Abstract
In our previous work, we demonstrated that the intracerebroventricular (i.c.v.) injection of an interleukin-1 receptor antagonist (IL-1ra) prevented the impairment in vasopressin secretion and increased survival rate in septic rats. Additionally, we saw a reduction in nitric oxide (NO) levels in cerebroventricular spinal fluid (CSF), suggesting that the IL-1ra prevents apoptosis that seems to occur in vasopressinergic neurons. Here, we investigated the effect of IL-1ra pre-treatment on the sepsis-induced increase in oxidative stress markers in the hypothalamus of rats. The animals were pre-treated by an i.c.v. injection of IL-1ra (9 nmol) or vehicle (0.01 M PBS) before being subjected to cecal ligation and puncture (CLP) or left as control (sham-operation or naive). After 4, 6, and 24 h, the animals were decapitated (n = 9/group) and the brain removed for hypothalamic tissue collection. Transcript and protein levels of IL-1, inducible nitric oxide synthase (iNOS), caspase-3, and hypoxia-inducible factor 1-alpha (HIF-1α) were measured by quantitative polymerase chain reaction (qPCR) and western blot, respectively. Hypothalamic mRNA levels of all these genes were significantly (P < 0.005) increased at 4, 6, and 24 h CLP, as compared to sham-operated animals. IL-1ra pre-treatment in these CLP animals significantly decreased IL-1 gene expression at all time points and also of iNOS, caspase-3, and HIF-1α at 24 h when compared to vehicle-treated CLP animals. The effect of the pre-treatment on protein expression was most clearly seen for IL-1β and iNOS at 24 h. Our results showed that blocking the IL-1-IL-1r signaling pathway by central administration of an IL-1ra decreases hypothalamic oxidative stress markers during sepsis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impairment of the secretion of arginine vasopressin (AVP), an important vasopressor hormone, is a major pathophysiological change associated with sepsis. AVP secretion during sepsis can be separated into two distinct phases [1–3]. In the initial phase, AVP secretion is increased in response to the drop in blood pressure, representing a normal endocrine response pattern, but in the late phase, its secretion is inappropriately low regardless of progressive, life-threatening hypotension [1–7].
The exact cause(s) of this drop in AVP secretion in the late phase of severe sepsis are still not fully elucidated. Thus, we consider that a better understanding of the cascade of events occurring in the hypothalamus during sepsis should aid in deciphering the causes of lower AVP secretion in the late phase of severe sepsis. The currently available data suggest the involvement of a large array of molecular factors in inducing alterations in AVP secretion during sepsis [8]. Among these, IL-1, NO, cleaved caspase 3, and HIF-1 have been pinpointed as important factors for the impairment of AVP secretion during the late phase of severe sepsis [1, 6, 8–10].
IL-1 concentrations, both in the periphery as well as centrally, are greatly increased in both phases of sepsis [8–11]. The activation of the immune system following an attack of the body by infectious agents leads to a well-described event known as cytokine storm, characterized by a robust increase of proinflammatory factors such as interleukin-6, NO, and tumor necrosis factor (TNF) into the systemic circulation. The rise of this systemic proinflammatory factors, caused by both direct and indirect mechanisms, subsequently contributes to the augmentation of IL-1 concentrations in parts of the central nervous system [8, 11]. In different parts of the brain, including the hypothalamus, IL-1 and other cytokines lead to the activation of iNOS, which in turn causes the excessive production of NO, this resulting in the expression of HIF-1 and several markers of oxidative stress and apoptosis [8–10, 12–15].
Recently, we saw the expression of these apoptosis markers in the supraoptic hypothalamic nucleus, an area harboring vasopressinergic magnocellular neurons [8–10]. Strikingly, the sepsis-induced increase in cellular apoptotic markers expressed in vasopressinergic neurons coincides with the previously observed diminished systemic AVP secretion, suggesting that apoptosis of vasopressinergic neurons might be a primary cause for the diminished AVP secretion in the late phase of sepsis.
