Abstract
Creutzfeldt-Jakob disease is a rare, but rapidly progressive, up to now untreatable and fatal neurodegenerative disorder. Clinical diagnosis of Creutzfeldt-Jakob disease (CJD) is difficult; however, it can be facilitated by suitable biomarkers. Aim of the present study is to compare levels of cerebrospinal fluid biomarkers (total tau protein, phosphorylated-tau protein, protein 14-3-3 and amyloid beta) in Slovak population of CJD suspect cases, retrospectively in over a 10-year period. One thousand three hundred sixty-four CSF samples from patients with suspect CJD, forming a homogenous group in terms of geographical as well as of equal transport conditions, storage and laboratory processing, were analysed. Definite diagnosis of Creutzfeldt-Jakob disease was confirmed in 101 patients with genetic form, and 60 patients with its sporadic form of the disease. Specificity of protein 14-3-3 and total tau in both forms CJD was similar (87 % for P14-3-3/85 % for total tau), sensitivity to P 14-3-3 and total tau was higher in sporadic Creutzfeldt-Jakob disease (sCJD) (90/95 %) than in genetic Creutzfeldt-Jakob disease (gCJD) (89/74 %). As expected, the total tau levels were significantly higher in CJD patients than in controls, but there was also significant difference between gCJD and sCJD (levels in gCJD were lower; p = 0.003). There was no significant difference in p-tau and Aβ 1-42 levels neither between both CJD forms nor between CJD patients and control group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human prion diseases are rare, transmissible, neurodegenerative lethal diseases. They are characterized by spongiform changes, neuronal loss and deposits of non-degradable, misfolded prion proteins (PrPsc) in the brain tissue. The conformationally altered protease-resistant proteins are considered to constitute the transmissible agent [1]. The most frequent human prion disease is Creutzfeldt-Jakob disease (CJD), which occurs in sporadic (sCJD), genetic (gCJD) and iatrogenic (iCJD) forms. While worldwide is the most frequent sCJD, (gCJD represents only 10–15 % of all CJD cases), Slovakia is peculiar by exceptionally high incidence (65–75 %) of the gCJD cases with the E200K mutation (gCJDE200K) [2] accumulated in geographic clusters [3]. Increased number of gCJD with the same mutation in Israel has an ethnical origin [4, 5].
Diagnostic criteria of WHO define possible, probable and definite diagnosis of CJD [6]. Definite diagnosis is confirmed by post-mortem histopathological examination of the brain tissue and by detection of PrPsc. Probable diagnosis of CJD is based on the typical clinical symptoms and results of clinical diagnosis such as EEG, analysis of the cerebrospinal fluid (CSF) and brain MRI [6–9]. CSF marker analysis is an important part of the probable CJD diagnosis. Investigated CSF biomarkers, such as protein 14-3-3 (P 14-3-3), total tau (t-tau) protein, phosphorylated form of tau protein (p-tau), and amyloid beta (Aß), indicate neuronal degeneration and/or activation of glial cells.
P 14-3-3 belongs to the family of proteins which are found in eukaryotic cells, with the highest expression in neuronal synapses [10]. It is a non-specific marker, and an increased CSF value indicates a pathological process with a massive neuronal death. The P 14-3-3 level is increased in the CSF of CJD-affected patients, but it can also be detected in inflammatory diseases and tumours of the CNS. Factors influencing the false-positive results of the P 14-3-3 in the CSF are blood cells and pleocytosis in the CSF, caused by inflammatory processes of the CNS [10].
Tau protein is a natural, structural protein of neuronal microtubules. Abnormal, misfolded tubular protein has a tendency to form thick fibres and to aggregate into neurofibrillary tangles in the patient’s brain. Abnormal tau protein has a tendency to transform into phosphorylated-tau protein (p-tau). The cutoff value for the t-tau protein in CJD diagnosis, based on international criteria, was determined to 1300 pg/ml [11, 12]. Tau protein analysis is not a part of the current diagnostic criteria. The t-tau value correlates with the patient’s age. This fact could be important for differentiation of the normal ageing from pathologic, neurodegenerative processes. According to published data, only the t-tau protein is the highly increased in CJD [13, 14], not its phosphorylated form. In CJD, the neuronal destruction and increasing level of t-tau are relatively fast, representing a significant difference to Alzheimer’s disease (AD) with a slower destruction process.
The pathological Aβ protein is the basis of senile or neuritic plaques in AD [15], and its level in the CSF is reduced. Differentiation is made between two main isoforms, shorter form Aβ 1-40 and longer one Aβ 1-42. Under normal circumstances, the soluble Aβ 1-40 isoform prevails, and Aβ 1-42 isoform aggregates faster. The Aβ 1-42 isoform exhibits the highest degree for adherence in plaques and has the most significant decline in values in AD patients [15]. In affected area, it participates in destruction of synapses, degenerative processes and progressive neuronal death.
