Abstract
Diabetes mellitus substantially increases the risk of stroke and enhances brain’s vulnerability to ischemia insult. In a previous study, Chikusetsu saponin IVa (CHS) pretreatment was proved to protect the brain from cerebral ischemic in normal stroke models. Whether CHS could attenuate cerebral ischemia/reperfusion (I/R) injury in diabetic mice and the possible underlying mechanism are still unrevealed. Male C57BL/6 mice were injected streptozotocin to induce diabetes. After that, the mice were pretreated with CHS for 1 month, and then, focal cerebral ischemia was induced following 24-h reperfusion. The neurobehavioral scores, infarction volumes, and some cytokines in the brain were measured. Apoptosis was analyzed by caspase-3, Bax, and Bcl-2 expression. Downstream molecules of adiponectin (APN) were investigated by Western blotting. The results showed that CHS reduced infarct size, improved neurological outcomes, and inhibited cell injury after I/R. In addition, CHS pretreatment increased APN level and enhanced neuronal AdipoR1, adenosine monophosphate-activated protein kinase (AMPK), and glycogen synthase kinase 3 beta (GSK-3β) expression in a concentration-dependent manner in diabetic mice, and these effects were abolished by APN knockout (KO). In vitro test, CHS treatment also alleviated PC12 cell injury and apoptosis, evidenced by reduced tumor necrosis factor alpha (TNF-α), malondialdehyde (MDA) and caspase-3 expression, and Bax/Bcl-2 ratio in I/R injured cells. Moreover, CHS enhanced AdipoR1, AMPK, and GSK-3β expression in a concentration-dependent manner. Likewise, short interfering RNA (sinRNA) knockdown of liver kinase B1 (LKB1), an upstream kinase of AMPK, reduced the ability of CHS in protecting cells from I/R injury. Furthermore, this LKB1-dependent cellular protection resulted from AdipoR1 and APN activation, as supported by the experiment using sinRNA knockdown of AdipoR1 and APN. Thus, CHS protected brain I/R in diabetes through AMPK-mediated phosphorylation of GSK-3β downstream of APN-LKB1 pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by hyperglycemia and long-term complications affecting the eyes, nerves, blood vessels, and kidneys [1, 2]. World Health Organization data shows that 347 million people worldwide are presently suffering from diabetes. Worldwide, health care expenditure for diabetes was US$471 billion in 2012 [3]. One prominent feature of T2DM is hyperglycemia. It is reported that hyperglycemia increases mortality and morbidity in stroke patients [4, 5] and T2DM substantially increases the risk of cerebral ischemia/reperfusion (I/R) injury [6]. Moreover, diabetic patients are more likely to experience the development of cerebral infarction, indicating that ischemia in diabetics is less likely to be reversible [7]. Unfortunately, effective intervention in I/R injury of diabetes is still under studying.
The possible mechanisms associated with severe damage caused by stroke in diabetic patients and animals may include degeneration of endothelial cells, disruption of blood–brain barrier (BBB), enhanced advanced glycation end product (AGE)-induced apoptosis, biochemical changes in cerebral capillaries, and alteration of neurotransmitter activity [8, 9]. With regard to cerebral I/R pathophysiology, it is reported that hyperglycemia exacerbates brain injury due to poor blood flow to the ischemic penumbra [10], the accumulation of lactate and intracellular acidosis in the ischemic brain [11], and the enhancement of inflammatory response [12]. Furthermore, diabetes is documented to enhance oxidative stress in the central nervous system of diabetic subjects [13]. Rizk and colleagues reported that focal I/R in diabetic rats increases neuronal apoptosis in the CA1 hippocampus, while neuronal necrosis in the cerebral cortex [14]. Also, hyperglycemia-induced selective vulnerability of certain areas of the brain otherwise resistant to ischemic damage has been noted to mediate aggravation of ischemic outcome in hyperglycemia [15].
Adenosine monophosphate-activated protein kinase (AMPK) plays a key role in regulating carbohydrate and lipid metabolism and is a potential therapeutic target in the treatment of metabolic diseases [16]. The benefit of exercise in diabetic patients is well known, and recent research indicates that AMPK plays a major role in this exercise-related effect. In several stressful situations, when cellular adenosine triphosphate (ATP) level falls down, the AMPK is phosphorylated and consequently activates its related proteins [17]. In ischemic condition, the Thr172 in the catalytic subunit of AMPK protein is reported to be phosphorylated [18]. Adiponectin (APN) is a cytokine produced predominantly in the adipose tissue and is abundantly present in the plasma [19]. There are pieces of evidence suggesting that APN and adiponectin receptors (AdipoR1 and AdipoR2) are widely expressed in the brain and play important roles in ischemia injury [20, 21]. Glycogen synthase kinase 3 beta (GSK-3β) has multiple functions, including cellular development regulation and tissue protection. Lots of papers reported that GSK-3β is involved in the protection of brain ischemia injury or brain trauma [22, 23]. The inhibition of GSK-3β was effective for regulating metabolism disorders. In addition, acute GSK-3β inhibition was proved to be a novel therapeutic strategy for acute myocardial injury in a diabetic model [24]. The activated form of AMPK is responsible for metabolic changes via the phosphorylation of GSK-3β, which is directly or indirectly related to glucose production [25]. It is reported that direct inactivation of GSK-3β inhibits the transcriptional activity of CREB through AMPK-induced phosphorylation of GSK-3β [26]. Therefore, GSK-3β might play crucial roles in cerebral protection in diabetes mellitus (DM) patients.
Over the centuries, the traditional Chinese medicine (TCM) has served as a major source of medicines for the prevention and treatment of diseases including DM. Aralia taibaiensis (AT), which has been used as a folk medicine for a long time, is used to treat diabetic and metabolic diseases in China, Korea, and Japan. But, its mechanism of action is few known for us. Previous study showed that the total saponin extract of AT (sAT) and the compound Chikusetsu saponin IVa (CHS, Fig. 1a) isolated from sAT dramatically stimulated insulin secretion [27]. Our recent study indicated a beneficial effect of CHS on myocardial cell subjected to high glucose in vivo and in vitro (not shown). The pieces of evidence suggested that CHS is a potential treatment for metabolic disorder and myocardium injury. However, whether CHS is effective in cerebral I/R in diabetes is still unknown. Therefore, we undertook the present study to determine whether CHS could protect the brain against I/R injury in streptozotocin (STZ)-induced diabetic mice and elucidate the hypothesis that APN, liver kinase B1 (LKB1), AMPK, and GSK-3β phosphorylation might be involved in the protection of CHS.