We, thus, hypothesized that the increase in IL-1, which is capable of stimulating the AVP release in the initial phase of sepsis, activates the iNOS-dependent NO production and, consequently, the strong expression of apoptotic genes in vasopressinergic neurons [8–10]. In a recent study [1], we observed that blocking the IL-1-IL-1r signaling pathway in the central nervous system by i.c.v. injection of IL-1ra, an antagonist of the IL-1 receptor, greatly reduced iNOS activity in the hypothalamus and diminished the NO concentration in CSF. More importantly, IL-1ra pre-treatment of septic rats caused a significant augmentation of AVP secretion in the late phase of sepsis. Even more strikingly, this modulation of sepsis-induced alterations in iNOS, NO, and AVP via blocking of the IL-1-IL-1r signaling pathway resulted in higher survival rate among septic rats. Nevertheless, the effects of IL-1ra on blocking the oxidative stress on the hypothalamus are still poorly understood. Therefore, in this study, we investigated the effect of IL-1ra pre-treatment on the sepsis-induced increase in IL-1, iNOS, CASP3, and HIF-1α transcript and protein levels in the hypothalamus of septic rats.
Material and Methods
Animals
All experiments in the present study were done in Wistar rats with an average body weight of 280 ± 30 g. The animals were obtained from the Central Animal Facility at the University of São Paulo, Campus at Ribeirão Preto, and they were housed in the same room under temperature- (25 ± 2 °C), and light- (06:00–18:00) controlled conditions in the animal facility of the School of Dentistry of Ribeirão Preto-USP. The animals were given free access to water and a commercial balanced rodent diet. All experiments in this study were carried out according to an Institutional Ethics Committee-approved protocol (CEUA protocol number: 12.1.1205.53.0).
Cecal Ligation and Puncture Surgery
Sepsis was induced in experimental rats by the surgical procedure of cecal ligation and puncture (CLP). We used this model because, like colon ascendents stent peritonitis (CASP), it has more similarity with human sepsis causing a polymicrobial infection and patterns of release of inflammatory mediators which differ from LPS injection [16].
Briefly, we gave to the animals an intraperitoneal injection (250 mg/kg) of tribromoethanol for induction of procedural sedation. A midline laparotomy was carried out under sterile conditions. The cecum was exposed, ligated below the ileo-cecal valve, and then punctured 10 times with a sterile 16-gauge needle. Subsequently, the abdominal cavity was closed by aseptic surgical suture. These rats were then given a subcutaneous injection of NaCl (0.9 %; 5 mL/250 g; body weight) solution to compensate for fluid loss during surgery. Control animals were either naive or sham-operated, whereby in the latter the cecum was manipulated but neither ligated nor punctured.
Working Solution of IL-1ra
Recombinant human IL-1ra (50 μg) was purchased from R&D systems (Minneapolis, MN, USA). Phosphate buffer saline (PBS), containing 0.1 % bovine serum albumin, was used to prepare stock and working solutions of IL-1ra.
Intracerebroventricular Cannulation
Under anesthesia induced by a mixture of ketamine and xylazine, a permanent 22-gauge stainless steel guide cannulae (0.7 mm OD, 10 mm long) was stereotaxically implanted into the right lateral ventricle. The position of the guide cannulae was 1.5 mm posterior to the bregma, 1.6 mm lateral to the midline, and 2.5 mm below the skull. The cannulae were fixed to the skull with screws and dental acrylic. All animals were given an antibiotic treatment after surgery, and they were allowed to recover for at least 5 days before experimentation. Animals showing misplaced or blocked cannulae, abnormal behavior, or abnormal patterns of weight gain were excluded from the study.
