Abstract
The developing brain is found highly vulnerable towards the exposure of different environmental chemicals/drugs, even at concentrations, those are generally considered safe in mature brain. The brain development is a very complex phenomenon which involves several processes running in parallel such as cell proliferation, migration, differentiation, maturation and synaptogenesis. If any step of these cellular processes hampered due to exposure of any xenobiotic/drug, there is almost no chance of recovery which could finally result in a life-long disability. Therefore, the developmental neurotoxicity (DNT) assessment of newly discovered drugs/molecules is a very serious concern among the neurologists. Animal-based DNT models have their own limitations such as ethical concerns and lower sensitivity with less predictive values in humans. Furthermore, non-availability of human foetal brain tissues/cells makes job more difficult to understand about mechanisms involve in DNT in human beings. Although, the use of cell culture have been proven as a powerful tool for DNT assessment, but many in vitro models are currently utilizing genetically unstable cell lines. The interpretation of data generated using such terminally differentiated cells is hard to extrapolate with in vivo situations. However, human umbilical cord blood stem cells (hUCBSCs) have been proposed as an excellent tool for alternative DNT testing because neuronal development from undifferentiated state could exactly mimic the original pattern of neuronal development in foetus when hUCBSCs differentiated into neuronal cells. Additionally, less ethical concern, easy availability and high plasticity make them an attractive source for establishing in vitro model of DNT assessment. In this review, we are focusing towards recent advancements on hUCBSCs-based in vitro model to understand DNTs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to increased industrialization in modern era, the chances of exposure to different known/unknown chemicals have been increased exponentially. Adults, children and developing foetus are routinely being exposed to variety of environmental chemicals including pollutants, drugs and new chemical entities (NCEs). Exposed people may not have any detrimental effect during short-term period, but it could have serious consequences during long-term exposure because of different systemic toxicities including neurotoxicity. The developing brain of child as well as of foetus has been found much more vulnerable towards the exposure of different environmental xenobiotics including organophosphate pesticides [1, 2]. The developing brain of foetus/newborn is always at higher risk against the exposure of environmental chemicals because of underdeveloped placental barrier as well as blood-brain barrier [3–5]. The high lipid contents and lower regeneration/post-mitotic nature of neurons work as oil in the fire and may enhance oxidative stress-mediated cell death after the exposures of environmental contaminants. The brain development is a very complex process which involves several other processes running in parallel such as cell proliferation, migration, homing, differentiation and synapse formation in highly regulated manner [6, 7]. Hampering any step at any stage of these cellular processes due to the exposure of xenobiotics/drugs/NCEs could lead to the life-long permanent disability [8–10]. Plethora of literature is available on public domain showing the susceptibility of developing human brain towards many toxicants/ chemicals and further development of neurological deficits/disorders [6–8]. Thus, the developing brain is much more critical towards the exposure of environmental chemicals in respect to developed adult brain which may not have severe consequences. Many studies of developmental neurotoxicity (DNT) involved traditional in vivo models using large number of experimental animals. Due to rapid industrialization, numbers of new chemicals are exponentially increasing in our ecosystem which needs to be tested precisely in term of developmental neurotoxicity in respect of animal welfare. For the assessment of developmental neurotoxicity of these environmental chemicals, in vivo animal models are not suitable as animal testing is complex, time consuming, costly and requires considerably high numbers of laboratory animals. To address the issue, 3Rs (reduce, refine and replace) concept has been adopted to develop alternative in vitro models which could reduce and refine the animal usage for rapid DNT evaluation. Moreover, the data generated through such in vivo animal studies are very difficult to recapitulate and extrapolate to human beings. Furthermore, the non-availability of human fetal brain tissue due to strict ethical problem makes this field very difficult and challenging. Therefore, to address these issues, many in vitro models derived from brain cells have been used for the assessment of neurotoxicity/developmental neurotoxicity which have shown less ethical dubious and provided more predictive and sensitive tool for functional studies at both cellular and molecular levels [9–11]. The major advantage of these cell-based in vitro models is their ability to reproduce various complex stages of brain development at cellular and molecular level. The literature is full of reports showing the use of these types of in vitro models for neurotoxicity/DNT studies involving different neuronal cell lines namely, rat pheochromocytoma PC12 cells [12–14], human neuroblastoma-SH-SY5Y cells [15–18], primary cultured neuronal/glial cells [19, 20], rat cerebellar granule cells [21–24] and cortical neurons [25, 26] to understand cellular and molecular mechanism of DNTs [27–31]. The established cell lines propagate rapidly and provide a homogenous population of cells which can be differentiated into neuronal-like cells by using various growth factors/neurotrophins. The only concern remains here is that these in vitro models utilizing cell lines which are genetically instable and terminally differentiated cells. Because of genetic instability, these cell line-based in vitro models may have different physiological outcomes after the exposure to toxicants in comparison to in vivo situations, and terminally differentiated nature ceases them to mimic the accurate evolutionary differentiation process. Thus, it is very difficult to extrapolate data generated through such in vitro models with the data generated by animals under in vivo situations. On the other side, the use of primary cultures of human neuronal/glial cells is hampered because of non-availability of developing/mature human brain tissues. In addition, primary cultures also contain post-mitotic neurons and have relatively limited lifespan [11, 32].
Stem cells are known to have self-renewal capability, long-term proliferation and plasticity potential towards the development of variety of cell types including brain neuronal and glial cells. Therefore, stem cells from different sources are particularly suited to study DNTs [32–36]. In the last few decades, establishment of stem cell-based in vitro model systems for DNT assessment has been the subject of high thrust in all over the world. Thus, we can say that stem cells are better promising and unparalleled tools for developing unique in vitro model systems to study developmental neurotoxicity. Theoretically, data generated from these in vitro model systems will be free from different concerns raised because of the genetic instability and terminal differentiation. Furthermore, it is easy to extrapolate the data generated employing human stem cells to predict/anticipate DNTs in human beings. Various types of stem cells based on their sources viz., embryonic stem cells [37–40], neural stem cells [41–43], bone marrow stem cells [44, 45] and umbilical cord blood stem cells [33–36, 43, 46–49] are being explored to develop alternative in vitro models for developmental neurotoxicity (DNT). The present review summarizes about the latest advancements/progresses achieved in the development and validation of human umbilical cord blood stem cells based in vitro models to evaluate the developmental neurotoxicity and their possible application in therapeutic pre-screening of various environmental chemicals, toxicants, pesticides and drugs.
