Abstract
Neurons are highly vulnerable to genotoxic agents. To restore genome integrity upon DNA lesions, neurons trigger a DNA damage response (DDR) that requires chromatin modifications and transcriptional silencing at DNA damage sites. To study the reorganization of the active RNA polymerase II (Pol II), which transcribes all mRNA-encoding genes, and the participation of the ubiquitin-proteasome system (UPS) in the neuronal DDR, we have used rat sensory ganglion neurons exposed to X-rays (4 Gy) ionizing radiation (IR). In control neurons, Pol II appears concentrated in numerous chromatin microfoci identified as transcription factories by the incorporation of 5′-fluorouridine into nascent RNA. Upon IR treatment, numerous IR-induced foci (IRIF), which were immunoreactive for γH2AX and 53BP1, were observed as early as 30 min post-IR; their number progressively reduced at 3 h, 1 day, and 3 days post-IR. The formation of IRIF was associated with a decrease in Pol II levels by both immunofluorescence and Western blotting. Treatment with the proteasome inhibitor bortezomib strongly increased Pol II levels in both control and irradiated neurons, suggesting that proteasome plays a proteolytic role by clearing stalled Pol II complexes at DNA damage sites, as a prelude to DNA repair. Neuronal IRIF recruited ubiquitylated proteins, including ubiquitylated histone H2A (Ub-H2A), and the catalytic proteasome 20S. Ub-H2A has been associated with transcriptional silencing at DNA damage sites. On the other hand, the participation of UPS in neuronal DDR may be essential for the ubiquitylation of Pol II and other proteasome substrates of the DNA repair machinery and their subsequent proteasome-mediated degradation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The RNA polymerase II (Pol II) transcribes all mRNA-encoding genes and some genes that encode snRNAs and miRNAs. It is well-known that the recruitment of Pol II to the promoter plays a key role in the regulation of transcription [1–3]. In particular, the largest Pol II subunit, Rpb1, contains an extended C-terminal domain (CTD), which is composed of tandem heptapeptide repeats, that associates regulatory factors of transcription and cotranscriptional RNA processing [4]. The transcription cycle includes three steps known as initiation, elongation, and termination. During the transition from the initiation to the elongation step, the CTD changes from a hypophosphorylated (Pol IIA) to a hyperphosphorylated (Pol IIO) form [2]. Phosphorylation on Ser2 of Pol IIO predominantly occurs in the elongation step, and this modification can be specifically recognized with the H5 antibody [5–7].
Several studies have shown that the largest Pol II subunit can be polyubiquitylated and that proteolysis by the ubiquitin proteasome system (UPS) regulates its concentration and may contribute to removing stellated Pol II from the DNA template during transcription [8–11]. Pol II transcription is severely affected by DNA damage, with DNA breaks in active transcribed genes resulting in Pol II stalling and transcriptional arrest [12, 13]. Permanently stalled Pol II complexes not only prevent transcription, but Pol II arrested in the DNA template blocks access of DNA repair factors to lesions [14, 15]. Consequently, removal of transcription-blocking lesions by polyubiquitylation and proteasome-mediated degradation of the Pol II complexes could resume transcription upon DNA damage [16]. Furthermore, several studies in nonneural cells have demonstrated the participation of UPS in the DNA damage response (DDR). In particular, the ubiquitylation of the histones H2AX and H2A is essential for chromatin remodeling at DNA damage sites [17] and proteasome seems to be involved in the proteolysis of some substrate proteins involved in DDR [18].
Neurons are very vulnerable to endogenous or exogenous genotoxic agents that produce DNA damage. The most common DNA lesions in neurons are single-stranded breaks produced by oxidative stress that are mainly repaired by the base excision repair (BER) pathway [19]. Another type of DNA lesions are double-strand breaks (DSBs) that may be induced by ionizing radiation (IR) and other genotoxic agents. They are particularly detrimental for the neurons as they affect genome stability and can trigger apoptosis [20, 21]. DSBs can be repaired by homologous recombination (HR) or nonhomologous end-joining (NHEJ). Since mature neurons lack sister chromatids that serve as a template to facilitate “error-free” repair by HR, DSBs need to be repaired by NHEJ, which involves processing and direct ligation of DSB ends [22, 23].
Defective DNA repair with an accumulation of DNA lesions and loss of genome stability underlies many neurodegenerative disorders in human patients and animal experimental models [24–27]. DNA damage interferes with the Pol II-dependent transcription of genes which encode essential proteins for neuronal transmission and intracellular signaling [28]. Moreover, a dysfunction of Pol II phosphorylation has been associated with the neurodegenerative disease amyotrophic lateral sclerosis [29]. In a previous work [21], we analyzed the spatiotemporal organization of the irradiation induced nuclear foci (IRIF) of DNA damage/repair in rat sensory ganglion neurons. Here, we use this neuronal model of DNA damage to analyze the involvement of Pol II and UPS in the DDR. Our results demonstrate that the active Pol IIO concentrates in numerous nuclear microfoci related to active transcription factories. They also indicate that the transient reduction of Pol IIO levels, at the early stage of the DDR, is dependent of proteasomal proteolysis. Finally, the persistent concentration of ubiquitylated proteins and proteasome 20S in IRIF supports the importance of the UPS in the neuronal DDR.
