Abstract
Cell transplantation strategies have provided potential therapeutic approaches for treatment of neurodegenerative diseases. Mesenchymal stem cells from Wharton’s jelly (WJMSCs) are abundant and available adult stem cells with low immunological incompatibility, which could be considered for cell replacement therapy in the future. However, MSC transplantation without any induction or support material causes poor control of cell viability and differentiation. In this study, we investigated the effect of the nanoscaffolds on WJMSCs differentiation into motor neuronal lineages in the presence of retinoic acid (RA) and sonic hedgehog (Shh). Surface properties of scaffolds have been shown to significantly influence cell behaviors such as adhesion, proliferation, and differentiation. Therefore, polycaprolactone (PCL) nanofibers were constructed via electrospinning, surface modified by plasma treatment, and grafted by collagen. Characterization of the scaffolds by means of ATR-FTIR, contact angel, and Bradford proved grafting of the collagen on the surface of the scaffolds. WJMSCs were seeded on nanofibrous and tissue culture plate (TCP) and viability of WJMSCs were measured by MTT assay and then induced to differentiate into motor neuron-like cells for 15 days. Differentiated cells were evaluated morphologically, and real-time PCR and immunocytochemistry methods were done to evaluate expression of motor neuron-like cell markers in mRNA and protein levels. Our results showed that obtained cells could express motor neuron biomarkers at both RNA and protein levels, but the survival and differentiation of WJMSCs into motor neuron-like cells on the PCL/collagen scaffold were higher than cultured cells in the TCP and PCL groups. Taken together, WJMSCs are an attractive stem cell source for inducing into motor neurons in vitro especially when grown on nanostructural scaffolds and PCL/collagen scaffolds can provide a suitable, three-dimensional situation for neuronal survival and differentiation that suggest their potential application towards nerve regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury (SCI) often leads to cell death and axonal degeneration resulting in functional motor and sensory loss [1]. In many cases, spinal cord is unable to regenerate or repair its functions following pathological trauma or disease [2–4]. Furthermore, current therapies are minimally effective and unable to restore lost function, thereby highlighting the need for new innovative therapies. Cell transplantation strategies are potential therapeutic approaches for treatment of neurodegenerative diseases to replace the damaged and lost cells after SCI [5–7]. Unfortunately, transplanted cells have low survival rate and much tendency to differentiate into either astrocytes or oligodendrocytes [8, 9]. Stem cell therapy can be complemented by fabrication of optimal scaffolds that can promote cell survival and differentiation towards specific neuronal lineages needed for effective spinal cord repair [10]. Design and fabrication of scaffolds using appropriate biomaterials for neural tissue engineering is critical for the creation of functional engineered tissues [11]. A variety of natural and synthetic polymers such as polycaprolactone (PCL) [12], poly(l-lactide-co-glycolide) (PLGA) [13], collagen [14, 15], and gelatin [16] have been investigated in a variety of studies for the fabrication of scaffolds for nerve tissue regeneration and to determine their supporting capacity of neuronal differentiation for a wide range of cell types.
PCL has good mechanical properties, biodegradability, and biocompatibility, which has been shown to be suitable for constructing scaffold for differentiation of stem cells to different types of cells [12, 17, 18]. In addition, the ability of PCL to support neural cells in vitro and in vivo has demonstrated previously [19–21]. However, hydrophobic feature of PCL is known as an adverse feature for cell attachment [22]. Surface plasma treatment and immobilization of scaffold with protein such as collagen are effective methods that have shown to improve surface characteristics such as hydrophilicity, cell attachment, expansion, proliferation, and infiltration [23–26]. In the present study, natural and synthetic polymers have been used to fabricate a near-perfect scaffold suitable for motor neuron differentiation. Among different methods for scaffold construction, electrospinning has drawn attention for neural repair because it is an easy, cost-effective technique and provides a proper matrix with high surface area to volume ratio and fiber diameters in the range of nanometer with sufficient pores to encourage mesenchymal stem cells (MSCs) adhesion, proliferation, and differentiation [27–29]. Intriguingly, inducing stem cells to differentiate into neural cells without any differentiation inducer on nanomaterial scaffolds have been reported [30]. Moreover, the interaction between cells and nanomaterial plays an important role, especially in controlling the stem cell differentiation procedure. Therefore, it can be concluded that different physicochemical properties of electrospun nanofibrous such as morphology, fiber diameter, and orientation may affect the quality of this interaction and in turn influence stem cells fate [31]. In the present study, we manipulated the electrospinning parameters to fabricate ideal nanofibrous scaffold of PCL for neural tissue engineering application, and following this pattern, collagen I was grafted to the surface of nanofibers. On the other hand, MSCs have become one of the most interesting cells for neural tissue engineering since these cells present high plasticity, proliferative, and differentiation capacity for treatment of neurodegenerative diseases [32, 33]. Notably, MSCs derived from Wharton’s jelly region of umbilical cord (WJMSC) represents an interesting alternate source for MSCs. WJMSCs have many advantages, such as, potentially available in large quantities, easily harvested and manipulated with no harm to the baby or mother, prolonged stemness properties in vitro up to 9–10 passages, limited number of ethical issues to be dealt, and most important are not tumorigenic. Interestingly, these cells express immunoprivileged and immunomodulatory phenotype, allowing for an allogeneic cell therapy source [34–37]. Furthermore, it is well established that WJMSC have capacity to differentiate into dopaminergic neuron and is the best candidate for regenerative SCI due to having high proliferation and differentiation capacity into neural cells [38].