Umbilical Cord Blood has Diverse Population of Stem Cells
Human umbilical cord blood (hUCB) is a perfect and one of the thriving source of haematopoietic stem cells (HSCs), non-haematopoietic stem cells (non-HSCs) and progenitor cells [48–56] and does not have any ethical concern as placenta is generally discarded after the birth of child. Therefore, the use of cord blood stem cells is non-controversial, very cheap and the most suitable biological material for DNT study. The non-invasive collection makes it comparatively cheaper and wonderful tool for establishing in vitro models of DNT [57]. Furthermore, it is very easy to grow these hUCB-HSCs, non-HSCs, mesenchymal stem cells (MSCs) and multipotent progenitor cells under in vitro conditions in undifferentiated state without any major loss in the pluripotency potential. In order to determine optimal conditions for in vitro expansion of human umbilical cord blood stem cells (hUCBSCs), researchers have tried various types of culture medium along with different permutation combinations of various cytokines, growth factors and physical parameters [58–61]. Various growth factors such as basic fibroblast growth factor (bFGF), stem cells factor (SCF), thrombopoietin (TPO), Fms-like tyrosine kinase-3 ligand (Flt-3 ligand), insulin-like growth factors (IGF-1&2) and cytokines (IL-3, IL-6, G-CSF, GM-CSF) have been found to be promising factors for efficient expansion of hUCBSCs specifically HSCs. These growth factors and cytokines are known to play key role in early haematopoiesis and prolonged undifferentiated proliferation of stem cells. TPO and Flt-3 ligand are known to be critical to regulate early proliferation and suppress apoptosis and ageing in hUCB-HSCs as well as in progenitor cells during in vitro culture conditions. Stem cell factor interacts with specific c-kit receptor and triggers signalling cascade to promote haematopoiesis and stemness and also maintains suitable microenvironment of haematopoietic stem cells and progenitor cells. Basic fibroblast growth factor (bFGF) significantly reduces cellular senescence and promotes stem cell self-renewal and differentiation [58, 62–68]. The protocols for isolation and long-term proliferation of hUCBSCs have been well established now.
Human umbilical cord blood-derived HSCs/non-HSCs as well as progenitor cells have high commitment towards different specialized lineages including brain cells of ectodermal origin. Buzanska and colleagues did pioneer work in this area and successfully isolated, characterized and differentiated these hUCBSCs into neuronal cells [46]. We and others have also successfully purified primitive HSCs (CD34+/CD133+/Thy1+) and CD34−/CD133− non-HSCs from whole human umbilical cord blood [34–36, 46, 48, 49]. We have reported the prolonged maintenance and substantial expansion of these human cord blood-derived CD34+/Thy1+ primitive HSCs, which also having extensive self-renewal, long term proliferation capacity and clonogenic capabilities. hUCBSCs express pluripotency markers such as Oct-3/4, Sox-2, Nanog and c-Myc which are usually expressed in pluripotent embryonic stem cells and thought to play key roles in maintaining pluripotency and self-renewal capabilities [49, 69–71]. Similarly, we also found high expression of these pluripotency markers namely, Oct-3/4, Sox-2, Nanog and c-Myc in CD34+/Thy1+ hUCB-HSCs, and moreover, the expression levels of these pluripotency markers were reduced during the differentiation of these hUCBSCs into neuronal cells [34–36]. These purified and characterized populations of hUCBSCs have great plasticity potential towards various specialized cell types of all three germ layers [72]. Although hUCB-HSCs are categorized as pluripotent stem cells, various other types of non-stem cell population are also present in the whole cord blood and must purify before carrying out to achieve quality differentiation for error free DNT studies. Additionally, human umbilical cord blood has been well accounted to contain a rich population of mesenchymal stem cells (MSCs) expressing many specific cell surface markers namely, CD29, CD44, CD90, CD105 and CD273 [73–76]. The connective tissue layer of human umbilical cord, Wharton’s jelly, is also a copious source of MSCs [77, 78]. The plasticity potential of MSCs is incomparable with umbilical cord blood stem cells as MSCs cannot be differentiated into various cell types of all three germ layers [79], but these cells have high potential to differentiate into neuronal cells under the influence of various neurogenic growth factors/neurotrophins [79–86]. Pluripotency of hUCBSCs could also have been enhanced by transfecting these cells with pluripotency-associated transcription factor genes namely, Sox-2, Oct-4, Klf-4 and c-Myc [87, 88]. Buzanska and colleagues successfully established hUCB-derived neural stem cell (NSC) line having the ability of higher growth, self-renewal capacity and plasticity potential towards neural cells [33, 46]. Our group has also demonstrated that Wnt/GSK3β/β-catenin signalling play a crucial role in the normal proliferation and maintenance of hUCBSCs, and pesticide-challenged cells rapidly enter into apoptosis [89]. Thus, easier accessibility of placental tissue/blood, diverse proliferating population and enormous plasticity makes these haematopoietic stem cells a very powerful tool to study developmental neurotoxicity of various xenobiotics, toxicants, pesticides and NCEs.
Human Umbilical Cord Blood Stem Cells Easily Differentiates into Neuronal Cells
Although embryonic stem cells have the maximum pluripotency power as well as plasticity potential, but they also have their own limitations and have been avoided for transplantation purposes or for other DNT studies. The main problems associated with the use of embryonic stem cells are ethical, religious and political as they represent a complete embryo. Additionally, unrestricted cell growth of these embryonic stem cells could lead to the formation of teratomas even after the differentiation into specific cell types [90, 91]. Neural stem cells derived from specific brain region of human may be the best source for developmental neurotoxicity as these cells do not have intergenomic epigenetic variations due to similar genetic material; however, this application is limited due to ethical problem and the least regenerative power of brain tissue [92]. These limitations are major hurdles to use human embryonic or neural stem cells for creating novel alternative in vitro model for DNT studies. Alternatively, hUCBSCs could serve the purpose and have been proved to be the most promising in vitro tools for DNT studies [33–36, 47]. There is no concern about teratoma formation in the neuronal cells derived from hUBSCs and also have almost no ethical concern. Moreover, these cells are considered as one of the most enriched source of stem cells [48, 49, 53]. The non-invasive collection method makes it excellent tools to study developmental neurotoxicity [34–36, 48, 54, 57].
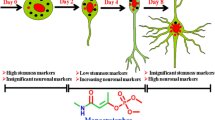
Pluripotent stem cells derived from human umbilical cord blood have similar potential of neuronal differentiation as neural stem cells derived from foetus [34–36, 93–99]. Neurons derived from these hUCBSCs have expression of different early and mature neuronal markers namely, nestin, musashi-1, nectin, neuronal nuclei (NeuN), post-synaptic density protein 95 (PSD95), synaptophysin (SYP), β-III tubulin (TUJ-1), growth-associated protein 43 (GAP43), various forms of neurofilaments (NF), neurotrophic growth factors and neuron-specific receptors N-methyl-d-aspartate (NMDA) and γ-aminobutyric acid (GABA) [34–36, 38, 53, 86, 94, 100–107]. Our group have also successfully isolated, maintained and differentiated hUCB-derived CD34+ HSCs into neuronal cells using nerve growth factor (NGF) and trans retinoic acid in serum free neurobasal medium. These differentiated cells displayed a typical neuron-like morphology and expressed significantly various early-stage, mid-stage and mature neuronal markers namely, nestin, synaptophysin, neuronal nuclei, PSD95, NFM, NFH, TUJ-1, MAP2, GAP43, PSA-NCAM, acetyl cholinesterase, neuron-specific receptors such as AMPA receptor, NMDA receptor (NR2A), neurogenesis transcription factor CREB and neuron-associated growth factors such as NGF and BDNF, etc. (Table 1). Moreover, we observed the decreasing expression of stem cell and pluripotency markers namely, CD34, CD133, c-MYC, OCT3, SOX2, Nanog and SHH throughout the neuronal differentiation [34–36].