Material and Methods
Animals and Treatments
Experiments were designed and performed to minimize the use of animals using a total of 60 young male Sprague–Dawley rats, distributed in a control (nonirradiated) and four experimental groups treated with X-ray ionizing radiation. The animals were housed with a 12-h light/dark cycle and had free access to food and water. The animals were kept, handled, and sacrificed in accordance with the directives of the Council of the European Communities and current Spanish legislation, and the experiments were approved by the Bioethical Committee of the University of Cantabria.
Exogenous DNA damage was induced by X-ray ionizing irradiation (IR) using an X-ray generator system (Maxishot-d, Yxlon, Int., USA). The animals’ heads were exposed to a sublethal dose of 4 Gy IR and processed at 0.5 h, 3 h, 1d and 3d post-IR. Some control and irradiated animals were treated with the proteasome inhibitor Bortezomib (Velcade, Millennium Pharmaceuticals Inc, MA, USA). The animals received two doses of bortezomib 0.2 mg/kg, dissolved in sterile saline and administered intravenously via the tail vein, on days 1 and 3 and were sacrificed 1 day post-administration of the second dose.
Immunofluorescence
For light immunocytochemistry, the animals (n = 3 per group) deeply anesthetized as described above were perfused with the fixative solution containing 3.7 % formaldehyde (freshly prepared from paraformaldehyde) in PBS. Tissue fragments of trigeminal ganglia were transferred to a drop of PBS on a slide, and neuronal dissociation was performed following the previously reported procedure [30]. The samples were sequentially treated with 0.1 M glycine in PBS for 15 min and 0.5 % Triton X-100 in PBS for 45 min. They were then incubated with the primary antibody overnight at 4 °C, washed with 0.05 % Tween 20 in PBS, incubated for 45 min in the specific secondary antibody conjugated with FITC or TexasRed (Jackson), washed in PBS, and mounted with the antifading medium ProLong (Invitrogen). Confocal images were obtained with a LSM510 (Zeiss, Germany) laser scanning microscope, using a 63× oil (1.4 NA) objective. In order to avoid overlapping signals, images were obtained by sequential excitation at 488 and 543 nm in order to detect FITC and Texas Red, respectively. Images were processed using Photoshop software.
The following primary antibodies were used. Mouse monoclonal antibodies anti-α-tubulin (Sigma-Aldrich), anti-γH2AX phospho-Ser139 (Millipore-Upstate), anti-ubiquitylated histone H2A (Millipore-Upstate), anti-RNA Polymerase II 8WG16 (Covance), and anti-RNA Polymerase II H5 (Covance). Rabbit polyclonal antibodies anti-53BP1 (Bethyl Laboratories) and anti-ubiquitin-protein conjugates (Biomol International). A rabbit polyclonal antibody against the 20S catalytic proteasome core has been previously characterized [31, 32].
Run-On Transcription Assays In Situ
Active transcription sites were labeled by the incorporation of 5′-FU into nascent RNA, as previously reported [33]. Briefly, rats (n = 3 per group) under anesthesia were given an i.p. injection of 5′-FU (Sigma, UK) at doses of 10 μl/g of a stock solution of 0.4 M 5′-FU in 0.9 % saline. The rats were killed at 30 min post-injection. The animals were fixed by perfusion with 3.7 % paraformaldehyde in HPEM buffer (2× HPEM: Hepes, 60 mM; Pipes, 130 mM; EGTA, 20 mM; and MgCl2 · 6H2O, 4 mM) containing 0.5 % Triton X-100. The incorporation of 5′-FU into nascent RNA was detected with a mouse monoclonal (clone BU-33) anti-BrdU antibody (Sigma-Aldrich) on dissociated neurons and then incubated with a FITC-conjugated secondary antibody (Jackson Laboratories) as previously described.
Immunoelectron Microscopy
For immunoelectron microscopy of RNA Pol IIO and 5′-FU incorporation sites into nascent RNA, control irradiated rats (n = 3 animals per group) were perfused with 3.7 % paraformaldehyde in 0.1 M cacodylate buffer. In the case of 5′-FU incorporation, this halogenated precursor was administrated as described above. Small tissue fragments of trigeminal ganglia were dehydrated with methanol, embedded in Lowicryl K4 M at −20 °C and polymerized with ultraviolet irradiation. Ultrathin sections were mounted on nickel grids and sequentially incubated with 0.1 M glycine in PBS for 15 min, 5 % BSA in PBS for 30 min, and the primary mouse anti-Pol IIO or mouse monoclonal anti-BrdU antibody for 1 h at 37 °C and then with a secondary antibody coupled to 15-nm gold particles (BioCell, UK; diluted 1:50 in PBS containing 1 % BSA). Following immunogold labeling, the grids were stained with lead citrate and uranyl acetate and examined with a JEOL 1011 electron microscope. As controls, ultrathin sections were treated as described above but with the primary antibody being omitted.