Experimental Protocol
Experimental rats were given an i.c.v. injection of IL-1ra (9 nmol in a total volume of 2 μL) or PBS (2 μL) as control or none (naive). After 15 min of IL-1ra or PBS injection, they were anesthetized with TBE and then sham-operated or subjected to CLP surgery. After 4 and 6 h (early phase of sepsis) or 24 h (late phase of sepsis), the rats were decapitated for brain removal. The hypothalamus was dissected from the brain and then stored at −70 °C until used for quantification of mRNA levels by qPCR and protein analysis by western blotting for IL-1, HIF-1α, cleaved casp-3, and iNOS.
Total RNA Extraction
The hypothalami were immersed in Trizol Reagent and processed according to the manufacturer’s instructions for extraction of total RNA. The concentration and purity of RNA were determined spectrophotometrically by absorbance readings at 260/280 nm.
cDNA Synthesis from Total RNA
Total RNA extracts from individual samples were used for cDNA synthesis by mixing with oligodT primers and Superscript II enzyme (Life Technologies). The mixture was incubated with RNase H to remove any remaining RNA. The cDNA was diluted with deionized water to a final concentration of 0.25 μg/μL and stored at −20 ° C.
Quantitative PCR
Quantitative analysis of mRNA for IL-1, iNOS, CASP3, HIF-1, and GAPDH was performed by real-time PCR using TaqMan technology. The primers for these genes were purchased from Life Technologies (Il1b-Rn00580432_m1, Casp3-Rn00563902_m1,Nos2-Rn00561646_m1,Hif1a-Rn00577560_m1,Gapdh-Rn01775763_g1). The reactions were performed in volumes of 20 μL, containing 2 μL cDNA, 10 μL TaqMan Fast Advance Master Mix, 1 μL TaqMan gene expression assay (Applied Biosystems), and 7 μL nuclease-free water. Fluorescence intensities of two standard deviations above the mean intensities of negative controls (without RNA) were considered for analysis. Expression of each gene was normalized using the expression of housekeeping gene: glyceraldehyde3-phosphate dehydrogenase (GAPDH) according to the 2−ΔΔCT method.
Western Blot Analysis
Hypothalamus tissue was placed in RIPA buffer (Sigma-Aldrich) with 10 % of diluted 1:10 protease inhibitor cocktail (P2714, Sigma-Aldrich) and 0.5 % of phenylmethylsulfonyl fluoride (PMSF, Sigma-Aldrich) 200 mM in methanol for homogenization by sonication. After shaking for 2 h on ice, the samples were centrifuged at 3500×g for 20 min at 4 °C to collect the supernatant. Total protein content was measured at 592 nm by means of a bicinchoninic acid (BCA) assay following the manufacturer’s instructions (BCA protein assay kit, Pierce). Equal amounts of total protein (50 μg) were diluted in 2× Laemmli buffer (Sigma-Aldrich) and separated by SDS-PAGE in a 10 % polyacrylamide gel (125 V for 1.5 h). Molecular mass markers of 10–245 kDa (Prism ultraprotein ladder, Abcam) were applied on the gel to visualize separation and transfer quality.
Following electrophoresis, the proteins were blotted onto a nitrocellulose membrane (0.45 μm; Millipore) in a tank blotting system. Western blotting was performed under a current of 200 V for 2 h in transfer buffer containing 20 % methanol. After remaining in blocking solution (5 % bovine serum albumin (BSA) in 0.1 M PBS with 0.2 % Tween 20 (Sigma-Aldrich) (PBS-T)) for 1 h, the membranes were rinsed in PBS-T and then incubated overnight under agitation at room temperature with specific antibodies diluted in PBS-T with BSA 1 %. For IL-1β detection, a rabbit polyclonal antibody (ab9787, Abcam) was used at a 1:500 dilution. iNOS detection was done with a rabbit polyclonal IgG antibody (ab153223, Abcam) at a 1:150 dilution and HIF-1α detection with a 1:500 diluted mouse monoclonal IgG (sc-53546, Santa Cruz Biotech). For cleaved caspase-3 detection, a rabbit monoclonal IgG antibody (9661S, Cell Signaling Tech) was used at a 1:200 dilution, and β-actin detection was done with a mouse monoclonal IgG antibody (sc-47778, Santa Cruz Biotechnology) at a 1:2000 dilution. Secondary HRP-conjugated antibodies (anti-rabbit (Abcam) and anti-mouse (Santa Cruz Biotechnology)) were diluted 1:10,000 in PBS-T supplemented with 1 % BSA and the membranes incubated under agitation for 2 h at room temperature. Membrane images were captured using a chemiluminescence reaction kit (Enhanced Chemiluminescence (ECL), GE Healthcare) and an image acquisition system (ChemiDoc MP System, BIO-RAD). ECL-detected protein bands were quantified by Image Lab™ software (BIO-RAD). The results were transformed into arbitrary units of optical density and expressed as their ratio to β-actin (internal control).