In addition, hUCB-MSCs have also been extensively studied for neurogenic potentials [105, 81, 108–111]. Recently, Zhang and colleagues [76] used condition medium-constituted olfactory ensheathing cells for the differentiation of mesenchymal stem cells into neuronal cells. These neuron-like cells were positive for neuron-specific enolase and having similar neuronal electrophysiological properties. Other groups have also demonstrated similar electrophysiological properties in differentiated neurons derived from hUCB pluripotent stem cells and MSCs [103, 112]. More specifically, hUCB-derived MSCs and other multipotent stem cells can also be differentiated into more specific neuronal cells like dopaminergic neurons which have higher expression of specific markers namely, DAT, TH, Nurr1, Pitx3 and dopamine transporter proteins [84, 85]. These types of cells can serve as an alternative regenerative medicine against Parkinson’s disease. Similar kind of study on hUCB non-haematopoietic multipotent stem cells showed differentiation of these cells into cortical GABAergic neurons with upregulated expression of GABAergic regulatory enzymes and transcription factors namely, MASH1 and DLX1 & 2 [86]. Even unspecified mononuclear cells derived from hUCB have capability to express neuronal markers namely, Musashi-1 and TUJ-1 and GFAP under the influence of specific growth factors/neurotrophins [113]. Seo and Cho have observed that differentiation of MSCs into neuronal cells induced the secretion of numerous trophic factors which can modulate different cellular processes such as neurogenesis, inflammation, angiogenesis and apoptosis [114].
Neurotrophins play an important role in the neuronal differentiation of hUCBSCs. Neurotrophin and NGF play very critical roles in the survival, maintenance and differentiation of sympathetic and sensory neuronal pathways. Binding of NGF to transmembrane tyrosine kinase receptor (TrkA) facilitates receptor dimerization and phosphorylation at cytoplasmic site which further facilitates phosphorylation of cytoplasmic adaptor protein (Shc) and initiates cell survival through AKT/MAPK pathway which suppresses c-JUN through CREB. NGF plays an important role in maintaining body haemostasis as binding of NGF/pro-NGF with lower affinity to p75NTR receptor could also lead to either survival through NF-kB or cell death through c-Jun N-terminal kinase activation [115]. It is well known that BDNF induces phosphorylation of MAPK/ERK and β-catenin through tropomyosin receptor kinase B (TrkB receptor) and triggers PI3K/AKT-dependent signalling pathways to stimulate neural differentiation and cell survival of hUCB-HSCs and MSC-derived neuronal cells [81, 89, 108]. We and others have described the importance of neurotrophins in the neuronal differentiation from hUCBSCs [34, 36]. Several studies showed that neurotrophins are induced endogenously during neuronal differentiation and make this process more viable [34, 36, 116]. Hafizi and colleagues have demonstrated the role of neuro miRNAs (mir-9 and mir-124) which played critical roles in differentiation of neuronal cells from CD133+/CD34+ hUCB-HSCs. Micro RNAs (miRNAs) could also play an important role in hUCBSC-derived in vitro DNT models and may precisely describe mechanism of developmental neurogenesis process [99]. Overall, the protocols are well established for the differentiation of stem cells into neural cells and more specific brain cells such as glial cells [117], cortical GABAergic neurons [86] and dopaminergic neurons [84, 85]. Thus, the morphological and physiological differentiation of hUCBSCs into neuronal cells in time-specific manner could serve as suitable in vitro model system for DNT studies without involving other issues related to ethics, cost and time.
Use of hUCBSc in the Development of In Vitro Model for Developmental Neurotoxicity
To take all the benefits into account, extensive efforts are being made worldwide to develop in vitro models for DNT studies by using hUCBSCs. Although, initially, these approaches seem very costly in terms of isolation, purification and maintenance of hUCBSCs, but once established, then we can work on relatively cheaper alternatives like replacement of neurotrophins with conditioned medium from specific neuronal cells [85, 118]. We and others have successfully proved that umbilical cord blood stem cells worked as in vitro tool to study DNTs of different chemicals/pesticides, and our studies also revealed neuronal cells derived from hUCBSCs have almost parallel expression of neuronal markers as reported during neurogenesis in foetus. We were able to differentiate hUCBSCs into neuronal cells in time-dependent manner and also characterized different stages of maturity namely, day 2, day 4 and day 8 by high throughout TaqMan low-density array-based real-time quantitative PCR and western blotting [34, 36]. We validated our differentiation process by observing decrease in the levels of different pluripotency markers namely, CD133, MYC, NANOG, SHH, KLF4, SOX2, POU5F1 and FRAP1as well as upregulation in the levels of different neuronal markers namely, NGF, BDNF, NFM, MBP, NFH, NCAM, STAT4, CHRM2 and NR42A during the progression of differentiation process (Table 1). An early increased and later downregulated level of early differentiation marker protein nestin further confirmed our differentiation process [34, 36]. Results were clearly indicating that hUCBSCs successfully differentiated into neuronal cells which could serve as novel in vitro model for developmental neurotoxicity studies. We divided differentiation process into four stages, undifferentiated, early differentiated, mid-differentiated and fully differentiated stages. We exposed these differentially differentiated cells with subtoxic doses of organophosphate pesticide monocrotophos (MCP) for a very short time, 3 h for transcriptional and 6 h for translational changes. We rule out the results of undifferentiated hUCBSCs in DNT studies because of their non-neuronal and different origin. We found higher damage in early and mid-differentiated neuronal cells with respect to fully mature neuronal cells [36]. Instead of us, several other groups also worked in this direction of developmental neurotoxicity and established the role of hUCBSCs for the assessment of DNTs of various environmental chemical entities [33–36, 43, 46, 47, 49, 102]. The researchers are also trying to establish 3D cultures of nervous system by using these hUCBSCs which seem to be the most promising and realistic in vitro model to study developmental neurotoxicity in human [33, 46, 119]. There is high probability of in vivo mimicking for developmental neurotoxicity of chemicals in in vitro 3D conditions compared to in vitro 2D conditions. Even hUCB-NSCs which were grown in bioengineered surface may have better comparable results to human being than in vivo animal data due to greater cell to cell interactions, controlled geometry and spatial distribution of the cells on the surface. This type of in vitro model of differentiating cells has been validated by exposing cells to known neurotoxicant MeHgCl [119]. Differentiated neuronal cells derived from hUCB-NSC line have been used for robust neurotoxicity assessment of a broad range of neurotoxic compounds of different categories [33]. Our data from hUCBSC-based in vitro model systems showed that MCP, a known organophosphorus pesticide, significantly altered neuron-specific MAPKs, oxidative stress, metabolism, apoptosis, neuronal and stem cell markers in early and mid-differentiation neurons. These differentiated and well-characterized neuronal cells were showing depleted dopaminergic and cholinergic receptors after the exposure of MCP, a known developmental neurotoxicant [36]. Additionally, our group did pioneer work on complete profiling of xenobiotic-metabolizing cytochrome P450s to establish developmental stage-specific bio-markers of exposure and effects using hUCB-CD34+ cell-derived differentiating neuronal cells. We have also reported that human HSC-derived developing neuronal cells expressed xenobiotic-metabolizing cytochrome P450s (CYP1A1, 2B6, 2E1 and 3A4), related receptor regulators (AHR, CAR and PXR) and phase II metabolizing enzyme GSTP1-1 during all the maturity periods. Furthermore, we also characterized responsiveness/functionalization of these CYPs using known inducers and inhibitors of CYPs along with neurotoxicant MCP [34]. Overall, results were not different from our other study that concluded early-stage differentiating neurons were more vulnerable towards toxicant compared to fully mature neurons. Thus, the HSC-derived developing neurons could be a homogenous in vitro tool to predict human-specific developmental neurotoxicity against various environmental chemicals and drugs (Fig. 1) [34, 36]. Recently, we explored the effect of 3-methylcholanthrene (MC), polycyclic aromatic hydrocarbon, on hUCB-HSC-derived developing neurons and reported stage-specific molecular mechanism of developmental neurotoxicity. Our findings suggest that MC significantly induces the expression and activity of AHR, CYP1A1 and GSTP1-1 and reduces the expression of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-d-aspartate (NMDA) receptors as well as cAMP response element binding protein (CREB). Additionally, MC hinders phosphorylation of neurogenesis transcription factor CREB through activating AHR and interferes with neuronal transmission which could lead to impaired neurogenesis/brain functions during brain development in neonates [35].