While the WHO diagnostic criteria of probable CJD includes semi-quantitative P 14-3-3 test, it does not include the quantitative determination of the tau protein. Discrepancies in experience with detection of these CSF biomarkers resulted in some criticism concerning P 14-3-3 [12, 16], as well as in different view on their diagnostic value.
The aim of this study is to analyse values of the investigated CSF biomarkers in the up to now largest homogenous investigated group of gCJDE200K patients, to compare them with data obtained in the Slovak sporadic CJD cases, as well as with previously published results in the literature, considering their diagnostic value and implementation in differential diagnosis of CJD.
Material and Methods
Samples Collection
The study includes 1364 samples of CSF, 1364 blood and 71 sera collected in the Department of Prion Diseases (National Reference Centrum for Prion Diseases in Slovakia) in the course of 2002–2014.
CSF samples were obtained from suspect cases of CJD from regional hospitals and transferred to our laboratory. The samples were shipped refrigerated or frozen, and prior to analysis, they were stored at −20 °C, while after analysis, they are stored in freezers at −80 °C.
Totaly, 101 confirmed gCJDE200K were obtained from the analysed 1364 CSF samples, and 60 samples were from patients affected by sCJD. Investigating the influence of the storage interval on tested samples, 20 CSF samples from gCJDE200K and 18 samples of sCJD with duration of storage (at −80 °C) for more than 10 years have been analysed. A control group was formed by 50 samples from previously suspected CJD cases. In which 25 of them were CJD excluded histopathologically, additional 25 patients were added in the control group retrospectively after having the negative results of the early in vivo diagnostic methods (DNA analysis for the presence of specific mutation and P14-3-3 detection) and the patient’s typical CJD clinical symptoms did not persist.
The same number (1364) of blood samples were examined for detection of the E200K mutation and distribution of the polymorphism M129 V. Possible age influence on obtained results was investigated comparing three groups of patients, consisting of those less than 50 years old, patients 51 to 65 years old and the ones older than 65 years old. Besides described investigations of CSF samples, t-tau determination has been performed also in 71 sera from patients with confirmed CJD.
The present study has been in line with rules approved by the ethical committee acting on the Slovak Medical University.
Protein Analysis in CSF
P 14-3-3 (ß isoform) was determined in CSF samples by Western blot according to Zerr et al. [17]. Heating up the samples in a buffer was followed by separation of the proteins based on different molecular weight by SDS/polyacrylamide gel electrophoresis. This was followed by a semi-dry blotting of proteins from the gel to a PVDF membrane, and blocking of free binding sites of the membrane in PBS containing 5 % low-fat milk. Detection was carried out by overnight incubation with primary antibody (pan 14-3-3 (K19) monoclonal antibody Santa Cruz Biotechnologies), and with the secondary antibody (Goat anti-rabbit IgG (H + L) HRP Jackson ImmunoResearch) the following day. Membranes were developed by enhanced chemiluminiscence (SuperSignal West Pico, PIERCE). Each Western blot was analysed by semi-quantitative method (negative result = no signal present; positive result = strong signal/band; weakly positive result = weaker signal compared to positive control). Each analysis was performed with positive and negative control. The sensitivity (% of positive results in patients with a confirmed diagnosis of CJD) and specificity (% of false-positive results in patients with other types of diagnosis than CJD) of the used method for detection of the P 14-3-3 were evaluated. A possible age influence on achieved results was investigated comparing three groups of patients consisting of patients less than 50 years old, 51 to 65 years old patients and those older than 65 years old.
T-tau, p-tau and Aβ 1-42 were measured in CSF sample collection retrospectively, using a commercially available sandwich ELISA kit (INNOTEST hTAU Ag, INNOTEST phospho-TAU (181P), INNOTEST amyloid β (1–42) manufactured by Innogenetics) following the manufacturer’s instructions. A positive t-tau test for CJD was considered at cutoff level >1300 pg/ml [11, 18, 19]. According to the manufacturer’s instructions, aimed at the diagnosis of AD, a pathological elevated p-tau level was considered at >61 pg/ml and pathological decreased Aβ 1-42 was considered at <450 pg/ml [11]. Besides individual t-tau and p-tau proteins, combined CSF levels of 1/t-tau and p-tau (pg/ml) and 2/t-tau and Aβ (pg/ml) were compared in sCJD with gCJDE200K, as well as between CJD with non-CJD patients.