Statistical Analysis
All results are expressed as mean ± SEM. For statistical analysis, we used two-way ANOVA followed by appropriate post hoc tests for each analysis. P values of <0.05 were considered statistically significant in all cases.
Results
Hypothalamic IL-1, iNOS, HIF-1α, and CASP3 Gene Expression Following IL-1ra Pre-treatment
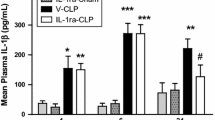
IL-1ra administration significantly decreased (P <0,05) IL-1 mRNA expression in CLP rats, at each time point when compared to vehicle-treated animals (Fig. 1). IL-1ra pre-treatment in CLP rats decreased iNOS mRNA expression when compared to vehicle-treated animals, only at 24 h. There was no significant difference in expression at different time points for any group of sham-operated animals. IL-1ra administration caused a significant decrease in HIF-1α mRNA levels at 4 and 24 h after CLP surgery compared to vehicle administration. CASP3 gene expression was strongly increased at each time point in vehicle-treated CLP animals as compared to sham-operated rats. However, the mRNA levels were significantly lower at 24 h in IL-1ra-treated CLP animals when compared to the vehicle-treated animals. There was no significant difference in IL-1, iNOS, HIF-1α, and CASP3 transcripts at the different time points in vehicle and IL-1ra-administered sham-operated control animals.
IL-1, iNOS, HIF-1α, and CASP3 transcript levels. a Administration of IL-1ra significantly (P < 0.05) decreased IL-1 mRNA expression at all time points in CLP animals as compared to vehicle treatment. b Administration of IL-1ra significantly (# P < 0.01) decreased iNOS mRNA expression at 24 h in CLP animals as compared to vehicle treatment. There was no significant difference between iNOS mRNA of IL-1ra and vehicle-treated rats at post-CLP 4 and 6 h. c Administration of IL-1ra significantly (P < 0.05) decreased HIF-1α mRNA expression at 4 and 24 h in CLP animals as compared to vehicle treatment at same time points. There was no significant difference between HIF-1α mRNA of IL-1ra and vehicle-treated rats at post-CLP 6 h. d Administration of IL-1ra significantly (# P < 0.05) decreased CASP3 mRNA expression at 24 h in CLP animals as compared to vehicle treatment at same time point. There was no significant difference between CASP3 mRNA of IL-1ra and vehicle-treated rats at post-CLP 4 and 6 h. There was no noteworthy effect of IL-1ra and vehicle treatments on the IL-1, iNOS, HIF-1α, and CASP3 mRNA expression in sham-operated animals at all time points. *P < 0.05, **P < 0.01, ***P < 0.001 difference of V-CLP against sham groups; the number sign represents a P < 0.05 difference of V-CLP against IL-1ra-CLP groups at 4, 6, and 24 h. Number of animals = 4 each column
Hypothalamic IL-1, iNOS, HIF-1α, and Cleaved Casp-3 Protein Levels Following IL-1ra Pre-treatment
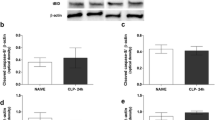