A general approach to show the use of human umbilical cord blood stem cell (hUCBSC)-derived neuronal cell-based in vitro model to study developmental neurotoxicity The applicability of hUCBSC-derived differentiating neuronal cell-based in vitro model to assess the developmental neurotoxicity of chemicals/drugs/xenobiotics/NCEs is unparalleled. Umbilical cord blood could be used as an enriched source for the isolation of pluripotent haematopoietic stem cells. During their neuronal differentiating, these cells may be exposed to unknown chemicals/drugs/xenobiotics/NCEs, and the effects of these compounds can be assessed by studying different markers involved in cell proliferation, neuronal differentiation, neuronal injuries and receptors at various stages of neuronal maturity such as days 2, 4 and 8. These neuronal cells derived from human umbilical cord stem cells can be used as a powerful tool to assess the developmental neurotoxicity in human beings
Conclusion
The Health Effect Test Guidelines OPPTS 8706300US by US Environmental Protection Agency (EPA, USA) and New OECD DNT Test Guidelines 426 (OECD, 2007) by Organization for Economic Cooperation and Developments mainly recommend animal use for developmental neurotoxicity studies in standard adult and developing animals. However, there is huge pressure on industries, education centres and regulatory bodies to develop methods for efficient screening of large number of chemicals/xenobiotics which may have danger of ‘silent pandemic’ or unknown adverse effects on developing brain of children. Experts from all over the world are giving more emphasis to develop in vitro models for DNT studies due to efficient screening by high throughput nature, lower cost, less assessment time and higher reliability. The preliminary screening from these in vitro models could refine animal number for such type of developmental neurotoxicity studies. First of all, there is need to define/develop new models/methods which could be used to explore the effect of different drugs/chemicals on developing nervous system afterword these alternative models/methods can be further recruited according to regulatory guidelines and requirements. Even before their regulatory acceptance, these models can prioritize different chemicals for in vivo developmental neurotoxicity assessment. We have to cover a long way before finding a suitable in vitro model who fulfills the entire requirements for integrating and interpreting developmental neurotoxicity data with respect to human beings. There should be a general understanding between regulatory agencies and scientists to know about limitations of each other in the development of these high throughputs in vitro model systems. These models should mimic the evolutionary conserved neurodevelopmental processes which make them mechanistically more relevant to human developmental neurotoxicity. Furthermore, these models should be able to decode the mechanism based on altering the cell to cell/organ to organ interactions and should be free from any ethical concern. To fulfil these requirements of least genomic variations, unavailability of human tissue and less ethical concern, neuronal cells derived from human umbilical cord blood seem to work as a powerful tool for the development of high throughput in vitro model to study developmental neurotoxicity. Human umbilical cord blood is imperishable and affluent source of haematopoietic, non-haematopoietic and progenitor stem cells. High preserving cost to preserve human umbilical cord makes it invaluable to ordinary peoples but works as positive thrust for research/industrial applications. The non-invasive collection methods, easy proliferation and high plasticity make it perfect to use for developmental toxicity studies including developmental neurotoxicity. Above all, hUCBSCs could be used for drugs/xenobiotics screening based on in vitro models of homologous cells as well as autologous cells which spur interest in designing a feedback validation process. Human umbilical cord blood derived haematopoietic, non-haematopoietic and mesenchymal stem cells as well as progenitor cells have the capability of self-renewal, long-term proliferation and easy differentiation into specific cells, and these properties make hUCBSCs as gold standard tool for establishing in vitro models of DNT. The differentiation of hUCBSCs is not only limited to nervous system but these cells can also give rise to many other cell types of different organs such as heart, kidney, retina, gut, bone, etc. and opens the new door to work on developmental toxicity for these organ too. Therefore, human umbilical cord blood stem cells have great potential to work as a fundamental tool for developing unique in vitro model system of developmental system toxicity including neurotoxicity to test broad spectrum of drugs/chemicals which almost seems impossible using animal-based in vivo model system. Models based on 3D growth of neuronal cells derived from umbilical cord blood stem cells have potential to decode the cell to cell/organ to organ interaction-based mechanism for developmental neurotoxicity. Initially, these researches may seem very costly, but it will become very cost-effective and useful after the development of suitable high throughput in vitro model(s) for developmental neurotoxicity.