Molecular Biological Analysis
For the sake of molecular biological analysis of the prion protein gene (PRNP), DNA was isolated from blood. Subsequently, genotype was determined by polymerase chain reaction followed by restriction (PCR-RFLP) assay using cleavage enzymes (enzyme BsmAI for codon 200, and enzyme HpyCH4 for codon 129). Cleavage fragments were visualized by electrophoresis. All samples (101) from the genetic CJD group had PRNP point mutation at codon 200 (E200K mutation) (gCJDE200K).
Statistical Analyses
Statistical analysis was performed using the program SPSS16.0. The Kolgomorov-Smirnov test showed that the results obtained with t-tau, p-tau and Aβ 1-42 proteins did not have a normal distribution, and therefore, the Mann-Whitney U test was used to compare these variables between diagnostic groups. For comparison of quantitative parameters between different genotypes, the non-parametric Kruskal-Wallis test was used; χ 2 test was used to determine differences between categorical variables. Spearman’s test was used for correlation between individual parameters. The text presents average data; value p < 0.05 was considered as statistically significant.
Results
Age Distribution of CJD Patients
Comparison of gCJD and sCJD cases in three age categories (younger than 50 years old, 51 to 65 years old, older than 65 years old) showed significantly different age distribution (p = 0.004). Sporadic CJD patients were older (p < 0.001) than gCJD cases.
Protein 14-3-3
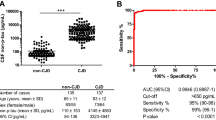
Specificity of the method varied between 75 and 90 % in the course of 2004–2014. Increasing age of the patients had no influence on specificity of the method, since the lowest specificity was observed in the group of 51 to 65 years old patients. Sensitivity of P14-3-3 in sCJD (90 %) was similar to gCJDE200K (89 %). P 14-3-3 signal intensity was stronger in sCJD than in the genetic form, where 19 % of the samples showed weak positive signal, while in case of sCJD, it was weak in only 0.6 % of the samples.
T-Tau Protein
It was retrospectively examined in the CSF samples stored in the Department of Prion Diseases since 2000. Obtained results showed significantly (p = 0.003) higher values of t-tau protein in sCJD (4718.7 pg/ml, with a range from 100 to 24,000 pg/ml) than in gCJDE200K (2115.9 pg/ml, with a range from 50 to 16,000 pg/ml) (Fig. 1a. Table 1). Sensitivity of the t-tau assay was lower in gCJDE200K (74 %) than in sCJD (95 %). Sensitivity of the method in gCJDE200K could be increased by reducing the threshold on 1000 pg/ml (84 %).
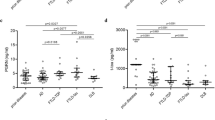
a–c Box plots of CSF a total tau, b amyloid beta, c P-tau in sCJD, gCJDE200K, non-CJD patients. Plots show 10, 25, 50, 75 and 90th percentiles and outliers, with significant difference in average CSF total tau protein between non-CJD and both forms of CJD, and no difference in average CSF amyloid beta and p-tau in non-CJD and CJD patients. t-tau: n (sCJD) = 60, n (gCJD) = 99, n (non-CJD) = 50. p-tau and Aβ1–42: n (sCJD) = 40, n (gCJD) = 72, n (non-CJD) = 50
Out of 18 tested samples of clinically suspect CJD, carriers of the E200K mutation without definite diagnosis confirmed by autopsy, total tau values were above the cutoff value of 1300 pg/ml in 10 cases (56 %). In case of eight of these patients, also the P 14 3-3 test was positive. However, values of t-tau were significantly higher in the confirmed cases of CJD (p = 0.016). In the group of 25 patients with histopathologically excluded CJD, 6 CSF samples have a t-tau above 1300 pg/ml (24 %). These are patients with AD, paraneoplastic neurological syndrome associated with cancer and stroke (based on data provided by clinical departments). Patients with t-tau < 1000 pg/ml from control group have AD, stroke, metabolic encephalopathy and paraneoplastic neurological syndrome.
The specificity for t-tau protein was 85 %, similar as for P 14-3-3 (87 %).