Materials and Methods
Drugs and Reagents
Chikusetsu saponin IVa (CHS), the purity being more than 98 %, was obtained from the New Drug Research and Development Center, Fourth Military Medical University. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and trypsin were purchased from GIBCO (Life Technologies, Grand Island, NY). Rosiglitazone (Ros), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), and 2,3,5-triphenyltetrazolium chloride (TTC) were obtained from Sigma (St. Louis, MO). The detection kits of malondialdehyde (MDA), superoxide dismutase (SOD), and caspase-3 were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, People’s Republic of China). Tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) enzyme-linked immunosorbent assay (ELISA) kits were purchased from Xitang Biology Technology Company (Shanghai, People’s Republic of China). APN ELISA kit was purchased from AdipoGen, Inc. (Incheon, South Korea). Polyclonal rabbit anti-mouse AMPK, GSK-3β, Bcl-2, β-actin, Bax, LKB1, AdipoR1, and AdipoR2 antibodies were purchased from Cell Signaling Technologies, MA, USA. All other chemicals used in this experiment were the purest grade commercially available.
Animals and Type 2 Diabetic Mouse Model
The experimental protocol was approved by the Ethics Committee for Animal Experimentation and was performed according to the Guidelines for Animal Experimentation of the Fourth Military Medical University and the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised in 1996. The mice and APN gene knockout mice (APN-KO) were provided by the Experimental Animal Center of the Fourth Military Medical University. DM was induced in male C57BL/6 mice 8–12 weeks old, weighing 23–25 g, by intraperitoneal injection of streptozotocin (STZ, Sigma, St Louis, MO, USA) at a dose of 50 mg/kg dissolved in 100 mM citrate buffer pH 4.5 for five consecutive days [28]. After 4 weeks, blood glucose levels were measured using Bayer’s BREEZE2 meter (Bayer Health Care LLC, Mishawaka, USA) by tail vein blood sampling. Mice with blood glucose levels of >11.1 mM were used for the present study. Mice were housed under controlled conditions with a 12-h light/dark cycle, at a temperature of 25 ± 2 °C, and humidity of 60–70 %. The mice were allowed free access to standard rodent diet and tap water.
Experimental Protocol
Mice were randomly divided into six groups: Sham, middle cerebral artery occlusion (MCAO), and MCAO pretreated with CHS (30, 60, and 120 mg/kg) or rosiglitazone (Ros, 2 mg/kg). CHS or Ros was given by gavage for 4-week duration of treatment starting after induction of diabetes, as shown in Fig. 1b. During the treatment period, general characteristics including water intake were assessed on a daily basis, while food consumption and body weight were monitored weekly. At termination, mice were weighed and then euthanized with an intraperitoneal injection of pentobarbital sodium (65 mg/kg body weight). Focal cerebral ischemia was induced by MCAO in mice using an intraluminal filament technique, as previously described [29]. After 1 h of MCAO, the filament was withdrawn and regional cerebral blood flow was restored to normal. Regional cerebral blood flow (rCBF) was measured by transcranial laser Doppler flowmetry (PeriFlux 5000; Perimed AB). A blood flow drop to 60 % of the baseline indicated successful blockage of the middle cerebral artery. After recovery from anesthesia, animals were kept in single cages for 24 h. The Sham group received only incision of the cervical skin to expose the common carotid artery, without occlusion of blood flow. The blood samples and brains were collected and stored at −80 °C until further analysis.
Neurobehavioral Evaluation and Infarct Assessment
After reperfusion, neurological assessment was performed by randomly choosing six rats from each group by staffs blinded to these groups. Neurological function was graded on a scale of 0 to 5 (0 = no deficit, 1 = failure to extend left forepaw fully, 2 = circling to the left, 3 = falling to the left, 4 = no spontaneous walking with a depressed level of consciousness, and 5 = dead). The animals were decapitated, and 2-mm-thick coronal sections from the brain were stained with 2 % TTC to evaluate the infarct volume, as described previously [28]. Infarction area was measured by subtracting the area of the noninfarcted ipsilateral hemisphere from that of the contralateral side. The infarct volume was calculated by integration of the infarct area in each section along the rostro-caudal axis.
Measurement of ROS
Six mice from each group were randomly selected for reactive oxygen species (ROS) measurement. The ipsilateral striatum was dissected out and minced on ice, incubated with 0.25 % trypsin at 37 °C for 60 min, and washed with DMEM containing 10 % FBS to harvest the cellular suspension. Free radical production was measured by the fluorescence method using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). DCFH-DA can cross cell membranes and is hydrolyzed by cellular esterases and then is oxidized to the fluorescent 2′,7′-dichlorofluorescein (DCF) in the presence of peroxides. Accumulation of DCF was measured using a spectrofluorometer (Shimadzu Corp., Japan).
Cell Culture
The neuron-like rat pheochromocytoma cell line PC12 cells were cultured in DMEM containing 10 % FBS and antibiotics (penicillin, 100 IU/mL; streptomycin, 100 μg/mL). The cultures were maintained at 37 °C in 5 % CO2 in a humidified incubator. Cell medium was replaced every 48 h. PC12 cells were incubated with DMEM containing CHS or with inhibitors under normoxic conditions for 6 h before I/R.
I/R Injury Model
PC12 cells were washed with phosphate-buffered solution (PBS) for one time and incubated in Earle’s balanced salt solution (116 mmol/L NaCl, 5.4 mmol/L KCl, 0.8 mmol/L MgSO4, l mmol/L NaH2PO4, 0.9 mmol/L CaCl2, and 10 mg/L phenol red). And then, the cells were incubated in a hypoxia chamber (Thermo Scientific, USA) containing a gas mixture of 95 % N2 and 5 % CO2 for 3 h. After ischemia, the cells were transferred back to full culture medium with oxygen for 6-h reperfusion. Normal control cells were incubated in a regular cell culture incubator under normoxic conditions.
Analysis of Cell Viability
After ischemia for 3 h followed by 6-h reperfusion pretreated with or without CHS, cell viability was assessed using a MTT assay. Briefly, cells in 96-well plates were rinsed with PBS, MTT (5 mg/mL) was added to each well, and then, they were incubated at 37 °C for 4 h. The medium with MTT was removed, and 150 μL DMSO was added to each well. The absorbance was measured at 490 nm using a microplate reader (Bio-Rad Laboratory, Hercules, CA). Cell viabilities are presented as values relative to those obtained when cells were treated with vehicle to control for variation between experiments.