Hypothalamic protein levels of IL-1, iNOS, HIF-1α, and cleaved casp-3 at 4 and 24 h of IL-1ra and vehicle pre-treatment are shown in Fig. 2. IL-1 and iNOS protein levels were greatly increased at 24 h in vehicle-administered CLP animals, when compared to vehicle and IL-ra pre-treated naive and CLP rats at 4 h. Furthermore, IL-1ra administration resulted in significantly decreased (P < 0.05) IL-1 and iNOS expression in CLP rats as compared to vehicle treatment at 24 h. There was no effect of IL-1ra administration on IL-1 and iNOS expression at 4 h as compared to naive and vehicle treatment. Moreover, no significant effect of IL-1ra pre-treatment was observed on cleaved casp-3 and HIF-1α protein expression. AU = Arbitray Unity
iNOS, IL-1, HIF-1α, and cleaved casp-3 protein levels. a Image of a representative western blot showing the effect of IL-1ra pre-treatment on the expression of IL-1β and iNOS expression in naive, vehicle, and IL-1ra pre-treated CLP animals. Administration of IL-1ra significantly (P < 0.05) decreased IL-1β (b) and iNOS (c) protein expression at 24 h in CLP animals as compared to vehicle treatment. No significant effect was observed on HIF-1α (d) and cleaved casp-3 (e) protein expression. Number of animals = 5 each column
Discussion
Sepsis is a life-threatening condition, which develops as a result of an overwhelming systemic inflammatory response to infection [8, 17, 18]. It is marked by widespread alterations in major biochemical regulatory pathways. Several studies have reported alterations in IL-1, NO, and AVP signaling pathways during sepsis [1, 6, 8–10, 19–25]. In the current study, in agreement with results of previous reports, we also noted that the hypothalamic gene and protein expressions of IL-1 and iNOS are significantly increased during sepsis. Additionally, mRNA levels of hypothalamic HIF-1α and CASP3 were also greatly enhanced.
Several studies have implicated excessive hypothalamic production of IL-1 with neuroendocrine alterations seen in sepsis [8, 22]. Specifically, in a previous study, we had observed an increase in IL-1β, iNOS, and cytochrome c expression and in annexin V affinity in neurons of supraoptic nuclei during sepsis in rats, with all these parameters representing strong evidence that apoptosis may affect AVP synthesis and secretion [9]. More recently, in another study, we observed that central IL-1ra treatment resulted in augmentation in systemic AVP levels, accompanied by a reduction in hypothalamic iNOS activity and diminished CSF NO levels in the late phase of sepsis [1].
We designed the current study, to gain insights into the mechanism of how an IL-1ra administration affects AVP secretion and hypothalamic nitric oxide. We observed that the hypothalamic mRNA and protein levels of IL-1β and iNOS are lowered following the IL-1ra administration. Furthermore, we also observed a significant reduction in hypothalamic HIF-1α and CASP 3 transcript levels. As these genes have been extensively involved in inducing neuronal apoptosis [9, 10], our findings suggest that by causing a reduction of HIF-1α and CASP3 gene expression, IL-1ra administration leads to blockade of apoptotic pathways in hypothalamic vasopressinergic neurons.