Combined CSF levels of both 1/t-tau and p-tau (pg/ml) (Fig. 2a) and 2/t-tau and Aβ (pg/ml) (Fig. 2b) compared in sCJD with gCJDE200K, as well as between CJD with non-CJD patients, showed statistically significant difference (p < 0.05). To compare biomarkers, ratio of p-tau/t-tau and Aß 1-42/t-tau non-parametric Man-Whitney test was used and had shown statistically significant difference between genetic and sporadic CJD (p < 0.001). The group is small to calculate cutoff value. For the (p-tau/t-tau) ×100, ratio is mean value in gCJD 4.09 (95 % confidence interval 2.67–5.51), in group of sCJD 1.6 (95 % confidence interval 0.52–2.68) and in non-CJD 16.1 (95 % confidence interval 9.53–22.62).
a, b Correlation CSF levels (pg/ml) of total tau and p-tau (a), total tau and amyloid beta (b) in sCJD, gCJDE200K and non-CJD patients (Spearman’s r = 0.451 for p-tau/t-tau, p < 0.001; Spearman’s r = 0.323 for Aβ/t-tau, p < 0.001) and comparison of ratio CSF levels of total tau and p-tau (c), total tau and amyloid beta (d), with statistically difference not only between non-CJD and CJD patients but also between both forms of CJD (Mann-Whitney test). n (gCJD) = 69 p-tau/t-tau(×100) mean v. = 4.09 (CI 95 % = 2.67–5.51); p < 0.001. t-tau/Aβ1–42 mean v. = 4.25 (CI 95 % = 3.40–5.1); p < 0.001. n (sCJD) = 40 p-tau/t-tau(×100) mean v. = 1.6 (CI 95 % = 0.52–2.68); p < 0.001. t-tau/Aβ1-42 mean v. = 12.17 (CI 95 % = 8.97–15.37); p < 0.001. n (non-CJD) = 30 p-tau/ t-tau(×100) mean v. = 16.08 (CI 95 % = 9.53–22.62); p < 0.001. t-tau/Aβ1-42 mean v. = 1.85 (CI 95 % = 0.82–2.88); p < 0.001
Codon 129
Distribution of M129 V polymorphism in examined patients showed in both sporadic and genetic CJD forms, a significant (75 %) prevalence of the M/M homozygosity (Table 1).
Homozygous M/M patients had a higher values of t-tau protein compared to M/V patients in both gCJDE200K (p = 0.007) and sCJD (p = 0.01). Homozygosity M/M on prion gene (PRNP) at codon 129 is considered as a risk factor for CJD [20]. Between the patients with sporadic CJD, there were homozygotes as well as heterozygotes with disease duration from 1 to 3 months from lumbar puncture (LP) until their death (Table 1). Patients with gCJDE200K died within 3 months after LP in a homozygous form M/M 70 %, and in heterozygous form M/V 11 % (Table 1).
Detection of Unspecific Biomarkers
In our study, Aβ 1-42 and p-tau did not show significant differences between CJD patients and patients with other neurodegenerative diseases. Average value of Aβ 1-42 amyloid in the CSF samples of 112 CJD patients was 498 pg/ml (with a range from 100 to 867), and in 50 control samples, it was 458 pg/ml (with a range from 180 to 890) (Fig. 1b, Table 2). Values in sCJD and gCJDE200K were not significantly different. Average values of p-tau in CSF samples of 112 patients with CJD were 41 pg/ml (20 to 83), and in 50 control samples, they were 48 pg/ml (20 to 120) (Fig. 1c, Table 2). Values in sCJD and gCJDE200K were not significantly different. In two gCJDE200K cases, in addition to the positive P 14-3-3 and high levels of t-tau, levels of p-tau were also significantly increased, while those of Aβ 1-42 were reduced. There was suspected parallel course of AD and CJD in these two patients based on the clinical symptoms. Both patients were demented with memory impairment, cognitive difficulties, hallucinations, impulsive behaviour and from the other clinical symptoms are observed myoclonus, aphasia, ataxia and gait disturbance. Diagnosis of CJD was confirmed histopathologically and by Western blot. Examination to establish AD diagnosis has not been performed.
Age of Patients
Comparison of biomarkers with the age of patients (Fig. 3a–f) (Fig. 3a–c) shows that age has not a significant effect on t-tau and Aß 1-42 in gCJD group; p-tau level is even slightly declining. T-tau and p-tau levels have in sCJD rising tendency; Aß 1-42 is decreasing (Fig. 3d–f) which is probably related to ageing processes.
a–f Correlation CSF levels (pg/ml) total tau, amyloid beta , p-tau and age in time of LP (samples delivery) in gCJDE200K (a–c) and sCJD (d–f) patients without statistical signification. T-tau: n (sCJD) = 60, n (gCJD) = 99, p-tau and Aβ(1-42), n (sCJD) = 40, n (gCJD) = 72; t-tau/age Spearman’s r = 0.64 (a) p = 0.53; Spearman’s r = 0.157 (d), p = 0,226); Aβ (1-42)/age Spearman’s r = 0.017 (b) p = 0,892; Spearman’s r = −0.096 (e), p = 0,549); p-tau/age Spearman’s r = −0.103 (c) p = 0,417; Spearman’s r = 0.202 (f), p = 0.218
Total Tau Protein Levels in Sera
Total tau protein was tested also in 51 sera from gCJDE200K patients and 20 sera from patients with sCJD. Most of the values (87 %) of the t-tau (using a commercial kit from the company INNOTEST hTau. INNOGENETICS) were lower than 100 pg/ml (average value was 67.5, with range from 20 to 450). Since the measuring accuracy indicated by the kit producer is within range from 125 to 1000 pg/ml, the detection limit is 87 pg/ml; the serum does not seem to be an appropriate biological material for this kit.