Biochemical Analysis
Blood samples were kept at 25 °C for 30 min. Serum was separated after centrifugation at 3000g for 20 min, and the supernatant was stored at −80 °C until use. PC12 cells were homogenized at 4 °C in cold buffer (1.5 mM Tris base–HCl, 1 mM DTT, 0.25 M sucrose, 1 mM MgCl2, 1.25 mg/mL pepstatin A, 10 mg/mL leupeptin, 2.5 mg/mL aproptonin, 0.5 mM PMSF, 2.5 mM EDTA, 1 mM EGTA, 0.1 M Na3VO4, 50 mM NaF, and 2 mM sodium pyrophosphate) and then centrifuged at 3000g for 10 min at 4 °C. The supernatant was collected and stored at −80 °C. Plasma and cellular TNF-α, IL-6, MDA, and SOD levels were determined using commercially available rat ELISA kits as per the manufacturer’s protocol.
Measurements of Plasma and Cellular APN Level
Plasma and brain APN levels were measured using an APN kit following the manufacturer’s protocol. The protein content of the samples was measured using the Bio-Rad protein assay kit with the use of bovine serum albumin as a standard. The values of plasma or brain APN were expressed as nanogram per milliliter in plasma or pictogram per kilogram in the brain.
Measurement of Caspase-3 Activities
Diabetic mice and PC12 cells were pretreated with or without CHS, followed by subjection to I/R. After incubation, the cells were rinsed with cold PBS and resuspended in chilled cell lysis buffer incubated for 20 min on ice and then centrifuged at 10,000g for 5 min. Caspase-3 activities under various experimental conditions were measured in the brain and PC12 cell extracts using a colorimetric assay kit from Nanjing Jiancheng Bioengineering Institute (Nanjing, People’s Republic of China). The levels of chromophore p-nitroanilide (pNA), after cleavage from the labeled substrate of caspase-3, DEVD-pNA, were determined by using a spectrophotometry with 405 nm and an emission wavelength of 505 nm.
sinRNA Transfection
APN and LKB1-specific short interfering RNA (sinRNA) molecules were chemically synthesized by Shanghai Genechem Company. Transfection was performed using Lipofectamine 2000, according to the manufacturer’s protocol. Following 48-h transfection, PC12 cells were pretreated with CHS and subjected to I/R.
Western Blotting Analysis
To determine levels of protein expression, we prepared extracts from brain tissues and PC12 cells. After reperfusion, the brain tissues were isolated from ischemic penumbra cortices and homogenized in ice-cold buffer. PC12 cells were scraped off the culture dishes and lysed by incubation with lysis buffer for 30 min. The homogenates from brain tissues and PC12 cells were centrifuged (10,000×g for 20 min at 4 °C), and the protein concentration of the supernatants was measured by a Bio-Rad protein assay kit.
Equal amounts of the protein samples were loaded onto 10 % sodium dodecyl sulfate–polyacrylamide gels, separated by electrophoresis, and transferred onto a polyvinylidene difluoride membrane (PVDF). The membranes were incubated in the presence of different primary antibodies at 4 °C overnight, and then, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The immunoreactive bands were detected using the ECL method. Optical densities of the bands were scanned and quantified image analysis systems (Bio-Rad, USA). β-Actin served as an internal control.
Statistical Analysis
All data are expressed as mean ± SD and were analyzed by SPSS 18.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used for statistical comparisons between the different groups. The neurological deficit scores were expressed as median (range) and analyzed with the Kruskal–Wallis test. P < 0.05 was considered to be statistically significant.
Results
CHS Conferred Neuroprotection in Diabetic Mice
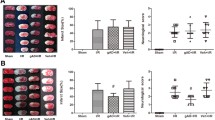
Type 2 diabetic mice showed hyperglycemia, hyperlipidemia, polydipsia, and polyphagia. Plasma glucose and lipid were significantly increased in the diabetic mice group compared with the controls, and the body weight in the type 2 diabetic group was significantly higher than that in the control group (P < 0.01). Compared with type 2 diabetic rats, the plasma glucose, lipid, and body weight in type 2 diabetic rats treated with CHS or Ros were reduced notably (P < 0.05). There was no statistical significance between Ros treatment group and CHS treatment group (Table 1). Pretreatment of diabetic mice with CHS for 1 month before MCAO was used to determine the protection of brain tissue from ischemic injury (Fig. 1b). Compared with the Sham group, MCAO group showed strong neurological deficits 24 h after reperfusion. Pretreatment with CHS significantly improved the neurological scores compared with the MCAO group (Fig. 2a). Meanwhile, assessment of infarct sizes 24 h after reperfusion showed a significantly smaller brain infarct volume in CHS +MCAO group compared with the MCAO group (Fig. 2b).
CHS pretreatment alleviated cerebral ischemic injury in diabetic mice. a Neurological scores 24 h after reperfusion in the diabetic mice with MCAO. CHS pretreatment significantly improved the neurological scores. b Infarct sizes 24 h after reperfusion in the diabetic mice with 60 min of MCAO. Compared with MCAO group, CHS pretreatment significantly inhibited the brain injury induced by I/R in the diabetic mice. c Caspase-3 activation. Substrates used for caspase-3 were Asp-Glu-Val-Asp-7-amino-4-trifluoromethylcoumarin (DEVD-AFC). d Bax, Bcl-2, and cleaved caspase-3 were detected by Western blot as mentioned in the “Materials and Methods” section. The CHS-treated group showed a lower ratio of Bax/Bcl-2 and cleaved caspase-3 compared with the MCAO group. ##P < 0.05 vs. the sham group; *P < 0.05, **P < 0.01 vs. the MCAO group. CHS Chikusetsu saponin IVa, Ros rosiglitazone (2 mg/kg), MCAO middle cerebral artery occlusion
Cell apoptosis is another effect of I/R injury and can serve as a measure of the extent of I/R injury. In this study, we found that CHS treatment alleviated cerebral apoptosis during I/R, evidenced by reduced amounts of caspase-3 (Fig. 2c). Western blot analysis of Bax/Bcl-2 and caspase-3 further confirmed this result. Bax/Bcl-2 and cleaved caspase-3 levels increased significantly in MCAO group compared with the Sham group. Moreover, the presence of CHS decreased MCAO-induced damages in concentration-dependent manners (Fig. 2d). There was no statistical significance between CHS (60 mg/kg) treatment group and Ros treatment group (P > 0.05). These results suggested that the neuroprotection of CHS against ischemic brain damage is via inhibition of apoptosis.
CHS Suppressed Oxidative Stress and Inflammatory Reaction
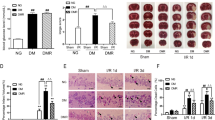
In this study, MDA and ROS were used as a marker of oxidative stress. After 24 h of reperfusion, MDA content showed a lower level in the CHS + MCAO group compared with the MCAO group (Fig. 3a). On the other hand, CHS pretreatment efficiently prevented the formation of ROS, compared with the MCAO group (Fig. 3b).