SDS-PAGE and Immunoblotting
Trigeminal ganglia from three animals per group were used. Ganglia were lysed using a Polytron PT-2000 (Kinematica®, Luzern-Switzerland) on ice and incubated for 30 min in the cold extraction buffer NETN [20 mM Tris–HCl pH 8.0, 500 mM NaCl, 1 mM EDTA] containing Benzonase (1 μl/1 ml lysis buffer) (Novagen) and supplemented with a protease and phosphatase inhibitor cocktail (Halt™ Protease and Phosphatase inhibitor single use cocktail, Thermo Scientific, USA). After centrifugation (12 min at 12,000 rpm) at 4 °C the supernatant was frozen. Proteins were separated on SDS-PAGE gels and transferred to nitrocellulose membranes by standard procedures. Monoclonal anti-RNA polymerase II (8WG16), anti-RNA Polymerase II (H5), anti-ubiquitylated histone H2A and anti-α-tubulin antibodies were used. Protein bands were detected with an Odyssey™ Infrared-Imaging System (Li-Cor Biosciences) according to Odyssey™ Western-Blotting Protocol. Immunoblots were developed with anti-mouse IRDye800DX or anti-rabbit IRDye700DX (Rockland Immunochemicals) secondary antibodies.
Results
RNA Pol IIO Distributes in Transcription Factories of the Euchromatin
Immunofluorescence for the active Pol IIO phosphorylated on Ser2 in sensory ganglion neurons showed a nuclear localization which excluded the nucleolus and smaller irregular areas, presumably corresponding to nuclear speckles of splicing factors [34]. In addition to weak diffuse nuclear staining, Pol IIO was concentrated in numerous microfoci throughout euchromatin regions identified as transcription factories (Fig. 1a), nuclear microdomains where a cluster of genes are transcribed together [35–37]. A similar pattern of extranucleolar transcription factories was observed with the in situ transcription assay after a 30-min pulse of exposure to 5′-FU, administrated by intraperitoneal injection (Fig. 1b). This transcription assay also revealed a strong 5′-FU incorporation into the nucleolus (Fig. 1b) which corresponds to the neuronal transcription of ribosomal genes [38, 39]. Transcription factories were homogeneously distributed throughout chromatin domains without a preferential localization at the nuclear interior or periphery. A similar organization pattern of transcription factories was found in the three main types of sensory ganglion neurons identified on the basis of their size [30]. Immunogold electron microscopy analysis of Pol IIO and nascent RNA detection (5′-FU incorporation) identified transcription factories in euchromatin regions (Fig. 1c, d). These factories appeared as small aggregates of amorphous material, ranging in size from ≈50 to 150 nm, and decorated by clusters of immunogold particles (Fig. 1c, d).
a Immunolocalization of the active RNA Pol llO in the nucleus of a control sensory ganglion neuron. Pol IIO appears concentrated in numerous nuclear microfoci corresponding to transcription factories. Note the absence of immunolabeling in the nucleolus. b In situ transcription assay with 5′-FU incorporation into nascent RNA revealed active transcription sites in the nucleolus and in numerous extranucleolar transcription factories. thirty-minute pulse of 5′-FU incorporation. Scale bar, 5 μm (a, b). c Detail of immunogold labeling for active RNA Pol IIO showing two transcription factories decorated with gold particles. d Detail of the incorporation of 5′-FU into transcription factories. Scale bar = 150 nm
Neuronal DNA Damage Induced by IR Transiently Downregulates Poll IIO Expression
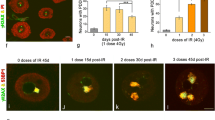
To investigate the dynamic behavior of Pol IIO during the DDR, trigeminal ganglion neurons were exposed to 4 Gy IR. The induction of a DDR was analyzed by double immunofluorescence for the histone H2AX phosphorylated on Ser133 (γH2AX), a DNA damage signal [40], and 53BP1, a key component of the NHEJ repair pathway [41, 42]. Numerous IRIF immunoreactive for γH2AX and 53BP1 were observed as early as 30 min post-IR, their number progressively reducing at 3 h, 1 day, and 3 days post-IR (Fig. 2a–e), indicating effective DNA repair of the majority of neuronal DNA lesions [21]. Immunofluorescence for Pol IIO with the H5 antibody demonstrated an early strong reduction of Pol IIO signal at 30 min post-IR, as compared with control neurons, followed by a progressive recovery of immunoreactivity at the following time points post-IR (Fig. 2f–j). This early transient drop of Pol IIO expression was confirmed by Western blotting, whereas no variations in the expression levels of the nonphosphorylated Pol IIA were detected (Fig. 2k).