Discussion
With respect to the predominant occurrence of sCJD worldwide, investigations of CSF biomarkers in patients with gCJD were done mostly as multicentre studies. [11, 19, 21]. A group of 54 gCJDE200K patients analysed in Israel [5] makes an exception. In our study, results of CSF and blood samples from genetic and sporadic CJD patients analysed for more than 10 years are presented, containing 101 samples from patients with gCJDE200K, which represent a valuable homogeneous group with the same conditions of transport, storage and samples processing. Considering the achieved results, diagnostic value of tested biomarkers for gCJDE200K and sCJD was analysed, compared and correlated with relevant data in the literature. Detection of the P14-3-3 is included into WHO diagnostic criteria of CJD [6]. Quality of the biological materials, especially the presence of blood in the CSF, significantly affects quality of the obtained results. According to our experience, red blood cells above 250 ery/ml may cause a false-positive result. Influence of blood contamination studied by Schmitz et al. [10] also confirmed that inoculation of CSF with erythrocytes (2500 cell/ml) has significantly increased the percentage of positive P14-3-3 results. Sensitivity of biomarkers in the CSF can be affected also by other factors such as age in time of the CJD onset, duration of the disease, lumbar puncture timing or the molecular subtype [5, 12, 21, 22]. Some limitation of the P 14-3-3 assay results from the visual inspection of immunoblots, leading to a subjective interpretation of bands [23]. When the signal in patients was too weak or negative, and the clinical symptoms consistent with CJD were persisting, another lumbar puncture was recommended after at least 2 months, since previous studies shows that repeated CSF analysis could be useful [24, 25].
In case of our patients, the sensitivity of P 14-3-3 in sCJD (90 %) and gCJDE200K (89 %) was similar and comparable with published data, where sensitivity ranged from 77 to 97 % in sCJD [5, 12, 17, 19, 26], and from 77 to 97 % in gCJD [5, 21, 27]. Regardless of the similar sensitivity in our compared CJD forms, the intensity of P 14-3-3 signal was stronger in sCJD. Specificity of the P14-3-3 test in the Slovak CJD group wass 87 % in average, varying between 75 and 90 % in the course of 2004–2014. Specificity could be influenced by the occurrence of atypical forms of CJD (e.g. age less than 50 years of age in sCJD [12]) and by selection of suspect patients over-evaluating their clinical symptoms, which might have increased number of false-positive results. Suggested influence of the number of examined samples was not confirmed, since despite the increasing annual number of tested CSFs in European countries (including Slovakia, where 120 CSFs were examined in 2011 and 196 CSFs in 2015), the specificity for P 14-3-3 remains generally stable, up to 92 % [11]. Assumed influence of age was not confirmed in our CJD patients. While the compared age groups showed significantly higher age in sCJD as in gCJDE200K, correlating with our previous data [3], no influence of the ageing on the P 14-3-3 specificity was observed, since the lowest values were found in the 51 to 65-year-old patient group. T-tau was examined in one of the largest dataset of patients with gCJDE200K from a single country. Sensitivity of t-tau in our genetic group was lower in comparison to sCJD, as well as to European studies [19, 21, 27]. T-tau (p-tau and Aß 1-42) in our patients were tested retrospectively, in CSF samples collected since 2000, so the quality of results could probably be influenced by duration of the sample storage. This statement is supported by observations of Schmitz et al. [10], that about 25 % of effectiveness of methods is decreasing in samples older than 9 years. Because of significantly higher values of t-tau, this influence is less evident in sCJD. Influence of the optimal cutoff value is demonstrated by Meiner et al., Satoh et al. [5, 28] and Coulthard et al. [16], who reported considerably increased t-tau sensitivity after reduced threshold value to 1000 pg/ml [5], or more precisely to 976 pg/ml [16].
Reducing the t-tau cutoff values from 1300 to 1000 pg/ml in the Slovak gCJDE200K cases, we have also observed that the t-tau sensitivity has increased from 74 to 84 %. That is comparable with the published data claiming 82 [19] and 86 % [21].
