Abstract
Dental tissue is emerging as a promising source of stem cells especially in nerve regeneration mainly due to their neural origin and ease of harvest. We isolated dental stem cells from three sources, namely, dental pulp (DPSCs), dental follicle (DFSCs), and apical papilla (SCAP), and explored the efficacy of each towards neural differentiation in comparison to bone marrow-derived stem cells. The neural differentiation potential was assessed by expression of various neural markers and neurosphere assay. We observed that DPSCs were inherently predisposed towards neural lineage. To further delineate the paracrine cues responsible for the differences in neural differentiation potential, we harvested the conditioned secretome from each of the stem cell population and observed their effect on colony formation, neurite extension, and neural gene expression of IMR-32, a pre-neuroblastic cell line. We found that neural differentiation was significantly enhanced when IMR-32 cells were treated with secretome derived from DMSCs as compared to the same from BMSCs. Th1/Th2/Th17 cytokine array revealed DPSC secretome had higher expression of the cytokines like GCSF, IFNγ, and TGFβ that promote neural differentiation. Thus, we concluded that DPSCs may be the preferred source of cells for obtaining neural lineage among the four sources of stem cells. Our results also indicate that the DPSC-secreted factors may be responsible for their propensity towards neural differentiation. This study suggests that DPSCs and their secretomes can be a potentially lucrative source for cell-based and “cell-free” (secretome) therapy for neural disorders and injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodegenerative diseases include a plethora of incurable and debilitating conditions that primarily affect the neurons in brain. Stem cell therapy (SCT) has been successful in animal models of Parkinson’s and Alzheimer disease models where partial recovery has been reported due to neuroprotective and immunomodulatory effects of the mesenchymal stem cells (MSCs) [1] and neural stem cells (NSCs) [2]. NSCs are most promising cell types to generate all types of neuronal cells and can differentiate into neurons in vivo but are not readily accessible. MSCs are readily available but they have restricted capacity for in vivo neural differentiation [3].
Bone marrow stem cells (BMSCs) represent a gold standard of adult stem cells used for regenerative medicine these days. But the main problem associated with use of BMSCs is their painful invasive isolation procedure, low cell number, and potential infection to the donor. Dental tissue is being considered as the most promising source for harvesting stem cells since it is harvested from a biological waste [4]. There are five sites discovered till date in a dental tissue from which stem cells can be harvested. These include stem cells from dental pulp named dental pulp stem cells (DPSCs) [5], exfoliated teeth, called stem cells from human exfoliated teeth (SHED) [6], from periodontal ligament, termed as periodontal ligament stem cells (PDLSCs) [7], from apical papilla, termed stem cells from apical papilla (SCAP) [8] and dental follicle, cells isolated from which are called dental follicle stem cells (DFSCs) [9]. Nosrat et al. have proposed that DPSCs can be successfully used as a source of viable cells for cell-based transplantation therapies [10]. Dental stem cells (DMSCs) and BMSCs share many biological characteristics like similar markers, e.g., CD-73 (cluster of differentiation-73), CD-105, CD-90, and abilities to give rise to three germ layer lineages; however, there are some variations in their differentiation potential, immunomodulatory activity, gene repertoire, and marker expression. There may be some differences in the proteome status between both cell types which still need to be delineated. These differences might be due to inherent heterogeneity or dedicated to some cell-specific differences in BMSCs and DMSCs. Different culture and isolation protocols established in different laboratories may also induce some variations. Although DMSCs are different from BMSCs, but they represent an important alternative for use in clinical settings and can be a better option in some cases than BMSCs [11].
As DMSCs are derivatives of neural crest, so they may be a more suitable stem cell candidate for treatment of neurodegenerative diseases as compared to other stem cells. In this study, we have explored the neural potential of three dental stem cell populations viz. DPSCs, SCAP, and DFSCs in comparison to BMSCs.
The benefits of stem cell transplantation might relate to a paracrine modulatory effect rather than the replacement of affected cells at the site of injury. So, the repertoire of secreted trophic and immunomodulatory cytokines produced by mesenchymal stem cells (MSCs) as the secretome is hypothesized have regenerative potential in tissue injury including neural trauma or degeneration. There are evidences that neurodegeneration is due to loss of various key neurotrophins like nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), etc. [12, 13]. Thus, an ideal source for neural regeneration should secrete these cytokines at higher levels. Based on this notion, we have evaluated the level of neural cytokines in the secretome of DMSCs and BMSCs and effect of the secretome from each stem cell population on various cellular aspects of a pre-neuroblastic cell line. The main aim of this study was to find a suitable cell source for neural differentiation which can contribute to various treatment modalities for different neurodegenerative diseases and cell-based transplantation therapies. We have also identified some of the secretome cues which can alter the neural differentiation potential of stem cells and hence account for differences in neural potential of different stem cell populations.