The aim of this study was to determine the effect of the nanofibrous scaffolds on WJMSCs differentiation into motor neuronal lineage. WJMSCs were induced to differentiate into motor neuron-like cells by using different signaling molecules and neurotrophic factors in vitro on scaffolds and tissue culture plate (TCP). Differentiated neurons were then characterized morphologically and for the expression of motor neuron markers was investigated using quantitative reverse transcription PCR and immunocytochemistry for PCL, PCL/collagen, and TCP groups.
Material and Methods
Materials
PCL (Mw 80,000) and collagen type I was obtained from Sigma-Aldrich, USA. Dichloromethane (DCM), N,N-dimethylformamide (DMF), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), and N-hydroxysuccinimide(NHS) was obtained from Merck, Germany. Dulbecco’s modified Eagle’s medium/Nutrient Mixture F12 (DMEM/F12), trypsin/EDTA, and B27 was obtained from GIBCO Invitrogen, USA. Collagenase type I, paraformaldehyde (PFA), fetal bovine serum (FBS), penicillin/streptomycin, fibroblast growth factor (FGF2), glial cell-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), and other chemicals, including isobutylmethylxanthine, 2-metcaptoethanol, sonic hedgehog (Shh), and retinoid acid (RA), were bought from Sigma-Aldrich, USA. Antibodies were obtained from Abcam, USA. RNeasy Plus Mini Kit was purchased from Qiagen, USA, and RevertAid First Strand cDNA Synthesis Kit was purchased from Fermentas, USA.
Methods
Fabrication and Modification in Solvent Ratio to Produce an Optimal Nanofibers
To obtain steady state condition for uniform, beadless, and well-defined fiber diameter for neural differentiation, PCL was electrospun using different ratios of binary solvent while all other electrospinning parameters were constant. PCL (10 % w/v) was dissolved in different ratio of DCM/DMF and stirred for 24 h at room temperature. Polymer solutions were fed to the needle tip using a syringe pump at a flow rate of 1.3 mL/h with an applied voltage of 15 kV. Prior to characterization and used for cell culture studies, the nanofibrous scaffolds was placed within a vacuum chamber for 48 h, to remove residual organic solvent. The morphology of nanofibers was characterized using a scanning electron microscope (SEM; Philips XL30, Netherland) at an accelerating voltage of 15 kV after specimens were coated with gold using a sputter coater. The fiber diameter was determined from SEM images by measuring 50 different locations for each sample using ImageJ analysis software (National Institutes of Health, USA).
Surface Modification and Collagen Immobilization
Surface modification of the optimal PCL scaffolds was performed in two stages: (1) plasma treatment and (2) collagen grafting. Before air plasma treatment, PCL nanofibers were rinsed with ethanol 70 % and distilled water. Surface plasma treatment was performed by low frequency plasma generator of 40 kHz frequency at 30 w with a cylindrical quartz reactor (Diener Electronics). Pure oxygen was introduced into the reaction chamber at 0.4 mbar, and then the glow discharge was applied for 5 min. Plasma-treated scaffolds were immersed in EDC/NHS solution (5 mg/ml) for 12 h. The nanofibers were then immersed in collagen solution (1 mg/ml) at 4 °C overnight.
Characterization of Collagen-Coated Fibers
To study the wettability of the nanofibers surface before and after surface treatment, water contact angle was measured by the sessile drop method with an optical bench-type contact angle goniometer (OCA Dataphysics model, Germany). Five samples were used for each test. The average value was reported with standard deviation (SD). Surface chemical modifications after plasma treatment and collagen grafting were investigated by attenuated total reflection Fourier transform infrared (ATR-FTIR, Bomem MB100, Canada). The amount of collagen-coated on PCL nanofibers was measured by the Bradford protein assay method. Briefly, the collagen-coated PCL nanofibers was immersed in 0.1 ml PBS together with 2-ml working reagent at room temperature for 2 h, and then the absorbance at 595 nm was measured. The collagen concentration was calculated from the collagen standard curve. To obtain a standard curve for collagen, several collagen solutions with defined concentrations were prepared and exposed to Bradford reagent according to above protocol and their absorbances at 595 nm were read and finally plotted versus concentrations.
Isolation and Culture of Mesenchymal Stem Cells from Wharton’s Jelly
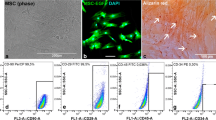
Umbilical cord was obtained from Shariati hospital (Tehran University of Medical Sciences) after filling consent forms. After being rinsed in PBS, the tissue was dissected into 3–5 cm pieces. In order to decrease possibility of contamination with endothelial cells, the vessels were removed from all pieces. Wharton’s jelly fragments were then incubated in media containing DMEM: F12, 10 % FBS, 1 % penicillin/streptomycin and 1 % collagenase type I at 37 °C for 3 h. Incubation with dispase enzyme was performed to assess more digested tissues. Cell pellets were obtained after centrifuging tissue pieces at 1500 rpm for 5 min at 4 °C. After discarding the liquid, the pellet was suspended in culture medium containing DMEM: F12 (1:1), 10 % FBS, 1 % penicillin/streptomycin and maintained at 37 °C in a 5 % CO2 incubator. After 3 days, any non-adherent cells were removed, and the medium was renewed daily until cells reached 80 % confluency. MSCs derived from Wharton’s jelly were characterized using flow cytometry for cell surface markers including CD105, CD90, CD73, CD45, and CD34.