In our group of suspect CJD patients, there were 18 carriers of the E200K mutation; however, autopsy was not done to any of them. High t-tau protein was present in 10 (56 %) of them, signalizing a considerable number of not confirmed genetic CJD cases. Evaluation of CSF tau proteins is not included in diagnostic criteria of CJD, but described observation along with results of the two compared methods indicate that testing of CSF tau protein could represent a valuable contribution to the differential CJD diagnosis.
Specificity of t-tau in our CJD patients was 85 %, but it was calculated in 1 year and in smaller set of samples. Chohan, Meiner and Coulthart et al. [5, 12, 16] have reported higher specificity for t-tau when compared to P14-3-3; our observation showed similar specificity for P14-3-3 and t-tau (87/85 %). Comparing the sensitivity of both methods for gCJDE200K, we have observed that it was higher to P 14-3-3 than to t-tau protein; both methods have decreased sensitivity in gCJD in comparison with sCJD. The reason is unclear, but maybe it is related to lower age of gCJD patients. Sensitivity of t-tau protein in our sCJD group was similar to the sensitivity of P 14-3-3 and comparable with the published findings [5, 12, 17, 21].
Analysing a possible influence of the M129 V polymorphism of the PRNP gene, our results were different compared to those described by Meiner et al. [5]. While in their study, correlation neither between t-tau values nor between P14-3-3 positivity and M129 V polymorphism was observed; our study has observed significantly higher t-tau values in methionine homozygous patients with both gCJDE200K (P = 0.007) and sCJD (p = 0.01). There was also another contrast to Meiner et al. [5] recorded previously, specifically in relation to methionine homozygotes and shorter duration of the disease in our group of gCJDE200K [3].
Moeko Noguchi-Shinohara et al. [29] reported significantly higher values of t-tau in CJD sera when compared to other neurological diseases. In our serum samples, as much as 87 % of t-tau values were under the measuring accuracy indicated by the producer of kits. At the moment, we have found methodical limitation for detection of serum t-tau protein in asymptomatic carriers of the E200K mutation as an early preclinical indicator of the disease development and progress. CJD and AD have several clinical and neuropathological features, resulting in diagnostic difficulties [26]. It is well known that reduced levels of Aβ 1-42 and increased levels of total tau protein are characteristic for numerous different types of dementia. According to Otto et al. [30], reduced levels of Aβ 1-42 in the CSF do not exclude diagnosis of CJD, although the value of Aβ 1-42 levels in the CSF of patients with CJD and AD do not show significant differences. However, these values are significantly different from those in other types of dementia or in patients without dementia. Cutoff values for Aβ 1-42 and the p-tau in CJD are not defined. Increased p-tau suggests a risk for AD [7]. In our tested group, we have observed decreased Aβ 1-42 values in both CJD and non-CJD patients, being in agreement with the assumption that Aβ 1-42 is not a specific CJD marker [11]. Since not only absolute values, but also ratio of their combinations are important for biomarkers, we have correlated t-tau with Aβ 1-42 and t-tau with p-tau. We have found statistically significant differences in the ratio of t-tau/Aβ 1-42 not only between CJD and non-CJD patients but also between gCJDE200K and sCJD. Alcolea et al. [31] have reported correlation between values of t-tau and Aβ 1-42, reflecting normal ageing process and depending on the Aβ42 status. Significantly, increased t-tau, p-tau values and decreased Aβ 1-42 according to the ageing can be detected neither in sCJD nor in gCJDE200K. Zanusso et al. [32] refer that ratio of p-tau/t-tau or of Aβ 1-42/p-tau may be helpful for distinguishing CJD from other dementias (AD, FDT). Lorens et al. [33] recommended introduction of p-tau/t-tau ratio into clinical investigations, which can be helpful to avoid misdiagnosis between sCJD and AD. Our observations have also confirmed the statistically significant difference in p-tau/t-tau ratio (Fig. 2a) not only between CJD and non-CJD patients but also between both investigated forms of CJD.
Conclusion
Comparison of CSF biomarkers in sCJD and gCJD has been done in a homogenous group of CJD patients with up to now largest number of gCJDE200K cases. Achieved results demonstrate that while the detection of absolute values of unspecific biomarkers as p-tau and Aß1-42 did not show significant differences between CJD and other neurodegenerative diseases, their combinations have differential diagnostic value.
Evaluation of specific t-tau and P 14-3-3 biomarkers have confirmed significant differences between sCJD or gCJD when compared to non-CJD control groups. The specificity of both diagnostic methods in our CJD patients was comparable, but sensitivity of these specific biomarkers was significantly higher in sCJD. Efficiency of t-tau method could be increased by reducing cutoff value from 1300 to 1000 pg/ml.