Material and Methods
Collection and Transport of Extracted Teeth
Teeth were obtained from donors (aged from 11 to 25 years) undergoing tooth extraction for orthodontic reasons as advised by the consulting orthodontist. Approved consent form was signed by the patient/guardian according to the guidelines of the Ethical Committee of PGIMER, Chandigarh. The extraction procedure was performed under standard conditions in local anesthesia. The extracted tooth was treated with a disinfectant solution and transported in Hanks’ balanced salt solution to the tissue culture laboratory.
Primary Culture of Dental and Bone Marrow Stem Cells
Five samples each of dental pulp, apical papilla, and dental follicle and three samples of bone marrow were isolated under sterile conditions. Culture of dental stem cells was established as reported previously [14].
Mesenchymal stem cells from the bone marrow were obtained from cultures already underway in the stem cell research facility for different clinical trials. MSCs were isolated using the protocol described by Chetan et al. [15]. DMSCs and BMSCs were cultured in 5 % CO2 atmosphere at 37 °C in a media composed of αMEM, penicillin/streptomycin, gentamycin, and glutamine supplemented with 10 % fetal bovine serum (FBS, USA origin, Sigma-Aldrich).
IMR-32 cell line was obtained from NCCS Pune, India, and cells were maintained in αMEM supplemented with 10 % FBS.
FACS Analysis
Cells were trypsinized with 0.25 % trypsin-EDTA, washed with PBS, and incubated in FITC/PE-labeled antibodies for CD-90, CD-105, CD-73, CD-34, and CD-45 in separate experiments. Cells were acquired and analyzed in FACS Canto (BD Biosciences).
Cell Differentiation
Differentiation into cells of osteo and adipo lineage was performed as reported previously using appropriate media and confirmed by alizarin red and oil red O staining [14, 16, 17]. Neural differentiation was done in neurobasal media supplemented with basic fibroblast growth factor (20 ng/ml), epidermal growth factor (20 ng/ml), B27 supplement (1 %), G5 supplement (0.5 %), and N2 supplement (0.2 %) (Invitrogen) for 41 days [18]. Hepatic differentiation was done in induction media for 14 days and maturation media for another 14 days. Induction media composed of αMEM supplemented with hepatocyte growth factor (20 ng/ml), epidermal growth factor (2 ng/ml), dexamethasone (0.5 μM), and ITS premix (50 mg/ml). Maturation medium contained αMEM supplemented with 20 ng/ml oncostatin M, 0.5 μM dexamethasone, and 50 mg/mL ITS+ premix. Hepatic differentiation was assessed with the help of a LDL assay kit (Abcam) as per manufacturer’s instructions.
Immunostaining
Neural antibodies anti-neurofilament (NFM) and anti-microtubule-associated protein (MAP-2) were used for characterizing neural cells as reported earlier [14]. Briefly, after fixation and permeabilization, cells were incubated with NFM (1:50) and MAP-2 (1:100) primary antibody overnight at 4 °C. Cells were photographed using Nikon Eclipse TS100 fluorescence microscope, and fluorescence intensity was compared using NIS-Elements D4.13.00 software supplied with microscope. Propidium iodide (PI) was used as counterstain for nucleus. Differentiated populations from BMSCs, DPSCs, SCAP, and DFSCs are denoted as N-BMSC, N-DPSC, N-SCAP, and N-DFSC wherever indicated.
Real-Time PCR Analysis
Total RNA from cells was isolated and complementary DNA (cDNA) synthesis was conducted using a kit-based method. Serial dilution of cDNA was used for relative quantitation of the expression of the neural cell markers β-tubulin III, nestin, and sox1 (ABI M7500). Relative expression of each gene was quantified for the assessment of neural differentiation. GAPDH was used as internal housekeeping control in each of the experiment.
Neurosphere Assay
Cells were plated in medium containing neurobasal medium (Invitrogen) enriched with B27 supplement, 20 ng/ml EGF, and 20 ng/ml FGF in ultra-low attachment six-well cell culture plates at a density of 1 × 103 cells/well. After 2–3 days, a small population of cells organized themselves in proliferating spheres, termed as “neurosphere.” These neurospheres were counted at different time intervals using Tali-based Image cytometer and cell counter (Applied Biosystems). MTT assay was performed on neurospheres to assess the viability of cells in nerosphere. NFM staining was carried out as per protocol described above to confirm neural differentiation of neurospheres.
Secretome Preparation
Stem cells (BMSC and DMSCs) were maintained in T-25 culture flasks and incubated with αMEM for 48 h at 37 °C in a 5 % CO2 atmosphere. Conditioned media from each stem cell type was collected on ice, centrifuged for 5 min at 3000 rpm, filtered to remove debris, and stored at −80 °C for future use. Whenever feasible, fresh secretome was used for secretome-related studies. Secretome was obtained between 3 and 7 passages and used in the ratio of 1:1 with the culture medium αMEM. All experiments were performed on secretome obtained after 48 h of stem cell culture.