References
Tilson HA (2000) Neurotoxicology risk assessment guidelines: developmental neurotoxicology. Neurotoxicology 21(1–2):189–194
Bal-Price AK, Hogberg HT, Buzanska L, Lenas P, van Vliet E, Hartung T (2010) In vitro developmental neurotoxicity (DNT) testing: relevant models and endpoints. Neurotoxicology 31(5):545–554
Stringari J, Nunes AK, Franco JL, Bohrer D, Garcia SC, Dafre AL, Milatovic D, Souza DO et al (2008) Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol Appl Pharmacol 227(1):147–154
Bridges CC, Joshee L, Zalups RK (2009) Effect of DMPS and DMSA on the placental and fetal disposition of methylmercury. Placenta 30(9):800–805
Ek CJ, Dziegielewska KM, Habgood MD, Saunders NR (2012) Barriers in the developing brain and neurotoxicology. Neurotoxicology 33(3):586–604
Rodier PM (1994) Vulnerable periods and processes during central nervous system development. Environ Health Perspect 102(Suppl 2):121–124
Rice D, Barone S Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108(Suppl 3):511–533
Johri A, Yadav S, Dhawan A, Parmar D (2008) Responsiveness of cerebral and hepatic cytochrome P450s in rat offspring prenatally exposed to lindane. Toxicol Appl Pharmacol 231(1):10–16
Powers CM, Wrench N, Ryde IT, Smith AM, Seidler FJ, Slotkin TA (2010) Silver impairs neurodevelopment: studies in PC12 cells. Environ Health Perspect 118(1):73–79. doi:10.1289/ehp.0901149
Powers CM, Badireddy AR, Ryde IT, Seidler FJ, Slotkin TA (2011) Silver nanoparticles compromise neurodevelopment in PC12 cells: critical contributions of silver ion, particle size, coating, and composition. Environ Health Perspect 119(1):37–44. doi:10.1289/ehp.1002337
Costa LG, Giordano G, Guizzetti M (2011) In vitro neurotoxicology: an introduction. Methods Mol Biol 758:1–9. doi:10.1007/978-1-61779-170-3_1
Kashyap MP, Singh AK, Siddiqui MA, Kumar V, Tripathi VK, Khanna VK, Yadav S, Jain SK et al (2010) Caspase cascade regulated mitochondria mediated apoptosis in monocrotophos exposed PC12 cells. Chem Res Toxicol 23(11):1663–1672. doi:10.1021/tx100234m
Kashyap MP, Singh AK, Kumar V, Tripathi VK, Srivastava RK, Agrawal M, Khanna VK, Yadav S et al (2011) Monocrotophos induced apoptosis in PC12 cells: role of xenobiotic metabolizing cytochrome P450s. PLoS One 6(3):e17757. doi:10.1371/journal.pone.0017757
Slotkin TA, Card J, Seidler FJ (2012) Chlorpyrifos developmental neurotoxicity: interaction with glucocorticoids in PC12 cells. Neurotoxicol Teratol 34(5):505–512
Tripathi VK, Kumar V, Singh AK, Kashyap MP, Jahan S, Pandey A, Alam S, Khan F et al (2014) Monocrotophos induces the expression and activity of xenobiotic metabolizing enzymes in pre-sensitized cultured human brain cells. PLoS One 9(3):e91946. doi:10.1371/journal.pone.0091946
Kashyap MP, Singh AK, Yadav DK, Siddiqui MA, Srivastava RK, Chaturvedi V, Rai N (2015) 4-Hydroxy-trans-2-nonenal (4-HNE) induces neuronal SH-SY5Y cell death via hampering ATP binding at kinase domain of Akt1. Arch Toxicol 89(2):243–258. doi:10.1007/s00204-014-1260-4
Li IH, Ma KH, Weng SJ, Huang SS, Liang CM, Huang YS (2014) Autophagy activation is involved in 3,4-methylenedioxymethamphetamine (‘ecstasy’)-induced neurotoxicity in cultured cortical neurons. PLoS One 9(12):e116565. doi:10.1371/journal.pone.0116565
Wan Z, Mah D, Simtchouk S, Kluftinger A, Little JP (2015) Human adipose tissue conditioned media from lean subjects is protective against H2O2 induced neurotoxicity in human SH-SY5Y neuronal cells. Int J Mol Sci 16(1):1221–1231
Kapoor N, Pant AB, Dhawan A, Dwievedi UN, Seth PK, Parmar D (2006) Cytochrome P450 1A isoenzymes in brain cells: expression and inducibility in cultured rat brain neuronal and glial cells. Life Sci 79(25):2387–2394
Kapoor N, Pant AB, Dhawan A, Dwievedi UN, Seth PK, Parmar D (2007) Differences in the expression and inducibility of cytochrome P450 2B isoenzymes in cultured rat brain neuronal and glial cells. Mol Cell Biochem 305(1–2):199–207. doi:10.1007/s11010-007-9544-0
Pearce IA, Cambray-Deakin MA, Burgoyne RD (1987) Glutamate acting on NMDA receptors stimulates neurite outgrowth from cerebellar granule cells. FEBS Lett 223(1):143–147
Costa LG, Fattori V, Giordano G, Vitalone A (2007) An in vitro approach to assess the toxicity of certain food contaminants: methylmercury and polychlorinated biphenyls. Toxicology 237(1–3):65–76
Kane CJ, Chang JY, Roberson PK, Garg TK, Han L (2008) Ethanol exposure of neonatal rats does not increase biomarkers of oxidative stress in isolated cerebellar granule neurons. Alcohol 42(1):29–36
Radio NM, Freudenrich TM, Robinette BL, Crofton KM, Mundy WR (2010) Comparison of PC12 and cerebellar granule cell cultures for evaluating neurite outgrowth using high content analysis. Neurotoxicol Teratol 32(1):25–35
Zurich MG, Eskes C, Honegger P, Berode M, Monnet-Tschudi F (2002) Maturation-dependent neurotoxicity of lead acetate in vitro: implication of glial reactions. J Neurosci Res 70(1):108–116. doi:10.1002/jnr.10367
van Vliet E, Morath S, Eskes C, Linge J, Rappsilber J, Honegger P, Hartung T, Coecke S (2008) A novel in vitro metabolomics approach for neurotoxicity testing, proof of principle for methyl mercury chloride and caffeine. Neurotoxicology 29(1):1–12
Jenkins SM, Ehman K, Barone S Jr (2004) Structure-activity comparison of organotin species: dibutyltin is a developmental neurotoxicant in vitro and in vivo. Brain Res Dev Brain Res 151(1–2):1–12. doi:10.1016/j.devbrainres.2004.03.015
Jameson RR, Seidler FJ, Qiao D, Slotkin TA (2006) Chlorpyrifos affects phenotypic outcomes in a model of mammalian neurodevelopment: critical stages targeting differentiation in PC12 cells. Environ Health Perspect 114(5):667–672
Lau K, McLean WG, Williams DP, Howard CV (2006) Synergistic interactions between commonly used food additives in a developmental neurotoxicity test. Toxicol Sci 90(1):178–187
Radio NM, Mundy WR (2008) Developmental neurotoxicity testing in vitro: models for assessing chemical effects on neurite outgrowth. Neurotoxicology 29(3):361–376
Slotkin TA, Seidler FJ (2009) Oxidative and excitatory mechanisms of developmental neurotoxicity: transcriptional profiles for chlorpyrifos, diazinon, dieldrin, and divalent nickel in PC12 cells. Environ Health Perspect 117(4):587–596. doi:10.1289/ehp.0800251
Breier JM, Gassmann K, Kayser R, Stegeman H, De Groot D, Fritsche E, Shafer TJ (2010) Neural progenitor cells as models for high-throughput screens of developmental neurotoxicity: state of the science. Neurotoxicol Teratol 32(1):4–15