The exact mechanism(s) by which IL-1 and NO are causing alterations in activities of vasopressinergic neurons during severe sepsis are still poorly understood. It has been noted that high NO concentrations cause metabolic hypoxia, which leads to impaired mitochondrial respiration through its competition with oxygen, and inhibits the transient and reversible cytochrome c oxidase [26, 27]. The impairment of mitochondrial respiration favors the generation of superoxide anions [27] and, therefore, of peroxynitrite and hydrogen peroxide (H2O2) [28], which in turn may further stimulate the expression/activity of iNOS [13] and hence increased NO production. Likewise, the state of hypoxia generated by the increase in NO can also induce the expression and stabilization of the HIF-1 subunits, thus leading to activation of the HIF-1 pathway during sepsis [29]. HIF-1 not only regulates the expression of several genes related to energy metabolism but also induces the expression of CASP3 and genes of the proapoptotic Bcl2 family [29, 30]. Moreover, HIF-1 can also be activated in other conditions, for example, when there is an increase in cytokines and growth factors like TNF, IL-1ß, and IGF-1 (insulin-like growth factor) [29], such as what happens in sepsis [9]. Therefore, our findings suggest that blocking the IL-1 pathway leads to decreased expression of IL-1 and iNOS, which in turn decreases the transcription of HIF-1 and CASP3 genes in vasopressinergic neurons of the hypothalamus. This condition may prevent apoptosis of vasopressinergic neurons and thus ameliorate the response of these neurons to the decrease in blood pressure and other sepsis-induced changes requiring an AVP response.
Previously, it has been shown that mutual communication between the immune system and the brain takes place at the circumventricular organs (CVOs), which are highly vascularized structures lacking a blood–brain barrier [11, 21, 31]. The vascular walls of the CVOs and perivascular glia constitutively express IL-1R1, which, when activated, induces IL-1β expression [11, 20]. Additionally in this context, IL-1β activates iNOS expression [11, 20], thus contributing to oxidative stress. In line with the increased expression of IL-1β and IL-1R1 seen in supraoptic neurons in previous work [9] and of IL-1 in the hypothalamus shown here, we observed that CLP-induced polymicrobial sepsis was accompanied by a progressively increased expression of the iNOS encoding gene until 24 h. IL-1ra administration blocked these pathways, which led to an attenuation of hypothalamus IL-1, iNOS, HIF-1α, and CASP3 expression preventing apoptosis and improving systemic AVP secretion.
In addition to the hypothalamus, sepsis-related oxidative stress and apoptosis have also been reported to occur in cardiovascular medullary autonomic regions of the brain as well as in areas related to cognitive functions, like the hippocampus [32, 33]. These results can explain, at least partially, the cardiovascular and cognitive dysfunctions seen during or following sepsis. Recently, a role for the microglia was reported in the development of cognitive impairment in septic rats, suggesting that neuronal damage seen in the hippocampus may be driven by these brain resident cells [34]. We presently conduct experiments on the role of microglial cells in the damage seen in hypothalamic neurons of our septic rats.
Moreover, as brain dysfunction can also alter neurotransmitter metabolism [35], neuropeptides like substance P, oxytocin, α-melanocyte-stimulating hormone, and neurotensin also deserve further attention. Higher levels of plasma neurotensin for example have been shown to result in lower survival rate of septic mice, while neurotensin antagonist and neurotensin null mice have high survival rates [36]. Hence, it should be interesting to investigate a possible IL-1ra effect on neurotensin concentrations in plasma and the brain. Similarly, IL-1ra effects on other cellular and biochemical mediators of sepsis should also be studied.
In conclusion, our results provide evidence that blocking of the IL-1/IL-1r signaling pathway by central administration of an IL-1r antagonist decreases oxidative stress in the hypothalamus in the late phase of sepsis, and this may be beneficial for survival. We believe that the mechanism for this effect of IL-1ra is through a reduction in the expression of IL-1, iNOS, HIF-1α, and CASP3 encoding genes in the hypothalamus of septic rats. As a consequence, this can favor vasopressin secretion and, thus, improve blood pressure and survival of animals.