a–e Representative examples of double immunolabeling for γH2AX and 53BP1 in dissociated sensory ganglion neurons from control (a) and irradiated neurons at 0.5 h, 3 h, 1 day, and 3 days (b–e). a Control neurons lack γH2AX signal and exhibit a diffuse nucleoplasmic labeling for 53BP1. b–d At 0.5 h, 3 h, 1 day, and 3 days post-IR, γH2AX and 53BP1 colocalize in numerous IRIF of variable size and they were distributed throughout the nucleus, excepting the nucleolus. e At 3 days post-IR, three large persistent IRIF appear intensely immunostained for γH2AX and 53BP1. Scale bar, 5 μm (a–e). f–j Immunostaining for active RNA Pol II in control neurons (f) and at different time points post-IR (g–j). g, h A reduction of RNA Pol IIO expression is observed at 0.5 and 3 h post-IR, progressively recovered at 1 and 3 days post-IR (i, j). Scale bar, 15 μm (f–j). k Western blot analysis of active hyperphosphorylated RNA Pol IIO and hypophosphorylated Pol IIA protein levels in sensory ganglion lysates from control and irradiated neurons. Protein levels of both types of Pol II were normalized to tubulin and the fold increase estimated. l Treatment with the proteasome inhibitor bortezomib increases Pol IIO levels in nonirradiated and radiated sensory ganglion neurons. Protein levels of both types of Pol II were normalized to tubulin and the fold increase estimated. m, n, o Representative examples of the in situ transcription assay with 5′-FU in control (m), 3 h post-IR (n), and bortezomib-treated and irradiated (o) sensory ganglion neurons. m, n Upon 3 h post-IR, an important reduction in the incorporation of 5′-FU into transcription factories is observed as compared with the control neuron. o A bortezomib-treated and irradiated neuron exhibits a notable incorporotaion of 5′-FU throughout the extensive euchromatin domains with a fine punctate pattern. The unstained irregular areas presumably correspond to nuclear speckles of splicing factors. Note the large size of the intensely labeled nucleolus. Scale bar, 5 μm
Since previous biochemical studies in nonneuronal cells have reported that Pol IIO may be ubiquitylated and degraded by the proteasome [16], we next investigate whether the transient reduction of Pol IIO upon IR is dependent on proteasome activity. To this end, animals pretreated with a single dose of the proteasome inhibitor bortezomib (0.5 mM) for 24 h were exposed to 4 Gy IR. Proteasome inhibition induced a significant increase in Pol IIO levels in both nonirradiated and irradiated ganglia at 3 h post-IR in comparison with control bortezomib-untreated ganglia (Fig. 2l; Supplementary Fig. 1). In contrast, no significant changes in Pol IIA levels were observed upon proteasome inhibition (Fig. 2l; Supplementary Fig. 1). These findings support the participation of the proteasome in regulating Pol IIO concentration during the neuronal DDR.
Consistent with the transient decrease of Pol IIO levels upon IR, the in situ transcription assay revealed a reduction of 5′-FU incorporation into transcription factories at 3 h post-IR as compared with nonirradiated neurons (Fig. 2m, n). Pretreatment with bortezomib in irradiated neurons induced a notable incorporation of 5′-FU into the euchromatin domains that showed a fine punctate pattern (Fig. 2o). This excluded several irregular areas, presumably corresponding to nuclear speckles [34]. Moreover, bortezomib treatment induced nucleolar hypertrophy and strong incorporation of 5′-FU into the nucleolus (Fig. 2o), as previously reported [39].
DNA Damage Foci Concentrated Ubiquitylated Proteins and 20S Proteasome
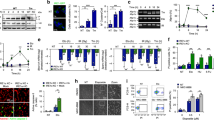
To determine the involvement of UPS in neuronal DDR upon IR treatment, we performed immunolabeling experiments to investigate the presence of ubiquitylated proteins in IRIF of DNA damage/repair. Double immunolabeling for γH2AX and ubiquitin-protein conjugates revealed the presence of ubiquitylated proteins in IRIF at all post-IR times studied (Fig. 3a–o). Since it is well-established in cultured nonneuronal cells that DSBs induced a chromatin remodeling mediated by the ubiquitylation of the histone H2A [17, 43], we investigated whether this histone modification also occurs in postmitotic neurons. Coimmunostaining for 53BP1 and ubiquitylated H2A (Ub-H2A) revealed the concentration of this histone variant in all IRIF (Fig. 4a–o). The IR-induced ubiquitylation of the H2A was confirmed by Western blot analysis (Fig. 4p). Next, we investigated whether the catalytic proteasome 20S complex is recruited to DNA damage/repair foci. Coimmunostaining for 20S proteasome and γH2AX revealed a diffuse nucleoplasmic pattern of the 20S proteasome in control neurons (Fig. 5a–c). In addition to the diffuse nucleoplasmic localization, 20S proteasome was concentrated in nuclear structures, identified as clastosomes [32], after 0.5 h post-IR (Fig. 5d–f). At this early stage of the DDR, γH2AX-positive IRIF did not concentrate 20S proteasome but they appeared in the proximity of clastosomes (Fig. 5d–f). Interestingly, from 3 h to 3 days post-IR 20S proteasome was redistributed and concentrated in IRIF where colocalized with γH2AX (Fig. 5g–o). The concentration of 20S catalytic proteasomes in IRIF supports their involvement in the molecular turnover of DNA repair factors which are proteasome substrates. Finally, a similar response of the ubiquitin proteasome system to DNA damage induced by IR was observed in the three main types of sensory ganglion neurons.
