Abstract
The conductivity and electric modulus formalism in tellurite-modified lithium borate glasses with composition (100–x) − (33.35Li2O–66.65B2O3) − (x)TeO2, (x = 0, 5, 10, 15, 20 and 25 mol%) have been prepared. Electric modulus, relaxation mechanism and conductivity parameters were investigated to understand the dielectric properties of the glass matrix in the temperature range between 403 and 583 K, and the frequency range between 100 Hz and 5 MHz. The experimental data of ac conductivity was fitted to Jonscher’s power law. The parameters like dc conductivity (σdc), frequency exponent (S) and crossover frequency (WH) were extracted. The conductivity of the glass matrix was found to exhibit anomalous increasing behaviour with TeO2 content. The decreasing trend in the values of the frequency exponent with the temperature rise demonstrates the correlated barrier hopping mechanism in the conduction process. The electric modulus data fitted to Davidson–Cole model and Kohlrausch–Williams–Watts model have revealed nearly the same values of β (Kohlrausch exponent), which depict the conductivity relaxation process. Other electrical parameters such as bulk resistance (Rb), capacitance (Ao) and stretching exponent (n) were also extracted by fitting the impedance data to the R-CPE model using Zview software. The dc conductivity of lithium boro tellurite glasses was found to follow Arrhenius behaviour and the corresponding activation energy was determined (0.5847–1.0004 eV).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Glass and glassy materials are the inorganic composites obtained due to fusion, chilled without crystallization [1]. Glass materials are amorphous, transparent and in short-range order in the structure of the crystal [2,3,4,5]. Borate-based glasses have special properties like low melting point, high transparency, and high thermal stability, making them suitable for technological applications [6,7]. Borate glasses are frequently used in the field of non-linear optical devices, optoelectronics and piezoelectric actuators [8]. The addition of transition metal oxides to the borate glass is very useful for optical amplifiers to give an intense fluorescence [9,10]. Borate glass matrix consists of boron with three oxygen atoms (BO3) and boron with four oxygen atoms (BO4) basic structural units. The mixture of these units gives di, tri, tetra and penta borate units in the glass matrix [11]. Borate oxide glasses also have both intermediate (IRO) and short-range order (SRO) components in the glass matrix when borate glass is changed with alkali oxide like Li2O, MgO, etc. [12].
Li2O is a glass matrix modifier and behaves like a good ionic conductor. Lithium oxide glass consists of Li+ ions that have more impact on electrical transport and physical and structural properties of the glass matrix. The amorphous B2O3 has boroxyl rings in which three oxygen atoms are part of the ring and three oxygen atoms are outside the ring. These rings are arbitrarily interconnected through loose BO3 units. Initially, with the addition of modifier oxide Li2O to B2O3 glass, the three-coordinated BO3 units are converted to four-coordinated tetrahedral boron BO4 units; thus, the network dimensionality and connectivity increase. This process will continue until the added modifier oxide reaches 33.3 mol%. When the modifier oxide exceeds 33 mol%, the reconversion of four-coordinated tetrahedral boron (BO4) to three-coordinate trigonal boron (BO3) takes place by creating a non-bridging oxygen atom (NBO). The creation of NBO atoms in the glass network decreases the connectivity and dimensionality of the borate glass network [13]. The formation of NBO in the glass network leads to the formation of more ionic bonds that impede high refractive index and high ionic conductivity values [14,15,16]. Glass containing Li2O has been widely used in solar cells, superionic conductors and battery applications [17].
Tellurium oxide (TeO2) acts as a conditional glass former, which can form glass with the help of network modifiers such as Li2O, Ag2O, MgO, ZnO and rare-earth oxides. The addition of a lower mole percentage of TeO2 enhances the conductivity and stability of the borate glass network [14,18,19]. Tellurite-based glasses are attractive to researchers due to their wide range of applications and unique properties [20].
Tellurite-modified glass matrices have technological and scientific applications in optical and microelectronics due to their appropriate optical and electrical properties. Tellurite-based glasses show high thermal expansion and are less hygroscopic, which produces non-linear results that are accountable for their applications in erasable recording, optical switching devices, and the field of laser [21,22,23]. To the finest of our knowledge, so far there is no literature report on the conductivity and impedance properties of tellurite-modified lithium borate glass with the composition used in our investigated samples. Therefore, in the current study, ac conductivity, dc conductivity, impedance, conduction mechanism and modulus formalism were analysed by various theoretical models using experimental data.