Colony Formation Assay
To evaluate efficiency of colony formation, single cells obtained after trypsinization of IMR-32 were plated (1 × 103 cells) per well of a six-well plate [19]. Cells were incubated with 50 % secretome from BMSCs/DMSCs and 50 % αMEM culture media. Media was changed every 3rd day. At the end of the incubation period, cells were fixed in 10 % neutral buffered formalin for 1 h and stained with crystal violet (0.1 %) for duration of 30 min and observed under a microscope. Colonies with >50 cells were counted. IMR-32 cells treated with secretome of BMSC, DPSC, SCAP, and DFSC are represented as IMR-BMSC, IMR-DPSC, IMR-SCAP, and IMR-DFSC, respectively.
Neurite Extension Assay
1 × 103 cells/well were seeded into six-well culture plates and treated with secretome from different stem cell sources. Neurite extension was measured by using software package provided with the microscope (Nikon Eclipse TS100) named NIS-Elements D4.13.00.
MTT Assay
Neurosphere (1 × 103/well) were seeded in a 96-well plate for cell viability studies. These were incubated with secretome and culture media (1:1) for a period of 48 h. MTT reagent was added as per protocol and cells were lysed by addition of 100 μl DMSO per well. The purple color conversion of MTT by live cells was measured at a wavelength of 565 nm (Tecan, M7500 pro).
ELISA for Neural Cytokines
The expression of nerve growth factor (NGF), brain-derived neurotropic factor (BDNF), and neurtophin 3 (NT-3) was performed on secretome derived from each of the four stem cells (BMSC, DPSC, SCAP and DFSC) using ELISA kits (Krishgen Biosystems) as per manufacturer’s instructions. Standard curve for different standards was obtained and optical density values of samples were extrapolated on standard curve to get the final concentration of respective cytokines present in different stem cell secretome.
Cytokine ELISA Array
The level of various cytokines in post 48 h secretome derived from cultures of each of the stem cell source was measured with human Th1/Th2/Th17 cytokines multianalyte ELISA array kit (MEH-003A) according to the manufacturer’s instructions.
Statistical Analysis
One-way ANOVA and unpaired Student’s T test were used to make statistical comparison. Each experiment was performed in triplicates. Statistical comparison involves values with average ± SD.
Results
Stem Cell Culture and Characterization
Dental stem cells were isolated from dental pulp, apical papilla, and follicle of impacted third molars of patients (Fig. 1a–d). hDMSCs (human DPSC, SCAP and DFSC) started migrating from the explants within the 3–4th days of seeding. Cells achieved their morphology and were ready for scaling up by the 13th–15th days (Fig. 1f–h). Confluent BMSC culture was achieved by day 18 (Fig. 1e). These cells were upscaled by further trypsinization upon reaching 70 % confluence and characterized at passage 3rd (3×). All further experiments were performed between the 3rd and 7th passage. All types of stem cells showed more than 90 % positivity for stem cell markers and negativity (<5 %) for endothelial and hematopoietic markers (Fig. S1a–d), thus confirming the stemness of the cell population while negativity for endothelial and hematopoietic markers indicated the purity of culture.
Extraction of dental tissue and primary culture of stem cells. a OPG of the patient showing third molar to be extracted. b Tooth dissected to harvest tissue. c Extracted tooth showing follicle and apical papilla d Three stem cell sites in isolated tooth—dental pulp, apical papilla, and dental follicle. e Differential interference contrast (DIC) microscopic images of primary culture of BMSCs. f Dental pulp stem cells coming out of an explant of pulp tissue. g Stem cells from apical papilla. h Dental follicle stem cells. Magnification 10×, scale bar 100 μm
Functionally, these stem cells were characterized by differentiation into osteocytes, adipocytes, and hepatic cells and as shown in Fig. S1e, which confirmed their multilineage differentiation potential.
Neural Differentiation Potential of Stem Cells
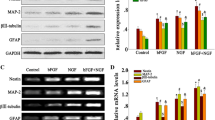
BMSCs and DMSCs were cultured into neural differentiation media for 30–41 days and neurons like cells with neural extensions were observed. Upon differentiation, all stem cells showed positive fluorescence staining with both NFM (Fig. 2a) and MAP2 (Fig. 2c). Comparative analysis of mean fluorescence intensities (MFI) showed that DPSCs had highest MFI (189.1 ± 33.4) for NFM as compared to SCAP (157 ± 24), BMSCs (141 ± 27.6), and DFSC (117 ± 17.7) (Fig. 2b). A similar intensity pattern was observed for MAP-2 antibody with DPSCs showing highest MFI (Fig. 2d). A 3D pattern of antibody binding on differentiated neural cell surfaces is shown in Fig. S2 (format embossed).