Confocal microscopy images from control (a–c) and irradiated (d–o) sensory ganglion neurons costained for ubiquitin-protein conjugates and γH2AX show the concentration of ubiquitylated proteins in IRIF of DNA damage chromatin. a–c Note the weak nucleaoplasmic signal of ubiquitylated proteins in control neurons. d–o Ionizing radiation increases nuclear expression of ubiquitin-protein conjugates and its accumulation in both transient and persistent IRIF where colocalize with γH2AX. Scale bar = 5 μm
Confocal microscopy images from control (a–c) and irradiated (d–o) sensory ganglion neurons co-stained for ubiquitylated H2A (Ub-H2A) and 53BP1 show the concentration of Ub-H2a in IRIF of DNA damage chromatin. Scale bar, 5 μm. p Western blot analysis of Ub-H2A protein levels in sensory ganglion lisates from controls and irradiated animals reveals the strong induction of Ub-H2A at 0.5 h post-IR, concomitantly with the formation of IRIF and the recruitment of the repair factor 53BP1. Note that Ub-H2A levels remain increased at all post-IR time points studied. Tubulin was used as a protein loading control
Confocal microscopy images from control (a–c) and irradiated (d–o) sensory ganglion neurons co-stained for 20S proteasome and γH2AX. In control neurons 20S proteasome exhibits a diffuse nucleoplasmic staining excluding the nucleolus (a–c). d–f At 0.5 h post-IR, 20S proteasome appears concentrated in nuclear domains identified as clastosomes and located in the vicinity of γH2AX-positive IRIF. g–o After 3 h, 1 day, and 3 days post-IR, 20S proteasome accumulates in γH2AX-positive IRIF. Scale bar = 5 μm
Discussion
Our results in sensory ganglion neurons demonstrate that the active RNA Pol II is concentrated in numerous microfoci throughout euchromatin domains which correspond to transcription factories, as indicated by the incorporation pattern of 5′-FU into nascent RNA. As far as we know, this is the first study on the nuclear distribution of active Pol II in neurons. These transcription factories are nuclear microdomains where several active genes are clustered and their primary transcripts co-transcriptionally processed [37, 44, 45]. Several studies have revealed that the nuclear architecture of chromosome territories and the spatial position of their genes influence on gene expression [46, 47]. For example, nuclear lamina exerts an inhibitory influence on the expression of genes located in its proximity, presumably related with the existence of a peripheral rim of condensed chromatin [48, 49]. In the case of sensory ganglion neurons, transcription factories appeared distributed throughout the nucleus without a preferential peripheral or interior localization. This spatial organization is consistent with the predominant euchromatic organization and the absence of the peripheral rim of heterochromatin reported in sensory ganglion neurons [30, 33]. With electron immunocytochemistry transcription factories appear as small aggregates of amorphous material, decorated with clusters of gold particles of Pol IIO immunoreactivity or 5′-FU incorporation. The presence of this amorphous material may reflect the high local concentration of Pol II complexes, transcriptional regulators and cotranscriptional processing factors of pre-mRNAs [37, 45]. Moreover, the concentration of Pol IIO in microfoci is also compatible with recent data of genome-wide screen analysis revealing that Pol II is enriched near the promoters of immediate early genes in rat cortical neurons [50]. The authors demonstrate that these genes harbor active chromatin marks and are poised for rapid induction of transcription by accumulating Pol II complexes just downstream of transcription start sites.
Our results support a proteasome-dependent downregulation of active Pol IIO during the early stage of neuronal DDR. Previous studies have shown that Pol II may be ubiquitylated and degraded by the proteasome [8, 12, 16, 51]. When DNA damage occurs in transcribed regions in neurons and other cellular types, it may induce persistent stalling of Pol II complexes at sites of DSBs with potentially disastrous consequences for gene expression [52]. Our observations support the notion that proteasome 20S plays an essential role in clearing arrested RNA Pol II machinery and exposing neuronal DSBs for the subsequent recruitment and assembly of DNA repair factors of the NHEJ pathway. Moreover, proteasomal degradation seems to be mainly involved in removing roadblock complexes of the hyperphosphorylated Pol IIO during processive transcript elongation. We demonstrate here that, in both control and IR exposed neurons, the proteasome inhibition induces the accumulation of hyperphosphorylated Pol IIO, but not of the hypophosphorylalated Pol IIA. The latter is preferentially associated with preinitiation transcriptional complexes [53]. Consistent with the accumulation of Pol IIO, the in situ transcription assay in bortezomib-pretreated neurons revealed a notable preservation of chromatin Pol II-dependent transcription at 3 h post-IR, a condition that induces transcriptional inhibition [21], present results]. Similarly, an intense Pol I-dependent nucleolar transcription was induced by proteasome inhibition. This effect seems to reflect a nucleolar reactive response to endoplasmic reticulum stress induced by proteasome inhibition [39]. In this context, it has been suggested that the transcription serves as a guardian of genome by sensing blocked Pol II complexes at DNA damage sites. Thus, the severity of DNA damage, the ability to remove transcription blocking lesions and the recovery of mRNA synthesis may determine cell survival or cell death [10].