Effect of CHS pretreatment on oxidative stress and inflammatory reaction after reperfusion in diabetic mice. CHS significantly reduced MDA production (a) and ROS formation (b) 24 h after reperfusion. IL-6 (c) and TNF-α (d) levels in plasma. ##P < 0.01 vs. the sham group; *P < 0.05, **P < 0.01 vs. the MCAO group. CHS Chikusetsu saponin IVa, Ros rosiglitazone, MCAO middle cerebral artery occlusion, ROS reactive oxygen species, MDA malondialdehyde, IL-6 interleukin-6, TNF-α tumor necrosis factor alpha
To assess the anti-inflammatory properties of CHS, plasma IL-6 and TNF-α levels were determined. As shown in Fig. 3c, d, plasma IL-6 and TNF-α levels were increased in the mice with MCAO. CHS treatment significantly reduced plasma IL-6 and TNF-α levels in the MCAO group comparable to that in the Sham group. There was no statistical significance between CHS (60 mg/kg) treatment group and Ros treatment group in type 2 diabetic rats (P > 0.05).
CHS Pretreatment Increased APN Plasma Level and APN Receptor Expression
In diabetic mice, blood samples were taken 24 h after reperfusion and APN expression was decreased in ischemia animals compared with the Sham group. Improvement of APN levels was detected in CHS-treated diabetic mice serum and brain tissues (Fig. 4a). Furthermore, the expression of APN in serum and brain tissues was downregulated by APN knockdown compared with the Sham and CHS groups (P < 0.01). AdipoR1 and AdipoR2 serve as receptors of APN and mediate APN-related signaling pathways. We further investigated whether or not brain APN receptors are altered in diabetic mice and whether they can be affected by CHS. Using Western blot, we observed that AdipoR1 was significantly increased in ischemic mice and the levels of the receptor were higher in CHS-treated mice. Meanwhile, there was no change in AdipoR2 levels (Fig. 4b). Moreover, CHS treatment alone also promoted an increase in the APN levels.
a Plasma and cerebral APN content and AdipoRs, AMPK, and GSK-3β expression in diabetic mice brain after CHS pretreatment. Plasma and cerebral APN levels (n = 6/group). APN levels in the plasma and brain were significantly decreased in the MCAO group compared with the sham group while increased in the CHS-treated group. b Pretreatment with CHS significantly upregulated the protein expression of AdipoR1 after ischemia-reperfusion injury in diabetic mice. Cerebral protein expression of AMPK and GSK-3β (c) and their phosphorylation status in sham and diabetic rats treated with or without MCAO. Mean band density was normalized relative to β-actin. Values are the mean ± SD. #P < 0.05, ##P < 0.01 vs. the sham group; **P < 0.01 vs. the MCAO group, &&P < 0.01 vs. the CHS treated group. CHS treated with CHS (120 g/kg) only, APN-KO APN knockout mice treated with CHS (120 g/kg)
After confirming the role of CHS on APN in cerebral I/R, we next tried to determine the underlying mechanism of CHS in cerebral I/R protection. A number of pathways involving molecules like AMPK, Akt, and STAT-3 have been reported to be associated with APN-mediated effects. Herein, we found that CHS activated AMPK via increasing phosphorylation of AMPK at Thr172, as well as phosphorylation of GSK-3β at Ser9, and further decreased in those of APN KO mice (Fig. 4c). From the above, it is clear that APN/AdipoR1/AMPK/GSK-3β pathway may be involved in the mechanism of CHS in cerebral I/R protection in vivo.
CHS Pretreatment Protected PC12 Cells from I/R-Induced Cytotoxicity and Apoptosis
To estimate the protective effect of CHS on PC12 cells, cell viability was tested. As shown by MTT assay, cell viability was markedly decreased after ischemia for 3 h followed by 6-h reperfusion. However, when cells were incubated with CHS, the cytotoxicity was significantly attenuated in a concentration-dependent manner, as shown in Fig. 5b. To further investigate the protective effect of CHS, TNF-α and MDA levels in cellular supernatant were estimated. Significant increases of TNF-α and MDA leakage rates were observed after I/R. Incubation with various concentrations of CHS significantly abolished these tendencies in a concentration-dependent manner (Fig. 5c, d).
CHS pretreatment protected PC12 cells from I/R-induced cytotoxicity and apoptosis. a Flow chart showing the timeline scheme of the experiment in vitro. b Protective effects of CHS on I/R-induced cytotoxicity in PC12 cells. PC12 cell viability was assessed by measuring the MTT reduction. Results were shown as fold of control. Cellular MDA (c) and TNF-α (d) contents. The cellular MDA and TNF-α contents were assessed as mentioned in the “Materials and Methods” section. e Effects of CHS on PC12 cell apoptosis induced by I/R. PC12. Cells were subjected to I/R with or without CHS pretreatment and then double stained with annexin-V/propidium iodide (PI). f Caspase-3 level in PC12 cells subjected to I/R. Values obtained from three independent experiments are expressed as mean ± SD. #P < 0.05, ##P < 0.01 vs. the control group; *P < 0.05, **P < 0.01 vs. the I/R group. I/R ischemic/reperfusion, CHS PC12 cells treated with CHS (40 μM) only, Ros PC12 cells pretreated with rosiglitazone (1 μM)
Apoptotic cells were estimated by flow cytometric analysis of annexin-V and propidium iodide-labeling cells, as shown in Fig. 5e. The normal PC12 cell apoptosis rate was only 4.6 %; after ischemia for 3 h followed by 6-h incubation with neurobasal medium, the apoptosis rate of the I/R group was increased to 41.2 %. Incubation with CHS for 6 h arrested the apoptosis in a concentration-dependent manner. This result was consistent with the changes of caspase-3 levels in the cells treated with CHS (Fig. 5f). There was no statistical significance between the CHS treatment group and Ros treatment group in PC12 cells subjected to I/R. We also found that CHS treatment alone had no injury effect on PC12 cells.
Effect of CHS on APN Receptors and the Downstream Signaling Pathways In Vitro
To assess the effect of CHS on APN receptors and the downstream protein expression in vitro, PC12 cells were cultured and treated with CHS and then subjected to I/R. From Western blot analysis, we found that the expression of AdipoR1 was increased significantly compared with the I/R group. AdipoR2 had no change between CHS groups and I/R group (Fig. 6).