Immunostaining for neural proteins and expression level of neural genes. a Fluorescent microscopic images of differentiated neural cells from four stem cell populations showing expression of NFM. b Mean fluorescence intensity of NFM protein in differentiated population. c MAP-2 expression in neuron neural cells after differentiation. d Mean fluorescence intensity of MAP-2 protein in differentiated population. e Gene expression levels of three neural genes in differentiated stem cells, n = 3 for each stem cell type. Magnification 10× and 20×, scale bar 20 μm. *p < 0.05; **p < 0.01; *** p < 0.001
Real-time PCR analysis was done for neural specific genes, β-tubulin III, nestin, and sox-1. The primer sequence for these genes is shown in Table 1. Gene expression analysis showed upregulation of all neural genes in DMSCs as compared to BMSCs. There was an increase in expression of β-tubulin in DPSCs (5.6-fold), SCAP (5.2-fold), and DFSC (2.8-fold) as compared to BMSCs (Fig. 2e). Maximum upregulation was observed in nestin (73.5-fold for DPSCs, 90.5-fold for SCAP, and 48.5-fold for DFSCs). Sox-1 also increased in DMSCs (1.5-fold in DPSCs, 2.4-fold in SCAP, and 1.7-fold in DFSCs) as compared to BMSCs.
Neurosphere analysis indicated that although all cells began forming neurospheres at day 3 of neurosphere induction, the size of neurospheres was more in the case of DMSCs as compared to BMSCs. The number of neurosphere increased significantly on the 7th day of culture (Fig. 3a–d). The maximum number of neurosphere was generated by DPSCs (Fig. 3e) while BMSCs produced the least number of neurospheres. We also assessed the viability of the neurospheres by MTT assay. BMSC-derived neurospheres were considered as control. We found that cell viability was approximately 100 % in all DMSCs (Fig. 3f). Characterization of these neurospheres was performed by immunostaining with NFM antibody and DAPI was used as counterstain. These neurospheres showed an intense positive staining for NFM, hence reflecting the neural nature of neurospheres (Fig. 4a–d). Neurosphere integrity assay revealed that neurospheres from DMSCs maintained their integrity during in vitro adhesion to culture wells for a period of 24 h while neurospheres obtained from BMSCs lost their integrity and burst to release the cells (Fig. S3).
Neurosphere formation at different days of non-adherent culture of stem cells in neural differentiation media. a–d DIC images of neurosphere formed at the 3rd–7th days of suspension culture in BMSCs, DPSCs, SCAP, and DFSCs, respectively. e Graph showing number of neurosphere formed at different days in above defined stem cell populations. f Percent viability in neurospheres derived from different stem cells at the 7th day of culture. Magnification 20×, scale bar 100 μm
Based on protein expression, morphological analysis, and neural gene expression data, it was clear that DMSCs (DPSC, SCAP, and DFSC) had a higher neural differentiation potential as compared to BMSCs, and overall, DPSCs showed highest potential to differentiate towards neural lineage. Next, we wanted to find out the secretome cues behind this difference in the differentiation potential. So, we selected IMR-32 cell line to observe the effect of secretome from BMSCs and DMSCs on colony formation, neurite extension, and neural gene expression.
Effect of Stem Cell Secretome on IMR-32 Cell Line
Effect of secretome derived from different stem cells on colony formation of IMR-32 cells depicted (Fig. 5a) the higher colony forming efficiency of DMSC secretome as compared to BMSC secretome with significantly high number of colonies formed in case of IMR-DPSCs (334), IMR-SCAP (365), and IMR-DFSC (397) as compared to IMR-32 control (254 ± 35.5). One interesting observation was that while treatment with DMSCs secretome increased the number of colonies in IMR-32 cells, treatment with BMSC secretome reduced the colony count to a significantly low level (83) (Fig. 5b).
Effect of secretome obtained from different stem cells on cellular aspects of IMR-32 cells. a Crystal violet stained colonies of IMR-32 cells after treatment with BMSC secretome (IMR-BMSC), DPSC secretome (IMR-DPSC), SCAP secretome (IMR-SCAP), and DFSC secretome (IMR-DFSC). b Quantification of number of colonies formed after secretome treatment. c Extended neuritis in IMR-32 cells after secretome treatment. d Average length of neuritis formed after secretome induced differentiation. Magnification-10× for condition (a) and 20× for condition (c), scale bar 100 μm. *p < 0.05; **p < 0.01; ***p < 0.001
We also assessed the neural differentiation ability of secretome, derived from four stem cell types since neurite extension is an indicator of functional network formation ability of neurons. It was observed that secretome from all stem cells induced neurite extensions in IMR-32 cells significantly. Minimum neurite extension was observed in IMR-32 cells treated with BMSC secretome (Fig. 5c). Neurite extension assay demonstrated that neurite lengths were higher in case of IMR-DMSCs as compared to IMR-BMSCs. Neurite length was highest in case of IMR-32 cells treated with DPSC secretome, IMR-DPSC (87.5 ± 3.1 μm) as compared to IMR-DFSC (63.2 ± 11.9 μm), IMR-SCAP (52.2 ± 11.3 μm) IMR-BMSC (20.6 ± 6.4 μm), and control IMR-32 cells (18.2 ± 5 μm) (Fig. 5d).