In the present work, we provide the first evidence in neurons that DNA damage foci concentrate Ub-H2A and catalytic proteasome 20S. Most mammalian neuronal types, including sensory ganglion neurons, have a predominant euchromatin architecture that correlates with their high transcriptional activity [33, 49]. However, decondensed chromatin facilitates genotoxic agents gaining access to DNA and disrupting its structure [54]. This makes neurons highly vulnerable to endogenous and exogenous genotoxic insults such as oxidative DNA damage or IR exposure [20, 21, 55], present results]. To restore genome integrity in DNA damage chromatin, epigenetic modifications of histones that promote recruitment of repair factors at DNA damage sites are required [56]. In this vein, our results in post-mitotic neurons demonstrated the concentration of ubiquitylated proteins, particularly Ub-H2A, in all IRIF of DNA damage chromatin. This epigenetic modification is consistent with previous experiments in nonneuronal cultured cells revealing that DNA-damage flanking chromatin concentrated Lys 63-linked ubiquitin conjugates and recruited the ubiquitin ligases RNF8 and RNF168, which initiate the ubiquitylation cascade (for a review, see [56, 57]). In sensory ganglion neurons, Ub-H2A is barely detected by both immunofluorescence and Western blotting in control neurons but it is strongly induced at 0.5 h post-IR, concomitantly with the formation of IRIF and the recruitment of signaling/repair factors γH2AX and 53BP1, respectively. It has been proposed that this histone ubiquitylation protects genome integrity by inducing transcriptional silencing and recruiting repair factors near the DNA lesions [17, 43]. Regarding the participation of the proteasome 20S in the neuronal DDR, this catalytic proteasome is enriched in control neuronal nuclei as previously reported [31, 32]. Proteasome 20S is aggregated in discrete nuclear foci, identified as clastosomes [32], as an early event in the neuronal DNA damage response, before being relocalized to γH2AX positive IRIF from 3 h post-IR onward. We propose that this recruitment of proteasome 20S to IRIF may reflect a local proteolytic activity in DNA damaged chromatin in order to degrade proteasome substrates of the DNA repair machinery during neuronal DDR. This function may contribute to sustaining a dynamic protein turnover in IRIF during the DDR. In this vein, the role of proteasome in the DNA repair response in yeast and human cultured cells has been proposed [18, 58]. Indeed, proteasome inhibitors sensitize tumor cells to DNA-damaging agents and cause a deficient DNA repair [59, 60]. Furthermore, we have previously demonstrated that proteasome inhibition with bortezomib can induce the formation of DNA damage foci in sensory ganglion neurons [55].
In conclusion, proteasome activity in sensory ganglion neurons plays an important role in regulating Pol IIO levels during DDR. Moreover, the recruitment of the catalytic 20S proteasome to DNA damage foci seems to be an important cellular mechanism in neuronal DDR.
References
Ptashne M, Gann A (1997) Transcriptional activation by recruitment. Nature 386:569–77
Saunders A, Core LJ, Lis JT (2006) Breaking barriers to transcription elongation. Nat Rev Mol Cell Biol 7:557–67
Jonkers I, Lis JT (2015) Getting up to speed with transcription elongation by RNA polymerase II. Nat Rev Mol Cell Biol 16:167–77
Hocine S, Singer RH, Grünwald D (2010) RNA processing and export. Cold Spring Harb Perspect Biol 2, a000752
Komarnitsky P, Cho EJ, Buratowski S (2000) Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev 14:2452–60
Warren SL, Landolfi AS, Curtis C, Morrow JS (1992) Cytostellin: a novel, highly conserved protein that undergoes continuous redistribution during the cell cycle. J Cell Sci 103:381–8
Guillot PV, Xie SQ, Hollinshead M, Pombo A (2004) Fixation-induced redistribution of hyperphosphorylated RNA polymerase II in the nucleus of human cells. Exp Cell Res 295:460–8
Ratner JN, Balasubramanian B, Corden J, Warren SL, Bregman DB (1998) Ultraviolet radiation-induced ubiquitination and proteasomal degradation of the large subunit of RNA polymerase II. Implications for transcription-coupled DNA repair. J Biol Chem 273:5184–9
McKay BC, Chen F, Clarke ST, Wiggin HE, Harley LM, Ljungman M (2001) UV light-induced degradation of RNA polymerase II is dependent on the Cockayne’s syndrome A and B proteins but not p53 or MLH1. Mutat Res 485:93–105
Ljungman M, Lane DP (2004) Transcription - guarding the genome by sensing DNA damage. Nat Rev Cancer 4:727–37