References
Wahab F, Tazinafo LF, Cárnio EC, Aguila FA, Batalhão ME, Rocha MJ (2015) Interleukin-1 receptor antagonist decreases cerebrospinal fluid nitric oxide levels and increases vasopressin secretion in the late phase of sepsis in rats. Endocrine 49:215–221
Landry DW, Levin HR, Gallant EM, Ashton RC, Seo S, D’Alessandro D, Oz MC, Oliver JA (1997) Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation 95:1122–1125
Oliveira-Pelegrin GR, Ravanelli MI, Branco LG, Rocha MJ (2009) Thermoregulation and vasopressin secretion during polymicrobial sepsis. Neuroimmunomodulation 16:45–53
Sharshar T, Blanchard A, Paillard M, Raphael JC, Gajdos P et al (2003) Circulating vasopressin levels in septic shock. Crit Care Med 31:1752–1758
Athayde LA, Oliveira-Pelegrin GR, Nomizo A, Faccioli LH, Rocha MJ (2009) Blocking central leukotrienes synthesis affects vasopressin release during sepsis. Neuroscience 160:829–836
Corrêa PB, Pancoto JA, de Oliveira-Pelegrin GR, Cárnio EC, Rocha MJ (2007) Participation of iNOS-derived NO in hypothalamic activation and vasopressin release during polymicrobial sepsis. J Neuroimmunol 183:17–25
Pancoto JA, Corrêa PB, Oliveira-Pelegrin GR, Rocha MJ (2008) Autonomic dysfunction in experimental sepsis induced by cecal ligation and puncture. Auton Neurosci 138:57–63
Wahab F, Atika B, Oliveira-Pelegrin GR, Rocha MJ (2013) Recent advances in the understanding of sepsis-induced alterations in the neuroendocrine system. Endocr Metab Immune Disord Drug Targets 13:335–347
Oliveira-Pelegrin GR, Basso PJ, Rocha MJ (2014) Cellular bioenergetics changes in magnocellular neurons may affect copeptin expression in the late phase of sepsis. J Neuroimmunol 267:28–34
Oliveira-Pelegrin GR, Basso PJ, Soares AS, Martinez MR, Riester KD, Rocha MJ (2013) Cleaved caspase-3 expression in hypothalamic magnocellular neurons may affect vasopressin secretion during experimental polymicrobial sepsis. J Neuroimmunol 258:10–16
Wong ML, Bongiorno PB, Rettori V, McCann SM, Licinio J (1997) Interleukin (IL) 1beta, IL-1 receptor antagonist, IL-10, and IL-13 gene expression in the central nervous system and anterior pituitary during systemic inflammation: pathophysiological implications. Proc Natl Acad Sci U S A 94:227–232
Zapelini PH, Rezin GT, Cardoso MR, Ritter C, Klamt F, Moreira JC, Streck EL, Dal-Pizzol F (2008) Antioxidant treatment reverses mitochondrial dysfunction in a sepsis animal model. Mitochondrion 8:211–218
Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JC, Dal-Pizzol F (2004) Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 32:342–349
Bozza FA, D’Avila JC, Ritter C, Sonneville R, Sharshar T, Dal-Pizzol F (2013) Bioenergetics, mitochondrial dysfunction, and oxidative stress in the pathophysiology of septic encephalopathy. Shock 1:10–16
Dal-Pizzol F, Ritter C, Cassol-Jr OJ, Rezin GT, Petronilho F, Zugno AI, Quevedo J, Streck EL (2010) Oxidative mechanisms of brain dysfunction during sepsis. Neurochem Res 35:1–12
Rittirsh D, Hoesel LM, Ward PA (2007) The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol 81:137–143
Vincent JL, Korkut HA (2008) Defining sepsis. Clin Chest Med 29:585–590
Annane D, Bellissant E, Cavaillon JM (2005) Septic shock. Lancet 365:63–78
Kovács KJ (2002) Neurohypophyseal hormones in the integration of physiological responses to immune challenges. Prog Brain Res 139:127–146
McCann SM, Kimura M, Karanth S, Yu WH, Mastronardi CA, Rettori V (2000) The mechanism of action of cytokines to control the release of hypothalamic and pituitary hormones in infection. Ann N Y Acad Sci 917:4–18