CHS activated APN receptors and the downstream signaling pathways in PC12 subjected to I/R. a Cerebral AdipoR1/2 protein expression. b Effect of CHS on phosphorylation of AMPK and GSK-3β. PC12 cells were pretreated with CHS in different doses and then subjected to I/R. Proteins were analyzed by Western blot. Values obtained from three independent experiments are expressed as mean ± SD. #P < 0.05, ##P < 0.01 vs. the control group; **P < 0.01 vs. the I/R group. I/R ischemic/reperfusion, CHS PC12 cells treated with CHS (40 μM) only
AMPK plays an important role in brain metabolism and cerebral survival response to ischemia. However, it is unknown whether or not AMPK could be affected by CHS. Therefore, we examined AMPK protein expression and its phosphorylation status in PC12 cells. CHS activated the phosphorylation of AMPK in PC12 cells subjected to I/R injury (Fig. 6). At the same time, the phosphorylation of GSK-3β at serine 9 which inhibits GSK-3β activity as an inactive form was upregulated in a dose-dependent manner in CHS-treated PC12 cells (Fig. 6).
The Roles of AMPK and GSK-3β Activation in PC12 Cells
To assess the roles of AMPK and GSK-3β activation by CHS in protecting PC12 cells from apoptosis, the AMPK inhibitory effects on caspase-3 levels were then measured. The inhibitory action on caspase-3 by CHS was significantly reversed by compound C (an AMPK inhibitor) (Fig. 7a). Therefore, the protection of cells by CHS might be associated at least in part with AMPK phosphorylation. In an effort to identify downstream molecule of AMPK, we next examined the effect of AMPK on GSK-3β phosphorylation. CHS treatment increased the phosphorylation of GSK-3β, but this increase was prevented by compound C pretreatment (Fig. 7b). Overall, our results demonstrated that the cytoprotective effect of CHS may be associated with the phosphorylation of GSK-3β downstream of AMPK.
The protective roles of AMPK and GSK-3β activation in PC12 cells and the relationships between AMPK and GSK-3β. a Effect of compound C on I/R-induced apoptosis in PC12 cells. PC12 cells were pretreated with CHS (40 μM) and/or AMPK inhibitor compound C (3 μM) for 6 h and then subjected to I/R. Cell lysates were used to assess caspase-3 level as instruction of kits. b Effect of compound C on CHS-induced GSK-3β phosphorylation in PC12 cells. PC12 cells were pretreated with CHS (40 μM) and/or AMPK inhibitor compound C and then subjected to I/R. Cell lysates were subjected for Western blotting. The role of LKB1 activation by CHS in PC12 cells. Phosphorylation of LKB1 by CHS was assessed in dose-dependent (c) and time-dependent (d) manner. Immunoblot analyses were performed on the lysates of cells that had been treated with CHS for the indicated time period and drug concentrations. Results were confirmed by three replicates. Relative phosphorylated LKB1 band intensities of immunoblot data were quantified (right). **P < 0.01 vs. the 0 h group or the vehicle-treated control (0 μM). e The role of CaMKK and LKB1 in the cytoprotective effect of CHS. PC12 cells were transfected with sinRNAs directed against LKB1 or pretreated with STO-609 (an inhibitor of CaMKK, 1 μg/mL); then, the cells were incubated with CHS for 6 h, followed by subjection to I/R. Cell lysates were used to assess caspase-3 level. Results were confirmed by three replicates. #P < 0.05, ##P < 0.01 vs. the control group; **P < 0.01 vs. the I/R group; &&P < 0.01 vs. the CHS-treated group
The Role of LKB1 Activation in PC12 Cells
To identify the upstream signal of AMPK activation by CHS, the dose and time course effect of CHS on LKB1 phosphorylation was assessed. CHS treatment resulted in a notable increase in the phosphorylation of LKB1 (Fig. 7c, d). To test the role of LKB1 in protecting PC12 cells against I/R injury, the effect of sinRNA knockdown of LKB1 on caspase-3 level change was determined. The protective effect of CHS against cell apoptosis elicited by I/R was reversed by sinRNA knockdown of LKB1 (Fig. 7e). In contrast, treatment with STO-609 (1 μg/mL), an inhibitor of Ca2+/calmodulin-dependent protein kinase kinase (CaMKK), failed to antagonize the beneficial effect of CHS as indicated by no change in caspase-3. Therefore, the cytoprotective effect of CHS against cell apoptosis may depend on LKB1, but not CaMKK.
LKB1 Phosphorylation by CHS Pretreatment Was Mediated by AdipoR1
To determine whether the activation of AdipoR1 by CHS pretreatment was associated with the phosphorylation regulation of LKB1 and AMPK, we administered AdipoR1 sinRNA and evaluated the expression of LKB1 and AMPK by Western blot. As shown in Fig. 8a, we found no effects on P-LKB1 and P-AMPK expression in the control sinRNA group. However, the expression of P-LKB1 and P-AMPK was decreased in the sinAdipoR1 + I/R + CHS group compared with the control sinRNA + CHS group (P < 0.05). To determine the relationships between APN and LKB1, AMPK, and GSK-3β phosphorylation, APN sinRNA was used. As the results shown, the expression of P-LKB1, P-AMPK, and P-GSK-3β was decreased in the sinAPN + I/R + CHS group compared with the control sinRNA + CHS group (P < 0.05).
a Effects of AdipoR1 sinRNA on LKB1 and AMPK phosphorylation in CHS-treated cells. PC12 cells were transfected with sinRNAs directed against AdipoR1 or nontargeting control sinRNA, continuously incubated with CHS for 6 h, and then subjected to I/R. The expression of LKB1 and AMPK was tested by Western blot. Results were confirmed by three replicates. b Effects of APN sinRNA on LKB1, GSK-3β, and AMPK phosphorylation in CHS-treated cells. **P < 0.01 vs. the I/R group; &&P < 0.01 vs. the CHS-treated group. I/R ischemic/reperfusion, CHS Chikusetsu saponin IVa
Discussion
Diabetes mellitus (DM) and ischemic stroke are common diseases that often arise together. DM is a leading cause of cerebrovascular disorders [30], and stroke is the second leading cause of long-term disability worldwide [31]. In China, the estimated overall prevalence of diabetes was 11.6 % and the prevalence of prediabetes was 50.1 % [32]. In diabetic patients, cerebral I/R injury is associated with enhanced damageability to disability and death. The main pathological disorders include cognitive decline, microvessel disease, and dementia [33, 34]. In animal studies, hyperglycemic cerebral ischemia leads to severe neurological deficit mediated by oxidative stress [35]. Some preventive or therapeutic methods against DM cerebral ischemic injury, such as glucose-lowering treatment and thrombolysis, are still without strong supporting pieces of evidence [36]. Therefore, it is vital to reduce the incidence of cerebrovascular events and ameliorate cerebral ischemic injury in diabetic patients. In the present study, we examined the protective potential of CHS in vivo and in vitro. Our current study indicated that CHS pretreatment increased the production of APN, which elicited protective effects against cerebral I/R injury through neuronal AMPK-mediated phosphorylation of GSK-3β in diabetic mice and PC12 cells subjected to I/R.