Somesh BP, Reid J, Liu WF, Sogaard TM, Erdjument-Bromage H, Tempst P, Svejstrup JQ (2005) Multiple mechanisms confining RNA polymerase II ubiquitylation to polymerases undergoing transcriptional arrest. Cell 121:913–23
Svejstrup JQ (2003) Rescue of arrested RNA polymerase II complexes. J Cell Sci 116:447–51
Pankotai T, Bonhomme C, Chen D, Soutoglou E (2012) DNAPKcs-dependent arrest of RNA polymerase II transcription in the presence of DNA breaks. Nat Struct Mol Biol 19:276–82
Donahue BA, Yin S, Taylor JS, Reines D, Hanawalt PC (1994) Transcript cleavage by RNA polymerase II arrested by a cyclobutane pyrimidine dimer in the DNA template. Proc Natl Acad Sci U S A 91:8502–6
Wilson MD, Harreman M, Taschner M, Reid J, Walker J, Erdjument-Bromage H, Tempst P, Svejstrup JQ (2013) Proteasome-mediated processing of Def1, a critical step in the cellular response to transcription stress. Cell 154:983–95
Wilson MD, Harreman M, Svejstrup JQ (2013) Ubiquitylation and degradation of elongating RNA polymerase II: the last resort. Biochim Biophys Acta 1829:151–7
Mailand N, Bekker-Jensen S, Faustrup H, Melander F, Bartek J, Lukas C, Lukas J (2007) RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell 131:887–900
Krogan NJ, Lam MH, Fillingham J, Keogh MC, Gebbia M, Li J, Datta N, Cagney G et al (2004) Proteasome involvement in the repair of DNA double-strand breaks. Mol Cell 16:1027–34
Englander EW (2013) DNA damage response in peripheral nervous system: coping with cancer therapy-induced DNA lesions. DNA Repair 12:685–90
Ferrer I, Serrano T, Alcantara S, Tortosa A, Graus F (1993) X-ray-induced cell death in the developing hippocampal complex involves neurons and requires protein synthesis. J Neuropathol Exp Neurol 52:370–8
Casafont I, Palanca A, Lafarga V, Berciano MT, Lafarga M (2011) Effect of ionizing radiation in sensory ganglion neurons: organization and dynamics of nuclear compartments of DNA damage/repair and their relationship with transcription and cell cycle. Acta Neuropathol 122:481–493
Lieber MR (2010) The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Ann Rev Biochem 79:181–211
Jeppesen DK, Bohr VA, Stevnsner T (2011) DNA repair deficiency in neurodegeneration. Prog Neurobiol 94:166–200
Rass U, Ahel I, West SC (2007) Defective DNA repair and neurodegeneration disease. Cell 130:991–1004
McKinnon PJ (2013) Maintaining genome stability in the nervous system. Nat Neurosci 16:1523–9
Baltanas F, Casafont I, Lafarga V, Weruaga E, Alonso JR, Berciano MT, Lafarga M (2011) Purkinje cell degeneration in pcd mice reveals large scale chromatin reorganization and gene silencing linked to defective DNA repair. J Biol Chem 286:28287–28302
Pan L, Penney J, Tsai LH (2014) Chromatin regulation of DNA damage repair and genome integrity in the central nervous system. J Mol Biol 426:3376–88
Hetman M, Vashishta A, Rempala G (2010) Neurotoxic mechanisms of DNA damage: focus on transcriptional inhibition. J Neurochem 114:1537–1549
Schwartz JC, Ebmeier CC, Podell ER, Heimiller J, Taatjes DJ, Cech TR (2012) FUS binds the CTD of RNA polymerase II and regulates its phosphorylation at Ser2. Genes Dev 26:2690–5
Pena E, Berciano MT, Fernandez R, Ojeda JL, Lafarga M (2001) Neuronal body size correlates with the number of nucleoli and Cajal bodies, and with the organization of the splicing machinery in rat trigeminal ganglion neurons. J Comp Neurol 430:250–63
Mengual E, Arizti P, Rodrigo J, Giménez-Amaya JM, Castaño JG (1996) Immunohistochemical distribution and electron microscopic subcellular localization of the proteasome in the rat CNS. J Neurosci 16:6331–41
Lafarga M, Berciano MT, Pena E, Mayo I, Castaño JG, Bohmann D, Rodrigues JP, Tavanez JP et al (2002) Clastosome: a subtype of nuclear body enriched in 19S and 20S proteasomes, ubiquitin, and protein substrates of proteasome. Mol Biol Cell 13:2771–82
Casafont I, Navascués J, Pena E, Lafarga M, Berciano MT (2006) Nuclear organization and dynamics of transcription sites in rat sensory ganglia neurons detected by incorporation of 5′-fluorouridine into nascent RNA. Neuroscience 140:453–62
Spector DL, Lamond AI (2011) Nuclear speckles. Cold Spring Harb Perspect Biol 3, a000646
Iborra FJ, Pombo A, Jackson DA, Cook PR (1996) Active RNA polymerases are localized within discrete transcription “factories’ in human nuclei. J Cell Sci 109:1427–36
Osborne CS, Chakalova L, Brown KE, Carter D, Horton A, Debrand E, Goyenechea B, Mitchell JA et al (2004) Active genes dynamically colocalize to shared sites of ongoing transcription. Nat Genet 36:1065–71
Rieder D, Trajanoski Z, McNally JG (2012) Transcription factories. Front Genet 3:221
Raska I, Shaw PJ, Cmarko D (2006) New insights into nucleolar architecture and activity. Int Rev Cytol 255:177–235