Buzanska L, Sypecka J, Nerini-Molteni S, Compagnoni A, Hogberg HT, del Torchio R, Domanska-Janik K, Zimmer J et al (2009) A human stem cell-based model for identifying adverse effects of organic and inorganic chemicals on the developing nervous system. Stem Cells 27(10):2591–2601. doi:10.1002/stem.179
Singh AK, Kashyap MP, Jahan S, Kumar V, Tripathi VK, Siddiqui MA, Yadav S, Khanna VK et al (2012) Expression and inducibility of cytochrome P450s (CYP1A1, 2B6, 2E1, 3A4) in human cord blood CD34(+) stem cell-derived differentiating neuronal cells. Toxicol Sci 129(2):392–410
Singh AK, Kashyap MP, Kumar V, Tripathi VK, Yadav DK, Khan F, Jahan S, Khanna VK et al (2013) 3-Methylcholanthrene induces neurotoxicity in developing neurons derived from human CD34+Thy1+ stem cells by activation of aryl hydrocarbon receptor. Neuromol Med 15(3):570–592. doi:10.1007/s12017-013-8243-0
Kashyap MP, Kumar V, Singh AK, Tripathi VK, Jahan S, Pandey A, Srivastava RK, Khanna VK et al (2015) Differentiating neurons derived from human umbilical cord blood stem cells work as a test system for developmental neurotoxicity. Mol Neurobiol 51(2):791–807. doi:10.1007/s12035-014-8716-7
Zimmer B, Kuegler PB, Baudis B, Genewsky A, Tanavde V, Koh W, Tan B, Waldmann T et al (2011) Coordinated waves of gene expression during neuronal differentiation of embryonic stem cells as basis for novel approaches to developmental neurotoxicity testing. Cell Death Differ 18(3):383–395
Visan A, Hayess K, Sittner D, Pohl EE, Riebeling C, Slawik B, Gulich K, Oelgeschlager M et al (2012) Neural differentiation of mouse embryonic stem cells as a tool to assess developmental neurotoxicity in vitro. Neurotoxicology 33(5):1135–1146
Hoelting L, Scheinhardt B, Bondarenko O, Schildknecht S, Kapitza M, Tanavde V, Tan B, Lee QY et al (2013) A 3-dimensional human embryonic stem cell (hESC)-derived model to detect developmental neurotoxicity of nanoparticles. Arch Toxicol 87(4):721–733. doi:10.1007/s00204-012-0984-2
Wilson MS, Graham JR, Ball AJ (2014) Multiparametric high content analysis for assessment of neurotoxicity in differentiated neuronal cell lines and human embryonic stem cell-derived neurons. Neurotoxicology 42:33–48
Gassmann K, Abel J, Bothe H, Haarmann-Stemmann T, Merk HF, Quasthoff KN, Rockel TD, Schreiber T et al (2010) Species-specific differential AhR expression protects human neural progenitor cells against developmental neurotoxicity of PAHs. Environ Health Perspect 118(11):1571–1577. doi:10.1289/ehp.0901545
Wang C, Liu F, Patterson TA, Paule MG, Slikker W Jr (2013) Utilization of neural stem cell-derived models to study anesthesia-related toxicity and preventative approaches. Mol Neurobiol 48(2):302–307. doi:10.1007/s12035-013-8501-z
Zychowicz M, Dziedzicka D, Mehn D, Kozlowska H, Kinsner-Ovaskainen A, Stepien PP, Rossi F, Buzanska L (2014) Developmental stage dependent neural stem cells sensitivity to methylmercury chloride on different biofunctional surfaces. Toxicol In Vitro 28(1):76–87
Lee JK, Jin HK, Bae JS (2010) Bone marrow-derived mesenchymal stem cells attenuate amyloid beta-induced memory impairment and apoptosis by inhibiting neuronal cell death. Curr Alzheimer Res 7(6):540–548
Grymula K, Tarnowski M, Piotrowska K, Suszynska M, Mierzejewska K, Borkowska S, Fiedorowicz K, Kucia M et al (2014) Evidence that the population of quiescent bone marrow-residing very small embryonic/epiblast-like stem cells (VSELs) expands in response to neurotoxic treatment. J Cell Mol Med 18(9):1797–1806. doi:10.1111/jcmm.12315
Buzanska L, Machaj EK, Zablocka B, Pojda Z, Domanska-Janik K (2002) Human cord blood-derived cells attain neuronal and glial features in vitro. J Cell Sci 115(Pt 10):2131–2138
Buzanska L, Habich A, Jurga M, Sypecka J, Domanska-Janik K (2005) Human cord blood-derived neural stem cell line-possible implementation in studying neurotoxicity. Toxicol In Vitro 19(7):991–999
McGuckin C, Forraz N, Baradez MO, Basford C, Dickinson AM, Navran S, Hartgerink JD (2006) Embryonic-like stem cells from umbilical cord blood and potential for neural modeling. Acta Neurobiol Exp (Wars) 66(4):321–329
McGuckin CP, Forraz N (2008) Potential for access to embryonic-like cells from human umbilical cord blood. Cell Prolif 41(Suppl 1):31–40
Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L et al (1989) Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A 86(10):3828–3832
Broxmeyer HE, Kurtzberg J, Gluckman E, Auerbach AD, Douglas G, Cooper S, Falkenburg JH, Bard J et al (1991) Umbilical cord blood hematopoietic stem and repopulating cells in human clinical transplantation. Blood Cells 17(2):313–329
Broxmeyer HE, Hangoc G, Cooper S, Ribeiro RC, Graves V, Yoder M, Wagner J, Vadhan-Raj S et al (1992) Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation in adults. Proc Natl Acad Sci U S A 89(9):4109–4113
Ali H, Bahbahani H (2010) Umbilical cord blood stem cells—potential therapeutic tool for neural injuries and disorders. Acta Neurobiol Exp (Wars) 70(3):316–324
Ballen KK, Barker JN, Stewart SK, Greene MF, Lane TA (2008) Collection and preservation of cord blood for personal use. Biol Blood Marrow Transplant 14(3):356–363
Buzanska L, Jurga M, Stachowiak EK, Stachowiak MK, Domanska-Janik K (2006) Neural stem-like cell line derived from a nonhematopoietic population of human umbilical cord blood. Stem Cells Dev 15(3):391–406. doi:10.1089/scd.2006.15.391
Lund TC, Boitano AE, Delaney CS, Shpall EJ, Wagner JE (2015) Advances in umbilical cord blood manipulation-from niche to bedside. Nat Rev Clin Oncol 12(3):163–174
Watt SM, Contreras M (2005) Stem cell medicine: umbilical cord blood and its stem cell potential. Semin Fetal Neonatal Med 10(3):209–220
Zandstra PW, Conneally E, Petzer AL, Piret JM, Eaves CJ (1997) Cytokine manipulation of primitive human hematopoietic cell self-renewal. Proc Natl Acad Sci U S A 94(9):4698–4703
Lu J, Aggarwal R, Pompili VJ, Das H (2010) A novel technology for hematopoietic stem cell expansion using combination of nanofiber and growth factors. Recent Pat Nanotechnol 4(2):125–135
Aguila JR, Liao W, Yang J, Avila C, Hagag N, Senzel L, Ma Y (2011) SALL4 is a robust stimulator for the expansion of hematopoietic stem cells. Blood 118(3):576–585
Jing Q, Cai H, Du Z, Ye Z, Tan WS (2013) Effects of agitation speed on the ex vivo expansion of cord blood hematopoietic stem/progenitor cells in stirred suspension culture. Artif Cells Nanomed Biotechnol 41(2):98–102. doi:10.3109/10731199.2012.712043
Petzer AL, Zandstra PW, Piret JM, Eaves CJ (1996) Differential cytokine effects on primitive (CD34 + CD38−) human hematopoietic cells: novel responses to flt3-ligand and thrombopoietin. J Exp Med 183(6):2551–2558