Cell Seeding on Scaffolds
Electrospun nanofibrous scaffolds were exposed to UV radiation for 2 h, washed three times with PBS and incubated with DMEM/F12 for 24 h before cell seeding. After the cells reached 85–90 % confluency, they detached by adding 1 ml of 0.25 % trypsin containing 0.1 % EDTA and keeping for 5 min to create a single cell suspension. Then, 5 × 104 cells were dropped onto the top of the scaffold and incubated in DMEM/F12 for 2 h to allow cells to attach on the surface of the scaffold. Fresh medium was then added for further incubation.
Assessment of Cell Attachment and Morphology of WJMSCs on Scaffolds
Morphological study of the in vitro cultured WJMSCs cells after 5 days post seeding on PCL, PCL/collagen nanofibrous scaffolds were performed by using SEM. The scaffolds fixed with 2.5 % glutaraldehyde for 1 h, followed by washing with PBS and dehydrated in series of sequentially increasing concentration of ethanol solutions (30, 50, 70, 80, 90, and 100 %) at 37 °C for 10 min per concentration. Finally cell-containing scaffolds were sputter coated with gold and then observed under SEM.
Cell Viability and Proliferation Assay
Scaffolds were seeded with human WJMSCs and cultured in DMEM/F12 for 1, 3, and 5 days. After fixation, a morphological evaluation of cell attachment was measured by 4′,6-diamidino-2-phenylindole (DAPI) staining. Viability and proliferation of the cells were performed with 3-(4,5-dimethylthiazoyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, 5 × 104 WJMSCs were loaded on nanofibers and TCP and let them to grow with complete media inside 96 well-plates for 1, 3, and 5 days. The media was then replaced with MTT dye (0.05 mg/ml) and incubated at 37 °C for 4 h. After removing MTT, DMSO was added to the wells and shacked for 15 min in dark place. The absorbance of the content of each well was measured at 570 nm using a spectrophotometric plate reader (Expert 96, Asys Hitch, Ec Austria). The experiment was repeated three times and the results were presented as means ± SD.
Motor Neuron-Like Cells Induction of WJMSCs on TCP and Scaffolds
For motor neuron induction, WJMSCs were exposed to cocktail of induction agent. Approximately, 2 × 105 WJMSCs were seeded on both the scaffolds and TCP and let them to grow for 24 h in previously defined media. Before treatment with the induction medium, the cells were pre-treated with DMEM:F12 (1:1), 20 % FBS, 2 % B27, 10 ng/ml FGF2, 250 μM isobutylmethylxanthine, 100 μM 2-metcaptoethanol and incubate for 24 h at 37 °C and 5 % CO2. In order to initiate motor neuron induction, the cells further induce with induction medium containing DMEM: F12 (1:1), 0.2 % B27, 100 ng/ml of Shh, and 0.01 ng/ml RA for 1 week. Then, induced media was replaced with media composed of DMEM: F12 (1:1), 0.2 % B27, 100 ng/ml GDNF, and 200 ng/ml BDNF and let them grow under this condition for 1 week (Liqing et al. 2011). After 15 days post induction, the differentiated cells were characterized for motor neuron biomarkers with immunocytochemistry and qRT-PCR.
RNA Isolation and Real-Time RT-PCR for Gene Expression Analysis
Total RNA was extracted from WJMSCs and differentiated cells following RNeasy Mini Kit protocol. Complementary DNA (cDNA) synthesis was then performed for 1 μg RNA by RevertAid First Strand cDNA Synthesis Kit. Designing of primers to analyze the expression of Nestin, Pax-6, NF-H, Islet-1, Chat, and HB9 (markers of differentiation) and GAPDH (internal control) genes was carried out by NCBI and gene runner software. The sequence of primers and annealing temperature has been shown in Table 1. Real-time PCR was performed (Corbet research, Rotor gene 5000) on 1 μg of cDNA, mixed with 10 μl of SYBR green, 300 nM of forward and reverse primers, and up to 20 μl of ddH2O. All the reactions were done as triplicate. Real-time PCR results were further analyzed using ΔΔCT equation.
Immunocytochemistry
After removal of the medium, the cells seeded in 24-well plate were fixed with 4 % PFA for 30 min at room temperature. The cells were then permeabilized with 0.2 % Triton X-100 for 30 min; the cells were blocked using blocking buffer containing 5 % bovine serum albumin (BSA) in PBS and incubated overnight at 4 °C with following primary antibodies: NF-H (SMI-32) (1:200), Chat (1:200), and Islet-1 (1:200) diluted in 5 % BSA in PBS overnight. Secondary antibodies included Alexa Fluor 594 donkey anti-mouse (1:700) and Alexa Fluor 498 donkey anti-mouse (1:500); the nuclei were counterstained with DAPI. For negative controls, only the secondary antibodies were used. No specific positive staining was detected in either case. Cells were examined by fluorescence microscope (Olympus BX51, Japan). All immunocytochemical experiments were repeated three times as independent experiments. The positively stained cells were counted in ten random fields (×200), and the ratio of the positive cells was calculated by dividing the number of positive cells over the total number of cells and was expressed as percentage, and three independent experiments were performed.