Wong ML, Rettori V, al-Shekhlee A, Bongiorno PB, Canteros G, McCann SM, Gold PW, Licinio J (1996) Inducible nitric oxide synthase gene expression in the brain during systemic inflammation. Nat Med 2:581–584
Parrillo JE (1993) Pathogenetic mechanisms of septic shock. N Engl J Med 328:1471–1477
Oliveira-Pelegrin GR, Aguila FA, Basso PJ, Rocha MJ (2010) Role of central NO-cGMP pathway in vasopressin and oxytocin gene expression during sepsis. Peptides 31:1847–1852
Oliveira-Pelegrin GR, de Azevedo SV, Yao ST, Murphy D, Rocha MJ (2010) Central NOS inhibition differentially affects vasopressin gene expression in hypothalamic nuclei in septic rats. J Neuroimmunol 227:80–86
Gabellec MM, Griffais R, Fillion G, Haour F (1995) Expression of interleukin 1 alpha, interleukin 1 beta and interleukin 1 receptor antagonist mRNA in mouse brain: regulation by bacterial lipopolysaccharide (LPS) treatment. Brain Res Mol Brain Res 31:122–130
Brown GC, Cooper CE (1994) Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 356:295–298
Erusalimsky JD, Moncada S (2007) Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol 27:2524–2531
Ghafourifar P, Asbury ML, Joshi SS, Kincaid ED (2005) Determination of mitochondrial nitric oxide synthase activity. Methods Enzymol 396:424–444
Sharp FR, Bernaudin M (2004) HIF1 and oxygen sensing in the brain. Nat Rev Neurosci 5:437–448
Bruick RK (2000) Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci U S A 97:9082–9087
Rivest S, Lacroix S, Vallières L, Nadeau S, Zhang J, Laflamme N (2000) How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med 223:22–38
Sharshar T, Gray F, Lorin de la Grandmaison G, Hopkinson NS, Ross E, Dorandeu A, Orlikowski D, Raphael JC, Gajdos P, Annane D (2003) Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet 362:1799–1805
Comim CM, Barichello T, Grandgirard D, Dal-Pizzol F, Quevedo J, Leib SL (2013) Caspase-3 mediates in part hippocampal apoptosis in sepsis. Mol Neurobiol 47:394–398
Michels M, Vieira AS, Vuolo F, Zapelini HG, Mendonça B, Mina F, Dominquini D, Steckert A, Schuck PF, Quevedo J, Petronilho F, Dal-Pizzol F (2015) The role of microglia activation in the development of sepsis-induced long-term cognitive impairment. Brain Behav Immun 43:54–59
da Silva FP, Machado MC, Sallet PC, Zampieri FG, Goulart AC, Torggler Filho F, Velasco IT, da Cruz Neto LM, de Souza HP (2014) Neuropeptide downregulation in sepsis. Inflammation 37:142–145
Piliponsky AM, Chen CC, Nishimura T, Metz M, Rios EJ, Dobner PR, Wada E, Wada K, Zacharias S, Mohanasundaram UM, Faix JD, Abrink M, Pejler G, Pearl RG, Tsai M, Galli SJ (2008) Neurotensin increases mortality and mast cells reduce neurotensin levels in a mouse model of sepsis. Nat Med 14:392–398
Acknowledgments
We thank Nadir Martins for the technical assistant and Klaus Hartfelder for providing the infrastructure for the qPCR analysis and reviewing a prior version of the manuscript.
Funding
Financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) is gratefully acknowledged. Fazal Wahab was a recipient of a FAPESP post-doctoral scholarship.
Conflict of Interest
The authors declare that they have no competing interests.
Compliance with Ethical Standards
This study involves the use of rats. All animal experiments in this study were carried out according to an Institutional Ethics Committee-approved protocol (CEUA protocol number: 12.1.1205.53.0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wahab, F., Santos-Junior, N.N., de Almeida Rodrigues, R.P. et al. Interleukin-1 Receptor Antagonist Decreases Hypothalamic Oxidative Stress During Experimental Sepsis. Mol Neurobiol 53, 3992–3998 (2016). https://doi.org/10.1007/s12035-015-9338-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9338-4