Detection of P 14-3-3 protein is unlike t-tau included in WHO diagnostic criteria. Achieved results demonstrate that detection of both compared biomarkers has an important role in the differential diagnosis of CJD. In genetic cases, confirmed CJD-specific mutation and positive results of both methods may decidedly contribute to the diagnosis of transmissible dementia and to protective preventive measures.
References
Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216:136–144
Mitrová E (1991) Some new aspects of CJD epidemiology in Slovakia. Eur J Epidem 7(5):439–449
Mitrová E, Belay G (2002) Creutzfeldt-Jakob disease with E200K mutation in Slovakia: characterization and development. Acta Virol 46:31–39
Korczyn AD (1994) Neurologic genetic diseases of Jewish people. Biomed Pharmacother 48:391–397
Meiner Z, Kahana E, Baitcher F, Korczyn AD, Chapman J, Cohen OS, Milo R, Aharon-Perez J et al (2011) Tau and 14-3-3 of genetic and sporadic Creutzfeldt-Jakob disease patients in Israel. J Neurol 258:255–262
WHO (2003) WHO manual for surveillance of human transmissible spongiform encephalopathies, including variant Creutzfeldt-Jakob disease, Geneva ISBN 92–4–154588-7
Zerr I, Pocchiari M, Collins S, Brandel JP, de Pedro Cuesta J, Knight RSG, Bernheimer H, Cardone F et al (2000) Analysis of EEG and CSF 14-3-3 proteins as aids to the diagnosis of Creutzfeldt-Jakob disease. Neurology 55:811–815
Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, Ladogana A, Schuur M, Haik S et al (2009) Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 132:2659–2668
Collins SJ, Sanchez-Juan P, Masters CL, Klug GM, van Duijn C, Poleggi A, Pocchiari M, Almonti S et al (2006) Determinatinants of diagnostic investigation sensitivities across the clinical spectrum of sporadic CJD. Brain 129:2278–2287
Schmitz M, Ebert E, Stoeck K, Karch A, Collins S, Calero M, Sklaviadis T, Laplanche JL et al (2016) Validation of 14-3-3 protein as a marker in sporadic Creutzfeldt-Jakob disease diagnostic. Mol Neurobiol 53(4):2189–2199
Stoeck K, Sanchez-Juan P, Gawinecka J, Green A, Ladogana A, Pocchiari M, Sanchez-Valle R, Mitrova E et al (2012) Cerebrospinal fluid biomarker supported diagnosis of Creutzfeldt-Jakob disease and rapid dementias: a longitudinal multicentre study over 10 years. Brain 135:3051–3061
Chohan G, Pennigton C, Mackenzie J, Andrews M, Everington D, Will R, Knight R, Green A (2010) The role of cerebrospinal fluid 14-3-3 and other proteins in the diagnosis of sporadic Creutzfeldt-Jakob disease in the UK: a 10-year review. J Neurol Neurosurg Psychiatry 81:1243–1248
Kapaki E, Kilidireas K, Paraskevas GP, Michalopoulou M, Patsouris E (2001) Highly increased CSF tau-protein and decreased beta-amyloid (1-42) in sporadic CJD: discrimination from Alzheimer’s disease? J Neurol Neurosurg Psychiatry 71(3):401–403
Otto M, Wiltfang J, Tumani H, Zerr I, Lantsch M, Kornhuber J, Weber T, Kretzschmar HA et al (1997) Elevated levels of tau-protein in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Neurosci Lett 225(3):210–212
Small DH, McLean CA (1999) Alzheimer’s disease and the amyloid beta protein: what is the role of amyloid? J Neurochem 73(2):443–449
Coulthart MB, Jansen GH, Olsen E, Godal DL, Connolly T, Choi BC, Wang Z, Cashman NR (2011) Diagnostic accuracy of cerebrospinal fluid protein markers for sporadic Creutzfeldt-Jakob disease in Canada: a 6-year prospective study. BMC Neurol 11:133
Zerr I, Bodemer M, Gefeller O, Otto M, Poser S, Wiltfang J, Windl O, Kretschmar HA et al (1998) Detection of 14-3-3 protein in the cerebrospinal fluid supports the diagnosis of Creutzfeldt-Jakob disease. Ann Neurol 43:32–40
Otto M, Wiltfang J, Cepek L et al (2002) Tau protein and 14-3-3 in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology 58:192–197
Sanchez-Juan P, Green A, Ladogana A, Cuadrado-Corrales N, Sanchez-Valle R, Mitrova E, Stoeck K, Sklaviadis T et al (2006) CSF tests in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology 67:637–643