Statistical Analysis
All data in this study was analyzed using ANOVA and student t test (SPSS 16.0). P values less than 0.05 were considered as significant.
Results
Effect of PCL Solvent Concentration on Fiber Morphology and Diameter
SEM micrographs of electrospun PCL in different ratio of DCM/DMF can be seen from Fig. 1. It was observed that there was difference in the diameter of fibers with different solvent fractions. As shown in Fig. 1, increasing the ratio of DMF up to 75 % was shown to preserve the electrospinnability, while fiber diameter was found to decrease. Mean value of fibers diameter for the samples electrospun with DCM/DMF (75:25), DCM/DMF (60:40), DCM/DMF (50:50), DCM/DMF (40:60), and DCM/DMF (25:75) were 2000, 1650, 1700, 980, and 750 nm, respectively.
The bead area was found to increase exponentially with increase in the concentration of DMF. Based on the obtained micrographs and fiber diameter comparison, it was decided that the most suitable ratio for nerve tissue engineering was DCM to DMF (25:75) and the reason was fiber diameter, consistency, continuity, and uniformity of fibers. The result showed that the ratio of the solvents had clear effect on the diameters and homogeneity of the fiber.
Characterization of Collagen-Coated PCL Nanofibers
To investigate the effect of plasma treatment and collagen grafting on the scaffolds surface chemistry, ATR-FTIR was performed (Fig. 2). In the PCL spectrum, a major absorption peak appeared at 1730 cm−1 according to the functional group, COO, in PCL. When collagen is immobilized onto the surface of the plasma-treated PCL nanofibers, three distinct peaks were observed. First, a broader peak which is located at 3000–3600 cm−1 can be ascribed to the stretching of the hydroxyl groups (−OH) or NH groups from grafted collagen groups. Second, a peak at 1648 cm−1 which might belong to vibration mode of amide I groups and third one at 1540 cm−1 which attributed to the amide II because of collagen immobilization. The results of contact angle measurement showed that the water contact angle value decreased from 140° for PCL nanofibers to 70° for PCL/collagen, indicating improved hydrophilicity of PCL/collagen nanofibrous scaffolds by covalent attachment of collagen on the surface of PCL nanofibers. By Bradford protein assay analysis, the amount of collagen on the surface of the PCL scaffolds was measured which determined that the amount of immobilized collagen on the surface was 15 μg/cm2.
Characterization of MSCs Derived from Wharton’s Jelly
When adherent cells grew to confluence, they were harvested, and then flow cytometry study was performed to characterize surface markers on mesenchymal stem cells. These cells were positive for CD90+ (93.6), CD105+ (90.7), and CD73+ (89.8) (mesenchymal markers) and negative for CD34 and CD4 (hematopoietic lineage markers) which indicated isolated cells were mesenchymal stem cells free of contamination of endothelial and hematopoietic cells (data is shown in unpublished article).
Assessment of Cell Adhesion and Viability
SEM micrographs of cell interaction with PCL and PCL/collagen electrospun nanofibrous scaffolds after 5 days of cell culture was shown in Fig. 3a, c. After seeding the same number of cells on scaffolds, SEM micrographs showed well attached and spread of cells on both of the scaffolds, but higher cell attachment and density on PCL/collagen scaffolds compared to PCL nanofibers were observed. These observations indicate a better integration of cells with PCL/collagen nanofibrous scaffolds. DAPI staining for cultured cells on PCL and PCL/collagen scaffold for 1, 3, and 5 days also showed the better attachment of cells on the PCL/collagen scaffolds (Fig. 3b, d) that was in agreement with SEM results. Viability and the rate of WJMSCs proliferation on PCL, PCL/collagen and TCP group were compared during period of culture with MTT assay (Fig. 4). The results of MTT assay for 1 day after cell cultures showed that there was no significant difference between cell viability of cultured cells on scaffolds and TCP on this day. However, in the following days, it was shown that PCL and PCL/collagen supported cell proliferation as much as TCP. We also compare the proliferation of the cells between collagen modified and unmodified scaffolds. Our data also confirmed the positive role of collagen on enhancement of the cell viability. The results obtained from MTT assay indicated that PCL/collagen nanofibrous scaffolds are suitable substrates than PCL nanofibers and TCP, in terms of cell attachment and proliferation.