CHS, a newly discovered and separated compound from sAT, belongs to triterpenoid saponin. Previous study of us had shown the anti hyperglycemic, hypolipidemic, and anti-oxidant activities of sAT in experimental T2DM rats [37]. The anti-injury ability of CHS in I/R-induced myocardial apoptosis in T2DM had also been well studied in our lab (data not shown). However, whether CHS has protective effects against cerebral I/R in T2DM rats was not known to us. In the current study, we found that CHS treatment decreased ischemic cerebral infarct size and neurological deficit in the mice with diabetes, respectively. We also found that CHS reduced fasting blood glucose, insulin, body weight, and lipids, which are induced by STZ in diabetic mice. To our knowledge, this was the first study demonstrated the protective role of CHS in diabetic mice with brain I/R injury.
It is well documented that inflammation and oxidative stress are two major mechanisms of I/R injury. Stroke triggers an inflammatory reaction for hours after the onset of a stroke, and this inflammation plays a central role in the pathogenesis of neuronal injury in ischemic stroke and especially in diabetic stroke [38]. In our experiments, CHS decreased the expression of IL-6 and TNF-α during brain I/R injury, demonstrating that CHS can exhibit protective effects during brain I/R injury through inhibiting the inflammatory cascade. Brain I/R triggers oxidative stress, which is an imbalance between the generation and elimination of ROS in the biological system. Our current results suggested that the production of ROS and MDA was significantly increased after cerebral I/R injury, while CHS pretreatment effectively neutralized the damage.
Neuronal apoptosis could be induced and even worsen under pathological situations, such as hyperglycemia complicated by ischemia [25]. Apoptosis represents the execution of an ATP-dependent death program often initiated by death ligand/death receptor interactions, such as Fas ligand with Fas, which leads to a caspase activation cascade [39]. Bax and Bcl-2 belong to the Bcl-2 family and are both involved in ischemia-induced neuronal cell apoptosis. Bcl-2 is an anti-apoptotic protein, while Bax is a pro-apoptotic one. In the current study, apoptotic events were revealed by the activation of caspase-3 as well as the ratio of Bax/Bcl-2. Our results suggested that with CHS pretreatment, the upregulation of Bax after I/R injury was inhibited, while the expression of Bcl-2 was enhanced, and these findings are consistent with the decreased activities of caspase-3 in vivo and in vitro.
APN is an abundant plasma protein secreted by the adipose tissue, which has at least three major functions, including insulin sensitization/metabolic regulatory function (in the liver and muscle), anti-inflammatory/vascular protective function, and anti-ischemic function [40]. Numerous studies revealed the correlation between the reduced APN level and increased morbidity/mortality of cardiovascular ischemic diseases and DM [41]. Some reports also demonstrated markedly increased I/R injury in APN-KO mice, and exogenous APN supplementation can significantly decrease myocardial apoptosis, infarct size, and impaired cardiac function [42]. In addition, other investigators found that the circulatory APN level was inversely correlated with the severity of acute liver and renal I/R injury [43, 44]. Although the presence of APN and its role in the brain still remain controversial, APN was found to be present in cerebrospinal fluid and might play an important role in physiological functions of the central nervous system (CNS) [45]. It has been reported that APN protected the brain against cerebral ischemic stroke [46]. AdipoR1 and AdipoR2, two APN receptors, have been identified and exert distinct biological properties in different tissues [47]. In mouse cortical neurons, both AdipoR1 and AdipoR2 are expressed, and AdipoR1 expression is more pronounced than AdipoR2 [48]. In our study, plasma APN levels were reduced in MCAO group and significantly elevated in the CHS-treated group, and the expression of AdipoR1, but not AdipoR2, was higher in CHS-treated diabetic mice after cerebral I/R injury. The different expressions between AdipoR1 and AdipoR2 might be due to their different distributions in the cortex area. The AdipoR1 expression from MCAO group showed a slight upregulation, but not significant difference compared with sham group. We presumed that the change might be due to the stress reaction.
AMPK can be activated by APN via APN receptors in various cell lines and tissues [49, 50]. Lots of literatures have shown that AMPK acts as a neuroprotective factor under several pathologic conditions, and AMPK deficiency indeed causes neurodegeneration [51]. The activation of AMPK through phosphorylation regulates apoptosis in response to energy depletion by inhibiting anabolic pathways and stimulating catabolic processes [52]. In this study, we found that AMPK signaling pathway was activated by CHS in diabetic mice and PC12 cells subjected to I/R. Although the mechanism of CHS’s cytoprotection involves AMPK activation, CHS does not directly activate AMPK in vitro (not shown). Therefore, proteins and/or components that lie upstream of AMPK might be the target(s) of CHS. CaMKK and LKB1 are two upstream kinases of AMPK in various cells, including brain cells. To determine whether any or both of these signaling pathways are involved in mediating the protective role of CHS, we used sinRNA of LKB1 and STO-609, an inhibitor of CaMKK, in PC12 cells. The results showed that sinLKB1, but not STO-609, abolished the cerebral protective effect of CHS, suggesting that the activation of AMPK by CHS was though LKB1.
GSK-3, originally identified as a regulator of glycogen metabolism, is now known as a multifaceted enzyme affecting a diverse range of biological functions, including cellular architecture, gene expression, and apoptosis. Emerging evidence suggests that GSK-3β participates in the regulation of metabolism disorders and cerebral ischemia injury. Inhibition of GSK-3β was recommended as a novel therapeutic target for DM treatment [53]. In particular, GSK-3β is well known to have critical roles in oxidative stress-induced neuronal cell death by enhancing the expression of proapoptotic proteins and by inhibiting the activity of anti-apoptotic proteins [54]. GSK-3β is inactivated by phosphorylation of serine, and its activity is increased by phosphorylation of tyrosine. TDZD-8, a GSK-3β inhibitor, was protective against cerebral I/R injury by inhibiting GSK-3β activity [55] and could stimulate a protection against acute myocardial ischemic injury in a DM rat model [24]. In the present study, CHS promoted the inhibitory phosphorylation of GSK-3β at serine 9. These results suggested that the protection of the brain by CHS might be associated at least in part with GSK-3β activation.