Palmer MS, Dryden AJ, Hughes JT, Collinge J (1991) Homozygous prion protein genotype predisposes to sporadic Creutzfeldt-Jakob disease. Nature 352:340–342
Ladogana A, Sanchez Juan P, Mitrova E, Green A, Cuadrado-Corrales N, Sanchez-Valle R, Koscova S, Aguzzi A et al (2009) Cerebrospinal fluid biomarkers in human genetic transmissible spongiform encephalopathies. J Neurol 256:1620–1628
Skinningsrud A, Stenser V, Gundersen AS, Fladby T (2008) Cerebrospinal fluid markers in Creutzfeldt-Jakob disease. Cerebrospinal Fluid Res 5
Baldeiras IE, Ribeiro MH, Pacheco P, Machado A, Santana I, Cunha L, Oliveira C (2009) Diagnostic value of CSF protein profile in a Portuguese population of sCJD patients. J Neurol 256:1540–1550
Sanchez-Juan P, Sanchez-Valle R, Green A, Ladogana A, Cuadrado-Corrales N, Mitrova E, Stoeck K, Sklaviadis T et al (2007) Influence of timing on CSF tests value for Creutzfeldt-Jakob disease diagnosis. J Neurol 254(7):901–906
Cohen OS, Chapman J, Korczyn AD, Warman-Alaluf N, Nitsan Z, Appel S, Kahana E, Rosenmann H (2015) CSF tau correlates with CJD severity and cognitive decline. Acta Neurol Scand . doi:10.1111/ane.12441May 25
Hainfeller JA, Wanschitz J, Jellinger K, Liberski PP, Gullotta F, Budka H (1998) Coexistence of Alzheimer-type neuropathology in Creutzfeldt-Jakob disease. Acta Neuropathol 96:116–122
Krasnianski A, Heinemann U, Ponto C, Kortt J, Kallenberg K, Varges D, Schulz-Schaeffer WJ, Kretzschmar HA et al (2016) Clinical findings and diagnosis in genetic prion disease in Germany. Eur J Epidemiol 31(2):187–196
Satoh K, Shirabe S, Eguchi H, Tsujino A, Eguchi K, Tsujihata M, Niwa M, Katamine S et al (2006) 14-3-3 protein, total tau and phosphorylated tau in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease and neurodegenerative disease in Japan. Cell Mol Neurobiol 26:45–52
Noguchi-Shinohara M, Hamaguchi T, Nozaki I, Sakai K, Yamada M (2011) Serum tau protein as a marker for the diagnosis of Creutzfeldt-Jakob disease. J Neurol 258:1464–1468
Otto M, Esselmann H, Schulz-Schaeffer W, Neumann N, Schröter A, Ratzka P, Cepek L, Zerr I et al (2000) Decreased β-amyloid 1-42 in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Neurology 54:1099–1102
Alcolea D, Martínez-Lage P, Sánchez-Juan P, Olazarán J, Antúnez C, Izagirre A, Ecay-Torres M, Estanga A et al (2015) Amyloid precursor protein metabolism and inflammation markers in preclinical Alzheimer disease. Neurology 85(7):626–633
Zanusso G, Fiorini M, Ferrari S, Gajofatto A, Cagnin A, Galassi A, Richelli S, Monaco S (2011) Cerebrospinal fluid markers in sporadic Creutzfeldt-Jakob disease. Int J Mol Sci 12:6281–6292
Llorens F, Schmitz M, Karch A, Cramm M, Lange P, Gherib K, Varges D, Schmidt C et al (2016) Comparative analysis of cerebrospinal fluid biomarkers in the differential diagnosis of neurodegenerative dementia. Alzheimers Dement 12(5):577–589
Acknowledgments
The study was supported by grant from EU Joint Program-Neurodegenerative Disease Research (JPND-DEMTEST Biomarker based diagnosis of rapid progressive dementias-optimization of diagnostic protocols, 01ED1201A) and by Center of Excellence in Environmental Health, ITMS No.26240120033, based on the supporting operational Research and development program financed from the European Regional Development Fund.
Thanks to Dana Vajcikova for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflicts of interests.
Additional information
Koscova S. and Slivarichova-Zakova Dana contributed equally
Rights and permissions
About this article
Cite this article
Koscova, S., Zakova Slivarichova, D., Tomeckova, I. et al. Cerebrospinal Fluid Biomarkers in the Diagnosis of Creutzfeldt-Jakob Disease in Slovak Patients: over 10-Year Period Review. Mol Neurobiol 54, 5919–5927 (2017). https://doi.org/10.1007/s12035-016-0128-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0128-4