Evaluation of WJMSCs Differentiation into Motor Neuron-Like Cell on TCP and Nanofibrous Scaffolds
To confirm the differentiation of WJMSCs to motor neuron-like cells and to demonstrate that this differentiation was not an artifact, three analyses were performed: morphological changes, expression of motor neuron biomarkers at both RNA, and protein levels. WJMSCs were induced to differentiate into motor neuron-like cells by using different signaling molecules including RA, Shh, GDNF, and BDNF for 15 days. To identify the neuronal differentiation morphologically, the differentiating cells were observed daily using a phase contrast microscope in TCP group (Fig. 5a). Most of the MSCs displayed changes in cellular morphology after exposed to motor neuron induction medium. The cells showed spindle fibroblast-like morphology after 24 h of culture in motor neuron pre-confluence culture medium. Seven days after differentiation, the cell bodies tended to be spherical, exhibiting bipolar neuron cell-like shape. These neuron cell-like cells continued to develop, the cells became exceedingly long that were in contact with neighboring cells at the end of the induction. However, there was no significant change in cell morphology in the untreated cells as control group they appeared as a monolayer of flat and fibroblast-like cells in morphology (Fig. 5a). Analysis of the expression of motor neuron markers including Nestin, PAX6, Islet-1, NF-H, Chat, and HB9 by means of qRT-PCR was performed 15 days post induction. Our results indicated that expression of motor neuron specific markers significantly (p < 0.001) up-regulated in cells grown on PCL/collagen compared with PCL and TCP groups. These results could clearly show the effect of collagen on increasing quality of stem cell differentiation in vitro. We could find significant decrease for the expression of Nestin and Pax6 between scaffolds and TCP and also between unmodified and collagen-coated scaffold groups (p < 0.001) (Fig. 5b). The increase in expression of motor neuron specific markers was accompanied by low expression of Nestin and PAX6, indicating a loss of multipotency phenotype and the differentiation of the cells. To confirm our data, the cells were further characterized by determining for the expression of motor neuron specific marker proteins. Immunocytochemistry analyses indicated that motor neuron biomarkers (NF-H, Chat and Islet-1) were highly expressed in cultured cells in PCL, PCL/collagen scaffolds, and TCP groups, but these markers were not expressed in control cells. Immunocytochemistry results also revealed higher expression of these markers in PCL/collagen compare to cells differentiated in PCL and TCP (Fig. 6a). The expression of NF-H (59 %), Chat (77 %), and Islet-1 (76 %) in PCL/collagen group were higher than the expression of NF-H (56 and 46 %), Chat (69 and 55 %) and Islet-1 (68 and 58 %) in PCL and TCP groups (p < 0.001) (Fig. 6b). Conclusively, our result showed that collagen-coated PCL nanofibers could provide better condition for differentiation of WJMSCs to motor neurons.
Characterization of motor neuron cell-like cells induced from WJMSCs. a Phase-contrast image of Wharton’s jelly stem cells before and after the induction in TCP group. Following the inductions, the cells changed from a flat cell shape to a round cell body, and axon of the cells became much longer on day 15. b Gene expression analysis of motor neuron biomarkers in differentiated MSCs. Differentiated cells were checked for RNA expression of motor neuron biomarkers 15 days post induction. Data showed that obtained cells could significantly express Islet-1, NF-H, Chat, and HB9. Additionally, expression of these markers is higher in cells treated with collagen compare with PCL and TCP. These results confirmed positive role of 3D culture and collagen in obtaining qualified motor neurons. RNA expression of Nestin and PAX6 as neuronal precursor biomarkers was shown down-regulation in cells treated with modified and unmodified PCL scaffolds. WJMSCs were used as a control, and GAPDH was the housekeeping gene control. Error bars show SD, n = 3 samples (***p < 0.001; *p < 0.05)
a Immunocytochemical analysis showed that differentiated WJMSCs were positive for NF-H, Islet-1, and Chat after 15 days post inductions (scale bar, 50 μm). b In histogram, percentages of cultured cells expressing markers NF-H, Islet-1, and Chat compared between PCL, PCL/collagen, and TCP. Each groups differed significantly from the others (p < 0.001). n = 3 biological samples, mean ± SD
Discussion
The fundamental approach in neural tissue engineering involves the fabrication of polymeric scaffolds with suitable chemical and mechanical properties with neural cells to produce a three-dimensional tissue substitute suitable for implantation [39]. This approach will be dependent on improved methodologies to promote cell survival and differentiation towards desirable cells. In this study, efficiency of the differentiation of the MSC-derived Wharton’s Jelly to motor neuron-like cells on nanofibrous scaffolds as a three-dimensional (3D) structure was examined. Also, the role of material topography in differentiation of the WJMSCs to motor neuron-like cells was investigated and compared with TCP as a two-dimensional (2D) topography. While numerous biomaterials are available, PCL was chosen because it has several advantages including biocompatibility, low cost and easy processability [40]. Moreover, electrospinning of polymers is receiving increasing attention for use in neural tissue engineering due to the ability to mimic the local tissue