Podesta M, Piaggio G, Pitto A, Zocchi E, Soracco M, Frassoni F, Luchetti S, Painelli E et al (2001) Modified in vitro conditions for cord blood-derived long-term culture-initiating cells. Exp Hematol 29(3):309–314
Ishigaki T, Sudo K, Hiroyama T, Miharada K, Ninomiya H, Chiba S, Nagasawa T, Nakamura Y (2009) Human hematopoietic stem cells can survive in vitro for several months. Adv Hematol 2009:936761. doi:10.1155/2009/936761
Dahlberg A, Delaney C, Bernstein ID (2011) Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood 117(23):6083–6090
Du Z, Cai H, Ye Z, Tan WS (2012) Optimization of SCF feeding regimen for ex vivo expansion of cord blood hematopoietic stem cells. J Biotechnol 164(2):211–219
Aljitawi OS (2012) Ex vivo expansion of umbilical cord blood: where are we? Int J Hematol 95(4):371–379. doi:10.1007/s12185-012-1053-6
Fan X, Gay FP, Lim FW, Ang JM, Chu PP, Bari S, Hwang WY (2014) Low-dose insulin-like growth factor binding proteins 1 and 2 and angiopoietin-like protein 3 coordinately stimulate ex vivo expansion of human umbilical cord blood hematopoietic stem cells as assayed in NOD/SCID gamma null mice. Stem Cell Res Ther 5(3):71
Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E, Czajka R, Wojakowski W et al (2007) Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia 21(2):297–303
Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM (2007) Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells 25(3):646–654
Orkin SH, Wang J, Kim J, Chu J, Rao S, Theunissen TW, Shen X, Levasseur DN (2008) The transcriptional network controlling pluripotency in ES cells. Cold Spring Harb Symp Quant Biol 73:195–202
Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, Mortier C, Bron D et al (2005) Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells 23(8):1105–1112
Baksh D, Yao R, Tuan RS (2007) Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 25(6):1384–1392
Jin W, Xing YQ, Yang AH (2009) Epidermal growth factor promotes the differentiation of stem cells derived from human umbilical cord blood into neuron-like cells via taurine induction in vitro. Vitro Cell Dev Biol Anim 45(7):321–327. doi:10.1007/s11626-009-9184-7
Sun T, Ma QH (2011) Repairing neural injuries using human umbilical cord blood. Mol Neurobiol 47(3):938–945. doi:10.1007/s12035-012-8388-0
Zeng Y, Rong M, Liu Y, Liu J, Lu M, Tao X, Li Z, Chen X et al (2013) Electrophysiological characterisation of human umbilical cord blood-derived mesenchymal stem cells induced by olfactory ensheathing cell-conditioned medium. Neurochem Res 38(12):2483–2489. doi:10.1007/s11064-013-1186-x
Forraz N, McGuckin CP (2011) The umbilical cord: a rich and ethical stem cell source to advance regenerative medicine. Cell Prolif 44(Suppl 1):60–69. doi:10.1111/j.1365-2184.2010.00729.x
Sabapathy V, Sundaram B, MS V, Mankuzhy P, Kumar S (2014) Human Wharton’s jelly mesenchymal stem cells plasticity augments scar-free skin wound healing with hair growth. PLoS One 9(4):e93726. doi:10.1371/journal.pone.0093726
Kang XQ, Zang WJ, Bao LJ, Li DL, Xu XL, Yu XJ (2006) Differentiating characterization of human umbilical cord blood-derived mesenchymal stem cells in vitro. Cell Biol Int 30(7):569–575
Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, Tukun A, Uckan D et al (2007) Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells 25(2):319–331
Lim JY, Park SI, Oh JH, Kim SM, Jeong CH, Jun JA, Lee KS, Oh W et al (2008) Brain-derived neurotrophic factor stimulates the neural differentiation of human umbilical cord blood-derived mesenchymal stem cells and survival of differentiated cells through MAPK/ERK and PI3K/Akt-dependent signaling pathways. J Neurosci Res 86(10):2168–2178. doi:10.1002/jnr.21669
Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW, Chang DY, Cho KG et al (2008) Neural induction with neurogenin1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells 26(9):2217–2228
Zwart I, Hill AJ, Girdlestone J, Manca MF, Navarrete R, Navarrete C, Jen LS (2008) Analysis of neural potential of human umbilical cord blood-derived multipotent mesenchymal stem cells in response to a range of neurogenic stimuli. J Neurosci Res 86(9):1902–1915. doi:10.1002/jnr.21649
Li X, Li H, Bi J, Chen Y, Jain S, Zhao Y (2012) Human cord blood-derived multipotent stem cells (CB-SCs) treated with all-trans-retinoic acid (ATRA) give rise to dopamine neurons. Biochem Biophys Res Commun 419(1):110–116
Yang S, Sun HM, Yan JH, Xue H, Wu B, Dong F, Li WS, Ji FQ et al (2013) Conditioned medium from human amniotic epithelial cells may induce the differentiation of human umbilical cord blood mesenchymal stem cells into dopaminergic neuron-like cells. J Neurosci Res 91(7):978–986. doi:10.1002/jnr.23225
Ali H, Bayatti N, Lindsay S, Dashti AA, Al-Mulla F (2013) Directed differentiation of umbilical cord blood stem cells into cortical GABAergic neurons. Acta Neurobiol Exp (Wars) 73(2):250–259
Giorgetti A, Montserrat N, Rodriguez-Piza I, Azqueta C, Veiga A, Izpisua Belmonte JC (2010) Generation of induced pluripotent stem cells from human cord blood cells with only two factors: Oct4 and Sox2. Nat Protoc 5(4):811–820
Takenaka C, Nishishita N, Takada N, Jakt LM, Kawamata S (2010) Effective generation of iPS cells from CD34+ cord blood cells by inhibition of p53. Exp Hematol 38(2):154–162
Kashyap MP, Singh AK, Kumar V, Yadav DK, Khan F, Jahan S, Khanna VK, Yadav S et al (2013) Pkb/Akt1 mediates Wnt/GSK3beta/beta-catenin signaling-induced apoptosis in human cord blood stem cells exposed to organophosphate pesticide monocrotophos. Stem Cells Dev 22(2):224–238. doi:10.1089/scd.2012.0220
Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L et al (2007) Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J 21(7):1345–1357
Blum B, Bar-Nur O, Golan-Lev T, Benvenisty N (2009) The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nat Biotechnol 27(3):281–287
Paspala SA, Vishwakarma SK, Murthy TV, Rao TN, Khan AA (2012) Potential role of stem cells in severe spinal cord injury: current perspectives and clinical data. Stem Cells Cloning 5:15–27. doi:10.2147/SCCAA.S28477
Chen N, Hudson JE, Walczak P, Misiuta I, Garbuzova-Davis S, Jiang L, Sanchez-Ramos J, Sanberg PR et al (2005) Human umbilical cord blood progenitors: the potential of these hematopoietic cells to become neural. Stem Cells 23(10):1560–1570
Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV et al (2004) A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med 200(2):123–135. doi:10.1084/jem.20040440