AMPK has been reported to directly inactivate GSK-3β through Ser9 phosphorylation in hepatocytes [56]. A previous study in differentiated hippocampal neurons and SY5Y cells has shown that 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) induces the phosphorylation of Akt and GSK-3β (pSer9) [28]. A recent study also showed a novel function of neuronal cytosolic enzyme adenylate kinase 1 (AK1) that regulates GSK-3β via AMPK [57]. To clarify the linkage between AMPK and GSK-3β in the protective role of CHS, compound C was used in the further study. The results demonstrated that the AMPK-dependent cerebral protection of CHS against I/R injury may be associated with the phosphorylation of GSK-3β.
Further studies also showed that inhibition of AdipoR1 or APN by sinRNA abolished LKB1, AMPK, and GSK-3β phosphorylation induced by CHS in PC12 cells. Meanwhile, silencing of AdipoR1 and APN or inhibition of AMPK phosphorylation abolished the anti-apoptotic effects. These findings provided the first evidence that CHS might prevent cellular apoptosis following cerebral I/R through an APN/AdipoR1-mediated AMPK/GSK-3β pathway in DM with I/R.
Summary
In conclusion, the present study demonstrated for the first time that CHS pretreatment attenuated cerebral I/R injury in STZ-induced diabetic mice; the protective effect may be mediated by AMPK-mediated phosphorylation of GSK-3β downstream of APN-LKB1 pathway (Fig. 9). The results suggested that CHS may have a potential role in the prophylaxis of cerebrovascular disease in diabetes.
Schematic diagram shows the protection mechanism of CHS pretreatment. CHS increases the production of APN which subsequently activates AdipoR1. Activated AdipoR1 promotes the p-LKB1, which enhances the expressions of AMPK and GSK-3β. Finally, the effects of blocking apoptosis, inflammation, and oxidative stress lead to a protection against cerebral I/R
References
Kim YM, Namkoong S, Yun YG, Hong HD, Lee YC, Ha KS, Lee H, Kwon HJ, Kwon YG, Kim YM (2007) Water extract of Korean red ginseng stimulates angiogenesis by activating the PI3K/Akt-dependent ERK1/2 and eNOS pathways in human umbilical vein endothelial cells. Biol Pharm Bull 30(9):1674–1679
Tuttle KR, McGill JB, Haney DJ, Lin TE, Anderson PW (2007) Kidney outcomes in long-term studies of ruboxistaurin for diabetic eye disease. Clin J Am Soc Nephrol 2(4):631–636
International Diabetes Federation (2012) IDF diabetes atlas, fifth edition, update 2012. http://www.idf.org/worlddiabetesday/toolkit/gp/factsfigures. Accessed 11 Dec 2013
Melamed E (1976) Reactive hyperglycemia in patients with acute stroke. J Neurol Sci 29(2–4):267–275
Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC (2001) Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 32:2426–2432
Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ (2012) Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol 11(3):261–271
Rehni AK, Nautiyal N, Perez-Pinzon MA, Dave KR (2014) Hyperglycemia/hypoglycemia-induced mitochondrial dysfunction and cerebral ischemic damage in diabetics. Metab Brain Dis. doi:10.1007/s11011-014-9538-z
Chen BH, Jiang DY, Tang LS (2006) Advanced glycation end-products induce apoptosis involving the signaling pathways of oxidative stress in bovine retinal pericytes. Life Sci 79:1040–1048
Mooradian AD (1997) Central nervous system complications of diabetes mellitus—a perspective from the blood-brain barrier. Brain Res Brain Res Rev 23(3):210–218
Duckrow RB, Beard DC, Brennan RW (1985) Regional cerebral blood flow decreases during hyperglycemia. Ann Neurol 17:267–272
OuYang YB, Mellergård P, Kristián T, Kristiánova V, Siesjö BK (1994) Influence of acid-base changes on the intracellular calcium concentration of neurons in primary culture. Exp Brain Res 101(2):265–271
Siesjö BK, Bendek G, Koide T, Westerberg E, Wieloch T (1985) Influence of acidosis on lipid peroxidation in brain tissue in vitro. J Cereb Blood Flow Metab 5:253–258
Yorek MA (2003) The role of oxidative stress in diabetic vascular and neural disease. Free Radic Res 37(5):471–480
Rizk NN, Rafols J, Dunbar JC (2005) Cerebral ischemia induced apoptosis and necrosis in normal and diabetic rats. Brain Res 1053(1–2):1–9
Ichikawa H, Kuriki A, Kinno R, Katoh H, Mukai M, Kawamura M (2012) Occurrence and clinicotopographical correlates of brainstem infarction in patients with diabetes mellitus. J Stroke Cerebrovasc Dis 21(8):890–897
Misra P, Chakrabarti R (2007) The role of AMP kinase in diabetes. Indian J Med Res 125(3):389–398
Mukherjee P, Mulrooney TJ, Marsh J, Blair D, Chiles TC, Seyfried TN (2008) Differential effects of energy stress on AMPK phosphorylation and apoptosis in experimental brain tumor and normal brain. Mol Cancer 7:37
Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H (2005) Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J 19:786–788
Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257:79–83
Ahima RS (2006) Metabolic actions of adipocyte hormones: focus on adiponectin. Obesity (Silver Spring) 14(Suppl 1):9S–15S
Thundyil J, Tang SC, Okun E, Shah K, Karamyan VT, Li YI (2010) Evidence that adiponectin receptor 1 activation exacerbates ischemic neuronal death. Exp Transl Stroke Med 2(1):15
Dash PK, Johnson D, Clark J, Orsi SA, Zhang M, Zhao J, Grill RJ, Moore AN, Pati S (2011) Involvement of the glycogen synthase kinase-3 signaling pathway in TBI pathology and neurocognitive outcome. PLoS One 6(9):e24648
Simao F, Matte A, Pagnussat AS, Netto CA, Salbego CG (2012) Resveratrol prevents CA1 neurons against ischemic injury by parallel modulation of both GSK-3beta and CREB through PI3-K/Akt pathways. Eur J Neurosci 36(7):2899–2905
Gross ER, Hsu AK, Gross GJ (2007) Diabetes abolishes morphine-induced cardioprotection via multiple pathways upstream of glycogen synthase kinase-3beta. Diabetes 56(1):127–136
Horike N, Sakoda H, Kushiyama A, Ono H, Fujishiro M, Kamata H et al (2008) AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. J Biol Chem 283(49):33902–339010
Lee JM, Seo WY, Song KH, Chanda D, Kim YD, Kim DK (2010) AMPK-dependent repression of hepatic gluconeogenesis via disruption of CREB.CRTC2 complex by orphan nuclear receptor small heterodimer partner. J Biol Chem 285(42):32182–32191