This observation pushed us to look into effect on neural gene expression profile of IMR-32 cells after secretome treatment. PCR analysis showed that there was a significant increase in the expression of all three neural genes (β-tubulin, nestin, and sox-1) when IMR-32 cells were treated with secretome derived from each of the stem cells source (except the levels of Sox-1 in IMR-BMSC cells) (Fig. 6a). During comparative analysis with IMR-BMSC cells, it was observed that IMR-DMSCs showed a significant upregulation of neural genes (β-tubulin III and sox-1). Maximum β-tubulin III expression was observed in IMR-DPSCs (12.1 ± 0.4-fold) while maximum sox-1 upregulation was seen in IMR-DFSCs (12.8 ± 0.5-fold). Expression of nestin was maximum in IMR-BMSCs (10.9 ± 0.2-fold).
Change in the expression levels of neural gene in IMR-32 cells after treatment of secretome and ELISA for neural cytokines. a Fold change in the expression level of three neural genes in IMR-32 cells after treatment with secretome from different stem cells. Fold change was calculated w.r.t. untreated cells taking them as baseline control. b quantification of NGF, BDNF, and NT-3 in four stem cell populations. *p < 0.05; **p < 0.01; ***p < 0.001
To explore the neural regulatory molecules present in secretome, we measured the expression of nerve growth factor (NGF), brain derived neurotropic factor (BDNF) and neurtophin 3 (NT-3) in secretome derived from DMSCs and BMSCs. We observed a significant increase of all three neural cytokines in secretome derived from DMSCs as compared to those from BMSCs with one exception (Fig. 6b). NGF was found to be significantly high in secretome of SCAP (794 ± 58.9) and DFSCs (780 ± 60.4) as compared to BMSC (720 ± 37), but there was not any significant difference between NGF levels between secretome of BMSCs and DPSCs. Significantly high levels of BDNF (700 ± 34.6, 1053 ± 83.2, 1040 ± 69.2) and NT3 (1106 ± 11.5, 1196 ± 80.8, 1396 ± 40.4) were observed in secretome derived from DPSCs, SCAP, and DFSCs, respectively, as compared to BDNF (620 ± 34.6) and NT-3 (603 ± 75) levels in BMSC secretome.
We also performed Th1/Th2/Th17 cytokine array on the secretome. An increase in GCSF (0.95-fold), IFN-γ (1.44-fold), and transforming growth factor- β (TGF-β, 1.41-fold) expression was observed in DPSC secretome. Conversely, IL-17 was found to be higher in BMSC secretome (1.82) as compared to DPSC secretome (1.41) (Table 2).
Discussion
The primary cultures generated from each of the cell lines conformed to the guidelines of international society for cellular therapy (ISCT) [20]. Stemness marker expression and trilineage differentiation ability into ectoderm (neural), mesoderm (osteo and Adipo) and endoderm (hepatic) further confirmed the stemness of cells.
Neurofilament is an important marker for axonal cytoskeleton of neurons and is a surrogate marker for neuronal injury and neurodegeneration [21]. MAP-2 is a marker for mature neurons [22] and it stabilizes the microtubule network by promoting the interaction between tubulin and microtubules. Significantly high MFI for both NFM and MAP-2 in case of neural differentiated DPSCs confirmed that these cells showed efficient and a more committed differentiation towards neural lineage as compared to other stem cell types. β-tubulin III, nestin, and sox-1 play an important role in maintenance, survival, and differentiation of neural cells [23–25]. An interesting study by Lee et al. has reported increase in protein levels of MAP-2 and β-tubulin III after neural differentiation in DMSCs [26]. High expression of β-tubulin III and nestin in the N-DMSCs as compared to N-BMSCs indicated that these two genes are playing the main role in differentiation of DMSCs towards neural lineage. Neurosphere assay is an important morphological assay to quantify the neural differentiation potential of stem cells derived from tissues of different origin [18]. A higher neurosphere count of N-DMSCs as compared to N-BMSCs clearly reflected the greater neurosphere forming ability of DMSC population. MTT assay confirmed the viability of neurospheres at the 7th day of differentiation. Taking together, DMSCs showed a significantly higher differentiation potential towards neural lineage as compared to BMSCs. Among DMSCs, DPSCs express the protein parameters to significantly higher extent compared to SCAP and DFSCs.