Zangiacomi V, Balon N, Maddens S, Lapierre V, Tiberghien P, Schlichter R, Versaux-Botteri C, Deschaseaux F (2008) Cord blood-derived neurons are originated from CD133+/CD34 stem/progenitor cells in a cell-to-cell contact dependent manner. Stem Cells Dev 17(5):1005–1016. doi:10.1089/scd.2007.0248
Stellos K, Panagiota V, Sachsenmaier S, Trunk T, Straten G, Leyhe T, Seizer P, Geisler T et al (2010) Increased circulating progenitor cells in Alzheimer’s disease patients with moderate to severe dementia: evidence for vascular repair and tissue regeneration? J Alzheimers Dis 19(2):591–600
Zanier ER, Montinaro M, Vigano M, Villa P, Fumagalli S, Pischiutta F, Longhi L, Leoni ML et al (2011) Human umbilical cord blood mesenchymal stem cells protect mice brain after trauma. Crit Care Med 39(11):2501–2510. doi:10.1097/CCM.0b013e31822629ba
Wang T, Choi E, Monaco MC, Campanac E, Medynets M, Do T, Rao P, Johnson KR et al (2013) Derivation of neural stem cells from human adult peripheral CD34+ cells for an autologous model of neuroinflammation. PLoS One 8(11):e81720. doi:10.1371/journal.pone.0081720
Hafizi M, Atashi A, Bakhshandeh B, Kabiri M, Nadri S, Hosseini RH, Soleimani M (2013) MicroRNAs as markers for neurally committed CD133+/CD34+ stem cells derived from human umbilical cord blood. Biochem Genet 51(3–4):175–188. doi:10.1007/s10528-012-9553-x
McGuckin CP, Forraz N, Allouard Q, Pettengell R (2004) Umbilical cord blood stem cells can expand hematopoietic and neuroglial progenitors in vitro. Exp Cell Res 295(2):350–359. doi:10.1016/j.yexcr.2003.12.028
Jang YK, Park JJ, Lee MC, Yoon BH, Yang YS, Yang SE, Kim SU (2004) Retinoic acid-mediated induction of neurons and glial cells from human umbilical cord-derived hematopoietic stem cells. J Neurosci Res 75(4):573–584. doi:10.1002/jnr.10789
Domanska-Janik K, Habich A, Sarnowska A, Janowski M (2006) Neural commitment of cord blood stem cells (HUCB-NSC/NP): therapeutic perspectives. Acta Neurobiol Exp (Wars) 66(4):279–291
Jurga M, Lipkowski AW, Lukomska B, Buzanska L, Kurzepa K, Sobanski T, Habich A, Coecke S et al (2009) Generation of functional neural artificial tissue from human umbilical cord blood stem cells. Tissue Eng Part C Methods 15(3):365–372. doi:10.1089/ten.tec.2008.0485
Jurga M, Forraz N, McGuckin CP (2010) Artificial human tissues from cord and cord blood stem cells for multi-organ regenerative medicine: viable alternatives to animal in vitro toxicology. Altern Lab Anim 38(2):183–192
Jurga M, Forraz N, Basford C, Atzeni G, Trevelyan AJ, Habibollah S, Ali H, Zwolinski SA et al (2012) Neurogenic properties and a clinical relevance of multipotent stem cells derived from cord blood samples stored in the biobanks. Stem Cells Dev 21(6):923–936. doi:10.1089/scd.2011.0224
Ali H, Jurga M, Kurgonaite K, Forraz N, McGuckin C (2009) Defined serum-free culturing conditions for neural tissue engineering of human cord blood stem cells. Acta Neurobiol Exp (Wars) 69(1):12–23
Ali H, Forraz N, McGuckin CP, Jurga M, Lindsay S, Ip BK, Trevelyan A, Basford C et al (2012) In vitro modelling of cortical neurogenesis by sequential induction of human umbilical cord blood stem cells. Stem Cell Rev 8(1):210–223. doi:10.1007/s12015-011-9287-x
Lim JY, Park SI, Kim SM, Jun JA, Oh JH, Ryu CH, Jeong CH, Park SH et al (2011) Neural differentiation of brain-derived neurotrophic factor-expressing human umbilical cord blood-derived mesenchymal stem cells in culture via TrkB-mediated ERK and beta-catenin phosphorylation and following transplantation into the developing brain. Cell Transplant 20:11-12
Wang L, Lu M (2014) Regulation and direction of umbilical cord blood mesenchymal stem cells to adopt neuronal fate. Int J Neurosci 124(3):149–159. doi:10.3109/00207454.2013.828055
Divya MS, Roshin GE, Divya TS, Rasheed VA, Santhoshkumar TR, Elizabeth KE, James J, Pillai RM (2012) Umbilical cord blood-derived mesenchymal stem cells consist of a unique population of progenitors co-expressing mesenchymal stem cell and neuronal markers capable of instantaneous neuronal differentiation. Stem Cell Res Ther 3(6):57
Huat TJ, Khan AA, Pati S, Mustafa Z, Abdullah JM, Jaafar H (2014) IGF-1 enhances cell proliferation and survival during early differentiation of mesenchymal stem cells to neural progenitor-like cells. BMC Neurosci 15:91
Sun W, Buzanska L, Domanska-Janik K, Salvi RJ, Stachowiak MK (2005) Voltage-sensitive and ligand-gated channels in differentiating neural stem-like cells derived from the nonhematopoietic fraction of human umbilical cord blood. Stem Cells 23(7):931–945
Sanchez-Ramos JR, Song S, Kamath SG, Zigova T, Willing A, Cardozo-Pelaez F, Stedeford T, Chopp M et al (2001) Expression of neural markers in human umbilical cord blood. Exp Neurol 171(1):109–115. doi:10.1006/exnr.2001.7748
Seo JH, Cho SR (2012) Neurorestoration induced by mesenchymal stem cells: potential therapeutic mechanisms for clinical trials. Yonsei Med J 53(6):1059–1067
Sofroniew MV, Howe CL, Mobley WC (2001) Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 24:1217–1281. doi:10.1146/annurev.neuro.24.1.1217
Park JW, Kang YD, Kim JS, Lee JH, Kim HW (2014) 3D microenvironment of collagen hydrogel enhances the release of neurotrophic factors from human umbilical cord blood cells and stimulates the neurite outgrowth of human neural precursor cells. Biochem Biophys Res Commun 447(3):400–406
Lee G, Chambers SM, Tomishima MJ, Studer L (2010) Derivation of neural crest cells from human pluripotent stem cells. Nat Protoc 5(4):688–701
Kitazawa A, Shimizu N (2007) Characterization of neurons differentiated from mouse embryonic stem cells using conditioned medium of dorsal root ganglia. J Biosci Bioeng 104(4):257–262
Buzanska L, Zychowicz M, Ruiz A, Ceriotti L, Coecke S, Rauscher H, Sobanski T, Whelan M et al (2010) Neural stem cells from human cord blood on bioengineered surfaces—novel approach to multiparameter bio-tests. Toxicology 270(1):35–42
Acknowledgments
We apologize to all authors whose reports on in vitro models for DNT could not be covered in this review. Dr. MP Kashyap acknowledges the support of the Urology Care Foundation, USA, and The Allergan Foundation, USA.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Singh, A.K., Kashyap, M.P. An Overview on Human Umbilical Cord Blood Stem Cell-Based Alternative In Vitro Models for Developmental Neurotoxicity Assessment. Mol Neurobiol 53, 3216–3226 (2016). https://doi.org/10.1007/s12035-015-9202-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9202-6