Cui J, Li YW, Jia N, Song XM, Duan JL, Weng Y et al (2013) Insulin-secretagogue activity of eleven plant extracts and twelve pure compounds isolated from Aralia taibaiensis. Life Sci 92(2):131–136
Wang Q, Peng Y, Chen S, Gou X, Hu B, Du J, Lu Y, Xiong L (2009) Pretreatment with electroacupuncture induces rapid tolerance to focal cerebral ischemia through regulation of endocannabinoid system. Stroke 40(6):2157–2164
Hata R, Mies G, Wiessner C, Fritze K, Hesselbarth D, Brinker G, Hossmann KA (1998) A reproducible model of middle cerebral artery occlusion in mice: hemodynamic, biochemical, and magnetic resonance imaging. J Cereb Blood Flow Metab 18(4):367–375
Baird TA, Parsons MW, Barber PA, Butcher KS, Desmond PM, Tress BM et al (2002) The influence of diabetes mellitus and hyperglycaemia on stroke incidence and outcome. J Clin Neurosci 9(6):618–626
Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A, Fineberg SE, Tierney WR (2002) Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology 59(1):67–71
Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Jiang Y, Dai M, Lu J, Xu M (2013) Prevalence and control of diabetes in Chinese adults. J Am Med Assoc 310(9):948–959
Remuzzi G, Schieppati A, Ruggenenti P (2002) Clinical practice. Nephropathy in patients with type 2 diabetes. N Engl J Med 346(15):1145–1151
Scheen AJ (2010) Central nervous system: a conductor orchestrating metabolic regulations harmed by both hyperglycaemia and hypoglycaemia. Diabetes Metab 36(Suppl 3):S31–S38
Kamada H, Yu F, Nito C, Chan PH (2007) Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke 38(3):1044–1049
California Acute Stroke Pilot Registry (CASPR) Investigators (2005) Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology 64(4):654–659
Weng Y, Yu L, Cui J, Zhu YR, Guo C, Wei G, Duan JL, Yin Y (2014) Antihyperglycemic, hypolipidemic and antioxidant activities of total saponins extracted from Aralia taibaiensis in experimental type 2 diabetic rats. J Ethnopharmacol 152(3):553–560
Serlin Y, Levy J, Shalev H (2011) Vascular pathology and blood-brain barrier disruption in cognitive and psychiatric complications of type 2 diabetes mellitus. Cardiovasc Psychiatry Neurol 2011:609202
King TD, Song L, Jope RS (2006) AMP-activated protein kinase (AMPK) activating agents cause dephosphorylation of Akt and glycogen synthase kinase-3. Biochem Pharmacol 71:1637–1647
Steffens S, Mach F (2008) Adiponectin and adaptive immunity: linking the bridge from obesity to atherogenesis. Circ Res 102(2):140–142
Goldstein BJ, Scalia R (2007) Adipokines and vascular disease in diabetes. Curr Diabetes Rep 7:25–33
Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA et al (2007) Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation 115(11):1408–1416
Cheng CF, Lian WS, Chen SH, Lai PF, Li HF (2012) Protective effects of adiponectin against renal ischemia-reperfusion injury via prostacyclin-PPAR- alpha-heme oxygenase-1 signaling pathway. J Cell Physiol 227(1):239–249
Zhang CHZ, Liao Y, Li Q, Chen MG, Zhao Q, Deng RH (2013) Recombinant adiponectin ameliorates liver ischemia reperfusion injury via activating the AMPK/eNOS pathway. PLoS One 8(6):e66382
Kos K, Harte AL, da Silva NF, Tonchev A, Chaldakov G, James S, Snead DR, Hoggart B, O’ Hare JP, McTernan PG, Kumar S (2007) Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab 92(3):1129–1136
Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423(6941):762–769
Guillod-Maximin E, Roy AF, Vacher CM, Aubourg A, Bailleux V, Lorsignol A et al (2009) Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J Endocrinol 200(1):93–105
Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C et al (2008) Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation 117(2):216–223
Kadowaki T, Yamauchi T (2005) Adiponectin and adiponectin receptors. Endocr Rev 26(3):439–451
Cai XJ, Chen L, Li L, Feng M, Li X, Zhang K et al (2010) Adiponectin inhibits lipopolysaccharide-induced adventitial fibroblast migration and transition to myofibroblasts via AdipoR1-AMPK-iNOS pathway. Mol Endocrinol 24(1):218–228
Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S (2009) AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem 109:17–23
Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA (2004) The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci 101:3329–3335
Cline GW, Johnson K, Regittnig W, Perret P, Tozzo E, Xiao L et al (2002) Effects of a novel glycogen synthase kinase-3 inhibitor on insulin-stimulated glucose metabolism in Zucker diabetic fatty (fa/fa) rats. Diabetes 51(10):2903–2910
Xi J, Wang H, Mueller RA, Norfleet EA, Xu Z (2009) Mechanism for resveratrol-induced cardioprotection against reperfusion injury involves glycogen synthase kinase 3beta and mitochondrial permeability transition pore. Eur J Pharmacol 604(1–3):111–116
Collino M, Thiemermann C, Mastrocola R, Gallicchio M, Benetti E, Miglio G et al (2008) Treatment with the glycogen synthase kinase-3beta inhibitor, TDZD-8, affects transient cerebral ischemia/reperfusion injury in the rat hippocampus. Shock 30(3):299–307
Park H, Kam T, Kim Y, Choi H, Gwon Y, Kim C (2012) Neuropathogenic role of adenylate kinase-1 in Ab-mediated tau phosphorylation via AMPK and GSK3b. Hum Mol Genet 21(12):2725–2737
Malhi H, Gores GJ, Lemasters JJ (2006) Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology 43(2 Suppl 1):S31–S44
Acknowledgments
This work was supported by the National Science Foundation of China (Nos. 81173514, 81303264, 81403134, 81403135, 81403182, and 81470174) and Excellent Civil Service Training Fund of Forth Military Medical University (No. 4138C4IDK6).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jialin Duan, Ying Yin, Jia Cui, Jiajia Yan, and Yanrong Zhu have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Duan, J., Yin, Y., Cui, J. et al. Chikusetsu Saponin IVa Ameliorates Cerebral Ischemia Reperfusion Injury in Diabetic Mice via Adiponectin-Mediated AMPK/GSK-3β Pathway In Vivo and In Vitro. Mol Neurobiol 53, 728–743 (2016). https://doi.org/10.1007/s12035-014-9033-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-9033-x