Further studies were carried in the IMR-32 cell line, a pre-neuroblastic cell line which is an ideal model system for research in various degenerative diseases [27, 28] and drug-induced neurotoxicity [29]. Differentiation of IMR-32 cell line into mature neurons has been reported previously by a synergistic application of BMP-6 and retinoic acid [30] or by vasoactive intestinal peptide [31]. We proposed to study the effects of secretome from different stem cell sources on neuron differentiation and maturation. High colony formation in case of IMR-DMSCs as compared to IMR-BMSCs may be due to higher levels of GCSF in DMSC secretome as compared to BMSC secretome.
Neurite extension assay is widely used as an indicator of neural differentiation [32]. In our study, increase in neurite length of IMR-32 on adding DMSC secretome directly demonstrated its higher neural differentiation potential. It may be appreciated that secretome from DPSCs induced maximum increase in neurite length and expression of neural genes, hence reflecting a more potent neural differentiation potential of DPSC secretome. Karaoz et al. have earlier reported that DPSCs show neuro-glia properties due to the intrinsic expression of specific neural stem cell markers [11]. Previous studies have also shown that even under non-neuronal inductive conditions, DPSCs express neural progenitor markers like nestin and glial fibrillary acidic protein (GFAP) [33, 34]. This effect may be due to the secretion of trophic factors like NGF, BDNF, and GDNF (glial cell line-derived neurotrophic factor) which can support growth of resident neurons and can also induce differentiation of DPSCs into neurons [10]. All these studies point to the potential role of autocrine/paracrine factors in lineage specific differentiation.
NGF, BDNF, and NT-3 are some of the major cytokines which play an important role in regulation of neuronal differentiation, synapse formation, and other cognitive functions by expression in a parallel or reciprocal manner [35, 36]. Significantly higher levels of these three cytokines in secretome of DMSCs in comparison to those of BMSCs may be the reason for greater neural differentiation potential of cells treated with DMSC secretome. Several cytokines secreted by Th1/Th2/Th17 cytokine loop also play an important role in neuronal regulation. GCSF has been reported to play a protective role in rescuing CNS neurons by an autocrine signaling mechanism. Ischemic conditions can induce the expression and release of GCSF which help neurons to survive from apoptotic death by activating various cell survival pathways [37, 38]. Expression of GCSF at a higher level in DPSC secretome might be responsible for increased neural potential of DPSCs. There are reports which indicated that during proliferative conditions, TNF-α can induce cell death in neural stem cells as evident from cell viability assays. IFN-γ was reported to have a non-toxic effect on the neural stem cells during proliferation phase. However, both were reported to be non-toxic during differentiation conditions [39]. Negligible levels of TNF-α (0.02-fold) in the secretome of all four types of stem cells indicated that there is no toxicity due to TNF-α. IFN gamma has been shown to enhance the neuronal differentiation by upregulating the expression of β-tubulin III and hence increasing the number of neural cells and decreasing astrocyte generation. So, during differentiation into neuronal-glia lineage, IFN-γ can tilt the balance towards neuronal lineage by increasing the expression of β-tubulin III [39]. Higher level of IFN-γ in the DPSC secretome can be suggestive of high neural potential of these stem cells among all four stem cells types. β-tubulin III was also upregulated (~12-fold) when IMR-32 cells were treated with DPSC secretome, thus reflecting a direct impact of increased levels of IFN-γ in enhancing the expression of β-tubulin III and ultimately a higher neural differentiation capacity of DPSCs. The expression of IFN-γ level in secretome of all four stem cell sources coincided well with β-tubulin III gene expression induced in IMR-32 cells after treatment with respective stem cell secretome. TGF-β has been reported to promote neuronal fate under both in vitro as well as in vivo conditions from progenitors in mouse cortical and hippocampal regions [40]. In our study, high levels of TGF-β in DPSC secretome (1.41-fold) may be responsible for supporting neuronal lineage and hence contributing to higher neural potential of DPSCs. IL-17 has been reported to have an inhibitory effect on neural differentiation as well as on neural stem cell proliferation [41]. It may be noted that IL-17 is expressed at a very high level in secretome from BMSCs (1.82-fold) while it is at low level in secretome from DPSCs. There are studies which indicated that IL-17 can regulate the levels of IFN-γ and a crosstalk is involved in their action [42]. Both IL-17 and IFN-γ are produced concomitantly and act synergistically to regulate various physiological functions [43]. High expression of IL-17 can abrogate the neural potential of BMSCs, neutralizing the effects of IFN-γ in BMSC secretome. The high levels of this inhibitory cytokine (IL-17) may thus account for reduced commitment of BMSCs towards neuronal phenotype as compared to DMSCs.