environment through the control of fiber alignment and diameter [13, 14, 41]. In addition, it was shown that the diameter and morphology of the nanofibers have a role in cell proliferation and differentiation [42]. To obtain proper nanofibers for neural differentiation, we first investigated the effect of the different ratios of the solvents on the fiber morphology and fiber diameter and then determined the effect of the nanofibrous scaffolds on differentiation of the WJMSCs into motor neuronal lineage. The results of SEM showed that the ratio of the solvent had an effect on the diameters and homogeneity of the electrospun fibers. Fiber diameter of PCL in our study was 400–500 nm that is in agreement with the size obtained by Binan et al. [43], which is appropriate for neurite outgrowth and neural differentiation. In order to improve the hydrophilicity of the PCL, type I collagen was immobilized on the nanofibers after surface modification by plasma treatment. The results of contact angle measurement and the presence of functional groups from ATR-FTIR spectra provided evidence for increased hydrophilicity of PCL/collagen scaffolds, and the amount of immobilized collagen on the surface was 15 μg/cm2. In order to differentiate Wharton’s jelly stem cell into motor neuron-like cells in this study, RA was utilized along with Shh similar to those carried out by Liqing et al. [44]. The combination of RA and Shh are commonly used for inducing the expression of transcription factors which play important roles in motor neuronal differentiation [45, 46]. Differentiated neurons in different groups were then characterized morphologically and for the expression of motor neuron markers including Nestin, PAX6, NF-H, Islet-1, HB9, and Chat in mRNA levels and protein by quantitative reverse transcription PCR and immunocytochemistry, respectively. To the best of our knowledge, this study is the first report for motor neuron-like cell differentiation of human WJMSCs in 2D and 3D culture. Among selected neuronal biomarkers, four markers including NF-H, Islet-1, Chat, and HB9 showed increase expression in cells induced through 3D culture compared with the ones differentiated in 2D culture experiments and were grown just under chemical-induced media. This is consistent with the idea that stem cells are differentiated in 3D culture, gain more similar phenotypes to in vivo conditions in regards to inducing correct cell morphology, gene expression, and biological behavior [47–49]. We also compare the expression results between collagen modified and unmodified scaffolds. Our data also confirmed the positive role of collagen on differentiation genes expression in induced MSCs [50, 51]. Unexpectedly, our data indicate significant decrease for expression of Nestin and PAX6 between 2D and 3D experiments groups and also among different types of modified and unmodified PCL scaffolds. Since PAX6 could also be expressed in stem cells [52] and Nestin expression is limited to the first days of differentiation [53], therefore, we propose that obtained cells has passed early stages of differentiation and are close to fully differentiated motor neuron-like cells. The results of immunocytochemistry also show that morphogenesis including RA and SHH are sufficient to induce motor neuron differentiation, but the results of nanofibrous scaffolds demonstrated that cell behaviors such as adhesion, growth, and differentiation can significantly influenced by surface properties of the scaffold [54, 55]. Selection of a proper cell and scaffolds for proliferation and differentiation into desire lineages are key element in regenerative medicine [56]. Wharton’s jelly stem cells are easily accessible, legally and ethically noncontroversial, and have low immunogenicity [57]. Additionally, these cells express the pluripotency markers Oct-4, Sox-2, and Nanog [58, 59], albeit at relatively lower levels than embryonic stem cells [60, 61]. Thus, we suppose that WJMSCs have the potential to express marker characteristic for motor neuron cell-like cells differentiation and could be used in human neurodegenerative disorders. Consequently, our results showed that collagen-coated PCL nanofibers could provide better condition for differentiation of WJMSCs to motor neurons. The results of our study open new opportunities for further investigations on the modification of nanofibrous scaffolds, and moreover, the combination of stem cells and nanomaterial expected to be an important tool in treating neural diseases such as spinal cord injury [62–64].
Conclusion
Nanostructures with suitable properties could serve as a platform to support the neuronal differentiation of MSCs. In this study, well-defined fiber diameter substrate suitable for neural differentiation was developed with controlled ratio of the solvents, surface modified with plasma and collagen grafting, and then motor neuron differentiation of WJMSCs in the presence of inducing factors on nanofibrous scaffolds and TCP was performed. Evaluation of the motor neuron specific markers by real-time PCR and immunocytochemistry showed that PCL/collagen nanofibrous scaffolds were suitable substrates for neuronal differentiation of WJMSCs and suggest that topographical cues, when applied in conjunction with targeted biochemical signals which can regulate the differentiation of stem cells into a specific cell.
References
Parr AM, Kulbatski I, Zahir T, Wang X, Yue C, Keating A, Tator CH (2008) Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience 155(3):760–770
Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, Stupp SI, Kessler JA (2008) Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci 28(14):3814–3823