Palanca A, Casafont I, Berciano MT, Lafarga M (2014) Reactive nucleolar and Cajal body responses to proteasome inhibition in sensory ganglion neurons. Biochim Biophys Acta 1842:848–59
Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A (2004) H2AX: the histone guardian of genome. DNA Repair 3:959–967
Noon AT, Goodarzi AA (2011) 53BP1-mediated DNA double strand break repair: insert bad pun here. DNA Repair 10:1071–1076
Callen E, di Virgilio M, Kruhlak MJ, Nieto-Soler M, Wong N, Chen HT, Faryabi RB, Polato F et al (2013) 53BP1 mediates productive and mutagenic DNA repair through distinct phosphoprotein interactions. Cell 153:1266–1280
Shanbhag NM, Rafalska-Metcalf IU, Balane-Bolivar C, Janicki SM, Greenberg RA (2010) ATM-dependent chromatin changes silence transcription in cis to DNA double-strand breaks. Cell 141:970–81
Canals-Hamann AZ, das Neves RP, Reittie JE, Iñiguez C, Soneji S, Enver T, Buckle VJ, Iborra FJ (2013) A biophysical model for transcription factories. BMC Biophys 6:2
Chakalova L, Fraser P (2010) Organization of transcription. Cold Spring Harb Perspect Biol 2, a000729
Bickmore WA, van Steensel B (2013) Genome architecture: domain organization of interphase chromosomes. Cell 152:1270–84
Rieder D, Ploner C, Krogsdam AM, Stocker G, Fischer M, Scheideler M, Dani C, Amri EZ et al (2014) Co-expressed genes prepositioned in spatial neighborhoods stochastically associate with SC35 speckles and RNA polymerase II factories. Cell Mol Life Sci 71:1741–59
Zuleger N, Robson MI, Schirmer EC (2011) The nuclear envelope as a chromatin organizer. Nucleus 2:339–49
Wilczynski GM (2014) Significance of higher-order chromatin architecture for neuronal function and dysfunction. Neuropharmacology 80:28–33
Saha RN, Wissink EM, Bailey ER, Zhao M, Fargo DC, Hwang JY, Daigle KR, Fenn JD et al (2011) Rapid activity-induced transcription of Arc and other IEGs relies on poised RNA polymerase II. Nat Neurosci 14:848–56
Gillette TG, Gonzalez F, Delahodde A, Johnston SA, Kodadek T (2004) Physical and functional association of RNA polymerase II and the proteasome. Proc Natl Acad Sci U S A 101:5904–9
Tornaletti S, Hanawalt PC (1999) Effect of DNA lesions on transcription elongation. Biochimie 81:139–46
Lu H, Flores O, Weinmann R, Reinberg D (1991) The nonphosphorylated form of RNA polymerase II preferentially associates with the preinitiation complex. Proc Natl Acad Sci U S A 88:10004–8
Misteli T, Soutoglou E (2009) The emerging role of nuclear architecture in DNA repair and genome maintenance. Nat Rev Mol Cell Biol 10:243–54
Palanca A, Casafont I, Berciano MT, Lafarga M (2014) Proteasome inhibition induces DNA damage and reorganizes nuclear architecture and protein synthesis machinery in sensory ganglion neurons. Cell Mol Life Sci 71:1961–75
Lukas J, Lukas C, Bartek J (2011) More than just a focus: the chromatin response to DNA damage and its role in genome integrity maintenance. Nat Cell Biol 13:1161–9
Doil C, Mailand N, Bekker-Jensen S, Menard P, Larsen DH, Pepperkok R, Ellenberg J, Panier S et al (2009) RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell 136:435–446
Motegi A, Murakawa Y, Takeda S (2009) The vital link between the ubiquitin-proteasome pathway and DNA repair: impact on cancer therapy. Cancer Lett 283:1–9
Jacquemont C, Taniguchi T (2007) Proteasome function is required for DNA damage response and fanconi anemia pathway activation. Cancer Res 67:7395–405
Takeshita T, Wu W, Koike A, Fukuda M, Ohta T (2009) Perturbation of DNA repair pathways by proteasome inhibitors corresponds to enhanced chemosensitivity of cells to DNA damage-inducing agents. Cancer Chemother Pharmacol 64:1039–46
Acknowledgments
This work was supported by the following grants: “Dirección General de Investigación” of Spain (BFU2011-23983; BFU2014-54754-P) and “Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED; CB06/05/0037)” from Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Animals
All procedures were approved by the Bioethical Committee of the University of Cantabria and were carried out according to the directives of the Council of the European Communities and current Spanish legislation.
Additional information
Iñigo Casafont and Ana Palanca contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
(DOCX 834 kb)
Rights and permissions
About this article
Cite this article
Casafont, I., Palanca, A., Lafarga, V. et al. Dynamic Behavior of the RNA Polymerase II and the Ubiquitin Proteasome System During the Neuronal DNA Damage Response to Ionizing Radiation. Mol Neurobiol 53, 6799–6808 (2016). https://doi.org/10.1007/s12035-015-9565-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9565-8