2016.05.024
Bell KF, Al-Mubarak B, Fowler JH, Baxter PS, Gupta K, Tsujita T, Chowdhry S, Patani R et al (2011) Mild oxidative stress activates Nrf2 in astrocytes, which contributes to neuroprotective ischemic preconditioning. Proc Natl Acad Sci U S A 108(1):E1–E2 . doi:10.1073/pnas.1015229108author reply E3-4
Haskew-Layton RE, Payappilly JB, Smirnova NA, Ma TC, Chan KK, Murphy TH, Guo H, Langley B et al (2010) Controlled enzymatic production of astrocytic hydrogen peroxide protects neurons from oxidative stress via an Nrf2-independent pathway. Proc Natl Acad Sci U S A 107(40):17385–17390. doi:10.1073/pnas.1003996107
Qosa H, Lichter J, Sarlo M, Markandaiah SS, McAvoy K, Richard JP, Jablonski MR, Maragakis NJ et al (2016) Astrocytes drive upregulation of the multidrug resistance transporter ABCB1 (p-glycoprotein) in endothelial cells of the blood-brain barrier in mutant superoxide dismutase 1-linked amyotrophic lateral sclerosis. Glia 64(8):1298–1313. doi:10.1002/glia.23003
Draheim T, Liessem A, Scheld M, Wilms F, Weissflog M, Denecke B, Kensler TW, Zendedel A et al (2016) Activation of the astrocytic Nrf2/ARE system ameliorates the formation of demyelinating lesions in a multiple sclerosis animal model. Glia 64(12):2219–2230. doi:10.1002/glia.23058
Baxter PS, Hardingham GE (2016) Adaptive regulation of the brain’s antioxidant defences by neurons and astrocytes. Free Radic Biol Med. doi:10.1016/j.freeradbiomed.2016.06.027
Simoni J, Simoni G, Moeller JF, Feola M, Griswold JA, Wesson DE (2012) Adenosine-5′-triphosphate-adenosine-glutathione cross-linked hemoglobin as erythropoiesis-stimulating agent. Artif Organs 36(2):139–150. doi:10.1111/j.1525-1594.2011.01431.x
Bahia PK, Rattray M, Williams RJ (2008) Dietary flavonoid (−)epicatechin stimulates phosphatidylinositol 3-kinase-dependent anti-oxidant response element activity and up-regulates glutathione in cortical astrocytes. J Neurochem 106(5):2194–2204. doi:10.1111/j.1471-4159.2008.05542.x
Liu B, Hu B, Shao S, Wu W, Fan L, Bai G, Shang P, Wang X (2015) CD163/hemoglobin oxygenase-1 pathway regulates inflammation in hematoma surrounding tissues after intracerebral hemorrhage. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association 24(12):2800–2809. doi:10.1016/j.jstrokecerebrovasdis.2015.08.013
Li X, Li C, Hou L, He M, Song G, Ren S, Han C (2015) Higher level of serum heme oxygenase-1 in patients with intracerebral hemorrhage. Int Surg 100(7–8):1220–1224. doi:10.9738/INTSURG-D-14-00086.1
Lu X, Chen-Roetling J, Regan RF (2014) Systemic hemin therapy attenuates blood-brain barrier disruption after intracerebral hemorrhage. Neurobiol Dis 70:245–251. doi:10.1016/j.nbd.2014.06.005
Gong Y, Tian H, Xi G, Keep RF, Hoff JT, Hua Y (2006) Systemic zinc protoporphyrin administration reduces intracerebral hemorrhage-induced brain injury. Acta Neurochir Suppl 96:232–236
Wagner KR, Hua Y, de Courten-Myers GM, Broderick JP, Nishimura RN, Lu SY, Dwyer BE (2000) Tin-mesoporphyrin, a potent heme oxygenase inhibitor, for treatment of intracerebral hemorrhage: in vivo and in vitro studies. Cell Mol Biol (Noisy-le-grand) 46(3):597–608
Koeppen AH, Dickson AC, Smith J (2004) Heme oxygenase in experimental intracerebral hemorrhage: the benefit of tin-mesoporphyrin. J Neuropathol Exp Neurol 63(6):587–597
Kwon KJ, Kim JN, Kim MK, Kim SY, Cho KS, Jeon SJ, Kim HY, Ryu JH et al (2013) Neuroprotective effects of valproic acid against hemin toxicity: possible involvement of the down-regulation of heme oxygenase-1 by regulating ubiquitin-proteasomal pathway. Neurochem Int 62(3):240–250. doi:10.1016/j.neuint.2012.12.019
Wang J, Dore S (2007) Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain 130(Pt 6):1643–1652. doi:10.1093/brain/awm095
Harada H, Sugimoto R, Watanabe A, Taketani S, Okada K, Warabi E, Siow R, Itoh K et al (2008) Differential roles for Nrf2 and AP-1 in upregulation of HO-1 expression by arsenite in murine embryonic fibroblasts. Free Radic Res 42(4):297–304. doi:10.1080/10715760801975735
Jiang C, Zuo F, Wang Y, Wan J, Yang Z, Lu H, Chen W, Zang W et al (2016) Progesterone exerts neuroprotective effects and improves long-term neurologic outcome after intracerebral hemorrhage in middle-aged mice. Neurobiol Aging 42:13–24. doi:10.1016/j.neurobiolaging.2016.02.029
Shihab PK, Al-Roub A, Al-Ghanim M, Al-Mass A, Behbehani K, Ahmad R (2015) TLR2 and AP-1/NF-kappaB are involved in the regulation of MMP-9 elicited by heat killed Listeria monocytogenes in human monocytic THP-1 cells. J Inflamm 12:32. doi:10.1186/s12950-015-0077-0
Wu H, Wu T, Hua W, Dong X, Gao Y, Zhao X, Chen W, Cao W et al (2015) PGE2 receptor agonist misoprostol protects brain against intracerebral hemorrhage in mice. Neurobiol Aging 36(3):1439–1450. doi:10.1016/j.neurobiolaging.2014.12.029
Wu H, Wu T, Han X, Wan J, Jiang C, Chen W, Lu H, Yang Q et al (2016) Cerebroprotection by the neuronal PGE2 receptor EP2 after intracerebral hemorrhage in middle-aged mice. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism: in press. doi:10.1177/0271678X15625351
Acknowledgements
This work was supported by the National Institute of Health R01NS078026 and R01AT007317 (JW) and by the American Heart Association Mid-Atlantic Affiliate Grant-in-Aid (13GRNT15730001 to JW) and Postdoctoral Fellowship Awards (15POST25090114 to X. Lan and 14POST20140003 to X. Han). The authors thank Tian Cheng and Jieru Wan for the Western blotting technical support, Wenzhu Wang for the blind analysis of immunostaining, and Claire Levine for the assistance with manuscript preparation. We thank Raymond Koehler, Zengjin Yang, and all the Wang lab members for their constructive suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Fig. 1
(DOCX 140 kb)
Supplementary Fig. 2
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Lan, X., Han, X., Li, Q. et al. (−)-Epicatechin, a Natural Flavonoid Compound, Protects Astrocytes Against Hemoglobin Toxicity via Nrf2 and AP-1 Signaling Pathways. Mol Neurobiol 54, 7898–7907 (2017). https://doi.org/10.1007/s12035-016-0271-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0271-y