Our study shows that DMSCs (DPSC, SCAP, and DFSC) may be a better candidate for neural differentiation as compared to BMSCs. DPSCs were the most potent and robust source which may be used for neural differentiation. A synergistic effect of Th1/Th2/Th17 cytokines, GCSF, IFN-γ, and TGF-β in addition to major neural cytokines BDNF and NT-3 may be responsible for the enhanced neural differentiation potential of DPSCs.
Conclusion
DPSCs have very highest tendency for neural differentiation as compared to other three types of stem cells studied. This effect is partially due to increased gene expression profile which might be related with the neural crest origin of the cells. Through our study, a very important contribution to this enhanced neural differentiation potential is reflected to those of secretome cues which are small soluble growth factors like GCSF, IFN-γ, TGF-β, NGF, BDNF, NT-3, etc. These secretome molecules can enhance neural differentiation by increasing the expression of neural genes, preventing apoptosis of neurons or by maintaining a neuronal fate during differentiation. Thus, a crosstalk of these molecules is regulating the differentiation of stem cells into neuronal lineage and holds a very important key to this difference. The intricate balance between these molecules in the cellular microenvironment regulates the genes and proteins which ultimately determines a cell fate. Further investigations can help in a better understanding of this balance and may be of great importance in designing secretome-based therapeutic modalities.
References
Kim KS, Kim HS, Park JM, Kim HW, Park MK, Lee HS, et al. (2013) Long-term immunomodulatory effect of amniotic stem cells in an Alzheimer’s disease model. Neurobiol Aging 34(10):2408–2420
Akerud P, Canals JM, Snyder EY, Arenas E (2001) Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson’s disease. J Neurosci 21(20):8108–8118
Lescaudron L, Boyer C, Bonnamain V, Fink KD, Leveque X, Rossignol J, et al. (2012) Assessing the potential clinical utility of transplantations of neural and mesenchymal stem cells for treating neurodegenerative diseases. Methods Mol Biol 879:147–164
Huang GT, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88(9):792–806
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97(25):13625–13630
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100(10):5807–5812
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364(9429):149–155
Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. (2008) Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34(2):166–171
Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, Mohl C, et al. (2005) Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol 24(2):155–165
Nosrat IV, Smith CA, Mullally P, Olson L, Nosrat CA (2004) Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur J Neurosci 19(9):2388–2398
Karaoz E, Demircan PC, Saglam O, Aksoy A, Kaymaz F, Duruksu G (2011) Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem Cell Biol 136(4):455–473
Arancio O, Chao MV (2007) Neurotrophins, synaptic plasticity and dementia. Curr Opin Neurobiol 17(3):325–330
Chen WW, Blurton-Jones M (2012) Concise review: can stem cells be used to treat or model Alzheimer’s disease? Stem Cells 30(12):2612–2618
Kumar A, Bhattacharyya S, Rattan, V (2015) Effect of uncontrolled freezing on biological characteristics of human dental pulp stem cells. Cell Tissue Bank
Mudrabettu C, Kumar V, Rakha A, Yadav AK, Ramachandran R, Kanwar DB, et al. (2015) Safety and efficacy of autologous mesenchymal stromal cells transplantation in patients undergoing living donor kidney transplantation: a pilot study. Nephrology (Carlton) 20(1):25–33
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, et al. (2011) Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol 56(7):709–721
Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, et al. (2005) Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 33(11):1402–1416
Zavan B, Michelotto L, Lancerotto L, Della Puppa A, D'Avella D, Abatangelo G, et al. (2010) Neural potential of a stem cell population in the adipose and cutaneous tissues. Neurol Res 32(1):47–54
Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ (1999) Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol 107(2):275–281
Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L (2013) Immunological characterization of multipotent mesenchymal stromal cells—the International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 15(9):1054–1061
Petzold A (2005) Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci 233(1–2):183–198
Seki T (2002) Expression patterns of immature neuronal markers PSA-NCAM, CRMP-4 and NeuroD in the hippocampus of young adult and aged rodents. J Neurosci Res 70(3):327–334
Draberova E, Del Valle L, Gordon J, Markova V, Smejkalova B, Bertrand L, et al. (2008) Class III beta-tubulin is constitutively coexpressed with glial fibrillary acidic protein and nestin in midgestational human fetal astrocytes: implications for phenotypic identity. J Neuropathol Exp Neurol 67(4):341–354