Thuret S, Moon LD, Gage FH (2006) Therapeutic interventions after spinal cord injury. Nat Rev Neurosci 7(8):628–643
Yang F, Murugan R, Ramakrishna S, Wang X, Ma YX, Wang S (2004) Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials 25(10):1891–1900
Parish CL, Arenas E (2007) Stem-cell-based strategies for the treatment of Parkinson’s disease. Neurodegener Dis 4(4):339–347
Winkler C, Kirik D, Bjorklund A (2005) Cell transplantation in Parkinson’s disease: how can we make it work? Trends Neurosci 28(2):86–92
Lindvall O, Kokaia Z, Martinez-Serrano A (2004) Stem cell therapy for human neurodegenerative disorders—how to make it work. Nat Med 10:42–50
Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG (2006) Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci 26(13):3377–3389
Parr AM, Kulbatski I, Tator CH (2007) Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J Neurotrauma 24(5):835–845
Dellatore SM, Garcia AS, Miller WM (2008) Mimicking stem cell niches to increase stem cell expansion. Curr Opin Biotechnol 19(5):534–540
Andrades JA, Nimni ME, Han B, Ertl DC, Hall FL, Becerra J (1996) Type I collagen combined with a recombinant TGF-beta serves as a scaffold for mesenchymal stem cells. Int J Dev Biol 1:107–108
Xie J, Willerth SM, Li X, Macewan MR, Rader A, Sakiyama-Elbert SE, Xia Y (2009) The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials 30(3):354–362
Lee JY, Bashur CA, Goldstein AS, Schmidt CE (2009) Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 30(26):4325–4335
Schnell E, Klinkhammer K, Balzer S, Brook G, Klee D, Dalton P, Mey J (2007) Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials 28(19):3012–3025
Li W, Guo Y, Wang H, Shi D, Liang C, Ye Z, Qing F, Gong J (2008) Electrospun nanofibers immobilized with collagen for neural stem cells culture. J Mater Sci Mater Med 19(2):847–854
Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S (2008) Electrospun poly(epsilon-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 29(34):4532–4539
Horne MK, Nisbet DR, Forsythe JS, Parish CL (2010) Three-dimensional nanofibrous scaffolds incorporating immobilized BDNF promote proliferation and differentiation of cortical neural stem cells. Stem Cells Dev 19(6):843–852
Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS (2005) Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials 26(25):5158–5166
Pettikiriarachchi JTS, Parish CL, Shoichet MS, Forsythe JS, Nisbet DR (2010) Biomaterials for brain tissue engineering. Aust J Chem 63(8):1143–1154
Wang TY, Forsythe JS, Parish CL, Nisbet DR (2012) Biofunctionalisation of polymeric scaffolds for neural tissue engineering. J Biomater Appl 27(4):369–390
Nisbet DR, Forsythe JS, Shen W, Finkelstein DI, Horne MK (2009) A review of the cellular response on electrospun nanofibers for tissue engineering. J Biomater Appl 24(1):7–29
Kim CH, Khil MS, Kim HY, Lee HU, Jahng KY (2006) An improved hydrophilicity via electrospinning for enhanced cell attachment and proliferation. J Biomed Mater Res B Appl Biomater 78(2):283–290
Duan Y, Wang Z, Yan W, Wang S, Zhang S, Jia J (2007) Preparation of collagen-coated electrospun nanofibers by remote plasma treatment and their biological properties. J Biomater Sci Polym 18(9):1153–1164
Ma Z, Gao C, Gong Y, Shen J (2005) Cartilage tissue engineering PLLA scaffold with surface immobilized collagen and basic fibroblast growth factor. Biomaterials 26(11):1253–1259
Wang Y, Lu L, Zheng Y, Chen X (2006) Improvement in hydrophilicity of PHBV films by plasma treatment. J Biomed Mater Res A 76(3):589–595
Ho MH, Hou LT, Tu CY, Hsieh HJ, Lai JY, Chen WJ, Wang DM (2006) Promotion of cell affinity of porous PLLA scaffolds by immobilization of RGD peptides via plasma treatment. Macromol Biosci 6(1):90–98
Shih YRV, Chen CN, Tsai SW, Wang YJ, Lee OK (2006) Growth of mesenchymal stem cells on electrospun type I collagen nanofibers. Stem Cells 24(11):2391–2397
Prabhakaran MP, Venugopal J, Chan CK, Ramakrishna S (2008) Surface modified electrospun nanofibrous scaffolds for nerve tissue engineering. Nanotechnology 19(45):455102
Venugopal J, Low S, Choon AT, Kumar AB, Ramakrishna S (2008) Electrospun-modified nanofibrous scaffolds for the mineralization of osteoblast cells. J Biomed Mater Res A 85(2):408–417
Chen YC, Lee DC, Hsiao CY, Chung YF, Chen HC, Thomas JP, Pong WF, Tai NH, Lin IN, Chiu IM (2009) The effect of ultra-nanocrystalline diamond films on the proliferation and differentiation of neural stem cells. Biomaterials 30(20):3428–3435
Zhao C, Tan A, Pastorin G, Ho HK (2013) Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol Adv 31(5):654–668
Vercelli A, Mereuta OM, Garbossa D, Muraca G, Mareschi K, Rustichelli D, Ferrero I, Mazzini L, Madon E, Fagioli F (2008) Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 31(3):395–405
Bouchez G, Sensebé L, Vourc’h P, Garreau L, Bodard S, Rico A, Guilloteau D, Charbord P, Besnard JC, Chalon S (2008) Partial recovery of dopaminergic pathway after graft of adult mesenchymal stem cells in a rat model of Parkinson’s disease. Neurochem Int 52(7):1332–1342
Forraz N, McGuckin CP (2011) The umbilical cord: a rich and ethical stem cell source to advance regenerative medicine. Cell Prolif 44(1):60–69
Romanov YA, Svintsitskaya VA, Smirnov VN (2003) Searching for alternative source of postnatal human mesenchymal stem cells; candidate MSC-like cells from umbilical cord. Stem Cells 21(1):105–110
Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S (2003) Matrix cells from Wharton’s Jelly from neurons and glia. Stem Cells 21(1):50–60
Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE (2005) Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells 23(2):220–229
Paldino E, Cenciarelli C, Giampaolo A, Milazzo L, Pescatori M, Hassan HJ, Casalbore P (2014) Induction of dopaminergic neurons from human Wharton’s jelly mesenchymal stem cell by forskolin. J Cell Physiol 229(2):232–244
Yang F, Xu CY, Kotaki M, Wang S, Ramakrishna S (2004) Characterization of neural stem cells on electrospun poly(L-lactic acid) nanofibrous scaffold. J Biomater Sci Polym Ed 15(12):1483–1497
Chen F, Lee CN, Teoh SH (2007) Nanofibrous modification on ultra-thin poly(e-caprolactone) membrane via electrospinning. Mater Sci Eng 27(2):325–332
Prabhakaran MP, Venugopal JR, Ramakrishna S (2009) Mesenchymal stem cell differentiation to neuronal cells on electrospun nanofibrous substrates for nerve tissue engineering. Biomaterials 30(28):4996–5003
Wang J, Ye R, Wei Y, Wang H, Xu X, Zhang F, Qu J, Zuo B, Zhang H (2012) The effects of electrospun TSF nanofiber diameter and alignment on neuronal differentiation of human embryonic stem cells. J Biomed Mater Res A 100(3):632–645
Binan L, Tendey C, De Crescenzo G, El Ayoubi R, Ajji A, Jolicoeur M (2014) Differentiation of neuronal stem cells into motor neurons using electrospun poly-L-lactic acid/gelatin scaffold. Biomaterials 35(2):664–674
Liqing Y, Jia G, Jiqing C, Ran G, Fei C, Jie K, Yanyun W, Cheng Z (2011) Directed differentiation of motor neuron cell-like cells from human adipose-derived stem cells in vitro. Neuroreport 22(8):370–373
Wu CY, Whye D, Mason RW, Wang W (2012) Efficient differentiation of mouse embryonic stem cells into motor neurons. J Vis Exp 9(64), e3813
Wichterle H, Lieberam I, Porter JA, Jessell TM (2002) Directed differentiation of embryonic stem cells into motor neurons. Cell 110(3):385–397
Luca AC, Mersch S, Deenen R, Schmidt S, Messner I, Schäfer KL, Baldus SE, Huckenbeck W, Piekorz RP, Knoefel WT, Krieg A, Stoecklein NH (2013) Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PLoS One 8(3), e59689
Niknamasl A, Ostad SN, Soleimani M, Azami M, Salmani MK, Lotfibakhshaiesh N, Ebrahimi-Barough S, Karimi R, Roozafzoon R, Ai J (2014 ) A new approach for pancreatic tissue engineering: human endometrial stem cells encapsulated in fibrin gel can differentiate to pancreatic islet beta-cell. Cell Biol Int 38(10):1174–1182
Asmani MN, Ai J, Amoabediny G, Noroozi A, Azami M, Ebrahimi-Barough S, Navaei-Nigjeh M, Ai A, Jafarabadi M (2013) Three-dimensional culture of differentiated endometrial stromal cells to oligodendrocyte progenitor cells (OPCs) in fibrin hydrogel. Cell Biol Int 37(12):1340–1349
Sumanasinghe RD, Bernacki SH, Loboa EG (2006) Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue Eng 12(12):3459–3465
Lund AW, Stegemann JP, Plopper GE (2009) Inhibition of ERK promotes collagen gel compaction and fibrillogenesis to amplify the osteogenesis of human mesenchymal stem cells in three-dimensional collagen I culture. Stem Cells Dev 18(2):331–341
Walther C, Gruss P (1991) Pax-6, a murine paired box gene, is expressed in the developing CNS. Development 113(4):1435–1449
Hendrickson ML, Rao AJ, Demerdash ON, Kalil RE (2011) Expression of nestin by neural cells in the adult rat and human brain. PLoS One 6(4), e18535
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4):677–689
Peyton SR, Putnam AJ (2005) Extracellular matrix rigidity governs smoth muscle cell motility in a biphasic fashion. J Cell Physiol 204(1):918–923
Troyer DL, Weiss ML (2008) Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells 26(3):591–599
Can A, Karahuseyinoglu S (2007) Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells 25(11):2886–2895
Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D (2006) Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 24(3):781–792
La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M, Di Stefano A, Giannuzzi P, Marasà L, Cappello F, Zummo G, Farina F (2009) Isolation and characterization of Oct-4þ/HLA-Gþ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol 131(2):267–282
Fong C-Y, Chak L-L, Biswas A, Tan J-H, Gauthaman K, Chan W-K, Bongso A (2011) Human Wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev Rep 7(1):1–16
Fong CY, Richards M, Manasi N, Biswas A, Bongso A (2007) Comparative growth behaviour and characterization of stem cells from human Wharton’s jelly. Reprod BioMed Online 15(6):708–718
Prabhakaran MP, Venugopal JR, Chyan TT, Hai LB, Chan CK, Lim AY, Ramakrishna S (2008) Electrospun biocomposite nanofibrous scaffolds for neural tissue engineering. Tissue Eng A 14(11):1787–1797
Ebrahimi-Barough S, Javidan JA, Saberi H, Joghataei MT, Rahbarghazi R, Mirzaei E, Faghihi F, Shirian S, Ai A, Ai J (2014) Evaluation of motor Neuron-like cell differentiation of hEnSCs on biodegradable PLGA nanofiber Scaffolds. Mol Neurobiol. doi:10.1007/s12035-014-8931-2
Ebrahimi-Barough S, Hoveizi E, Norouzi Javidan A, Ai J (2015) Investigating the neuroglial differentiation effect of neuroblastoma conditioned medium in human endometrial stem cells cultured on 3D nanofibrous scaffold. J Biomed Mater Res A. doi:10.1002/jbm.a.35397
Acknowledgments
The author wish to thank Iran University of Medical Sciences and Iranian National Science Foundation (grant number 91001166).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bagher, Z., Azami, M., Ebrahimi-Barough, S. et al. Differentiation of Wharton’s Jelly-Derived Mesenchymal Stem Cells into Motor Neuron-Like Cells on Three-Dimensional Collagen-Grafted Nanofibers. Mol Neurobiol 53, 2397–2408 (2016). https://doi.org/10.1007/s12035-015-9199-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9199-x