Wislet-Gendebien S, Hans G, Leprince P, Rigo JM, Moonen G, Rogister B (2005) Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells 23(3):392–402
Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R (1998) A role for SOX1 in neural determination. Development 125(10):1967–1978
Lee JH, Um S, Song IS, Kim HY, Seo BM (2014) Neurogenic differentiation of human dental stem cells in vitro. J Korean Assoc Oral Maxillofac Surg 40(4):173–180
Neill D, Hughes D, Edwardson JA, Rima BK, Allsop D (1994) Human IMR-32 neuroblastoma cells as a model cell line in Alzheimer’s disease research. J Neurosci Res 39(4):482–493
Misiti F, Sampaolese B, Mezzogori D, Orsini F, Pezzotti M, Giardina B, et al. (2006) Protective effect of rhubarb derivatives on amyloid beta (1-42) peptide-induced apoptosis in IMR-32 cells: a case of nutrigenomic. Brain Res Bull 71(1–3):29–36
Kaur N, Dhiman M, Perez-Polo JR, Mantha AK (2015) Ginkgolide B revamps neuroprotective role of apurinic/apyrimidinic endonuclease 1 and mitochondrial oxidative phosphorylation against Abeta25-35-induced neurotoxicity in human neuroblastoma cells. J Neurosci Res 93(6):938–947
Sumantran VN, Brederlau A, Funa K (2003) BMP-6 and retinoic acid synergistically differentiate the IMR-32 human neuroblastoma cells. Anticancer Res 23(2B):1297–1303
Pence JC, Shorter NA (1990) In vitro differentiation of human neuroblastoma cells caused by vasoactive intestinal peptide. Cancer Res 50(16):5177–5183
Park KS, Lee RD, Kang SK, Han SY, Park KL, Yang KH, et al. (2004) Neuronal differentiation of embryonic midbrain cells by upregulation of peroxisome proliferator-activated receptor-gamma via the JNK-dependent pathway. Exp Cell Res 297(2):424–433
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. (2002) Stem cell properties of human dental pulp stem cells. J Dent Res 81(8):531–535
Davidson RM (1994) Neural form of voltage-dependent sodium current in human cultured dental pulp cells. Arch Oral Biol 39(7):613–620
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14(1):7–23
Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, et al. (1990) NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron 5(4):501–509
Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, et al. (2005) The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest 115(8):2083–2098
Jung KH, Chu K, Lee ST, Kim SJ, Sinn DI, Kim SU, et al. (2006) Granulocyte colony-stimulating factor stimulates neurogenesis via vascular endothelial growth factor with STAT activation. Brain Res 1073-1074:190–201
Wong G, Goldshmit Y, Turnley AM (2004) Interferon-gamma but not TNF alpha promotes neuronal differentiation and neurite outgrowth of murine adult neural stem cells. Exp Neurol 187(1):171–177
Vogel T, Ahrens S, Buttner N, Krieglstein K (2010) Transforming growth factor beta promotes neuronal cell fate of mouse cortical and hippocampal progenitors in vitro and in vivo: identification of Nedd9 as an essential signaling component. Cereb Cortex 20(3):661–671
Li Z, Li K, Zhu L, Kan Q, Yan Y, Kumar P, et al. (2013) Inhibitory effect of IL-17 on neural stem cell proliferation and neural cell differentiation. BMC Immunol 14:20
Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, et al. (2010) IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest 120(1):331–342
Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, et al. (2009) Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 119(10):1424–1432
Acknowledgments
AK received fellowship support from CSIR, Govt. of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest
Electronic supplementary material
Figure S1
FACS characterization and multilineage differentiation of primary stem cells. a. FACS cytogram of BMSCs, b. DPSC, c. SCAP, d. DFSC to show the expression level of different stem cell markers, for DPSC (n = 5), SCAP (n = 5) and DFSC (n = 5) and BMSC (n = 3), e. Multilineage potential of different stem cell populations in cellular derivatives of osteocytes (alizarin red staining), adipocytes (oil red O staining is visible in lipid droplets) and hepatocytes (final images are merged images of LDL Dylight fluorochrome-red for low density lipoprotein uptake and LDL receptor-green). Magnification is 10X for osteocytes and 20X for adipocytes and hepatocytes, scale bar 100 μm (GIF 17 kb)
Figure S2
Intensity surface plots representing a 3D reconstruction of antibody binding intensities of NFM and MAP-2 antibody expression on surface of neural cells obtained after neural differentiation (GIF 7 kb)
Figure S3
Neurosphere integrity assay. DIC images of neurospheres generated from different stem cells post seven days of differentiation and after 24 h of maintenance on adherent cell culture plate. Magnification-20X, scale bar 100 μm (GIF 5 kb)
Rights and permissions
About this article
Cite this article
Kumar, A., Kumar, V., Rattan, V. et al. Secretome Cues Modulate the Neurogenic Potential of Bone Marrow and Dental Stem Cells. Mol Neurobiol 54, 4672–4682 (2017). https://doi.org/10.1007/s12035-016-0011-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0011-3