2 Experimental
Glass samples with compositions (100–x) (33.35Li2O–66.65B2O3) − xTeO2, where x = 0, 5, 10, 15, 20 and 25 mol%, have been prepared by the method of melt quenching. High-quality Sigma Aldrich Li2CO3, B2O3 and TeO3 chemicals were used. A group of 12 g of the above-mentioned chemicals was taken in a silica crucible and heated in an electrical furnace in the temperature range between 1100 and 1150°C depending on the chemical composition. The molten liquid was swirled to ensure homogeneity and further quenched between two brass plates. The prepared samples were kept for annealing at 400°C for 3 h to remove the thermal stress developed during quenching. All samples were well polished for a uniform thickness, and silver paste was coated on both sides. The ac conductivity, impedance and modulus were measured by the Wayne Kerr LCR analyzer 6500B in the frequency range of 100 Hz – 5 MHz with different temperatures.
3 Results and discussion
3.1 AC conductivity response with temperature and frequency
To study the non-linearities in conductivity behaviour, it is necessary to analyse the ac conductivity of the lithium boro tellurite (LBT) series. The typical plot of LBT3 glass conductivity variation with frequency at different temperatures is shown in figure 1. In solids, the mechanism and nature of conductivity dispersion could be analysed using Jonscher’s power law [24,25].
where σdc is the dc conductivity, ωH is the crossover frequency and S is the temperature-dependent power law exponent (0 ≤ S ≤ 1) [26,27]. The power law exponent S signifies the degree of modification of the glass structure, which indicates the type of hopping mechanism concerning the localized sites in the glass [28]. The ac conductivity (σac) is obtained from the complex impedance data.
The ac conductivity, which depends on frequency, throws light on conduction due to the hopping mechanism by small polarons [29]. The plateau region at the lower frequency side matches the frequency-dependent dc conductivity, whereas the higher frequency side corresponds to the ac electrical conductivity and shows dispersion with an increase in temperature. To study the conduction mechanism of the ac conductivity for the present samples, a suitable theoretical model has been used to understand the hopping mechanism using temperature-dependent exponent values. The parameters S and σdc values are obtained from the theoretical fit of different models. The comparison of σdc values obtained from different models is presented in table 1. The inset of figure 2 for the LBT1 sample shows σdc increases with an increase in temperature. Barton and Namikawa [30,31] consider that at higher temperatures, the number of charge carriers increases as a result hopping also increases. The polarization effect at the electrode is observed at lower frequencies and is absent at the higher frequency side [32]. AC conductivity is found to be less near the lower frequency end, which may be due to more ion accumulation, while at the higher frequency end the conductivity increases, which is observed by Jonscher’s Power law [33,34]. The obtained power law exponent values of LBT glasses depend on the barrier height for the hopping mechanism between two regions [35]. The various reports involved in hopping mechanism models indicate that the exponent S lies between 0.1 and 1 depicting that it highly depends on temperature and frequency [36]. The variation of the exponent S with temperature (T) is plotted in figure 2 to identify the type of conduction mechanism. Figure 2 shows that the exponent S decreases with a temperature rise. The temperature dependency of S found that the correlated barrier hopping (CBH) model was suitable for the electrical conduction mechanism in the current samples. According to this model [37], the exponent S is given by
where, WH is the barrier height, KB is the Boltzmann constant, and T is the temperature.
To compare the conductivity values, the experimental data for the real part of the dielectric constant fitted to the modified Cole–Cole model is shown in figure 3 [38]. The conductivity values of the LBT series obtained by this model were found to match those values obtained by Jonscher’s power law model, as listed in table 1.
where ε∞, εS, τ, α, ω, σdc and n are the dielectric constant corresponding to the higher frequency side, the dielectric constant at the lower frequency side, characteristic relaxation time, empirical distribution parameter whose values lie between 0 and 1, angular frequency, dc conductivity and the exponent, respectively.
3.2 DC electrical conductivity and activation energy
The structure-property plays a vital role in the conductivity of the glass and the structure decides the mobility of ion concentration and transport of mobile ions. The modification of the structure may be due to an increase in dopant concentration and temperature. The effect of temperature on the conductivity of the glass samples in our study has revealed Arrhenius's nature, which is clear from figure 4.
The Arrhenius equation is given by
where σ0 is the pre-exponential factor, T is the temperature, Ea is the activation energy and K is the Boltzmann constant. The activation energy values of the glass samples were deduced by fitting the experimental data curve σdcT vs. 1000/T using equation (4) and they are shown in table 1.
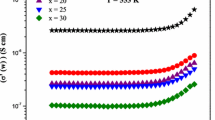
The model, which describes the temperature dependence of activation energy, was suggested by Shengelaya et al. [39] to explain the small polaron hopping mechanism [40,41]. The variation of activation energy and dc conductivity with TeO2 content is shown in figure 5. It is observed from figure 5 that the activation energy was found to increase with TeO2 concentration up to 10 mol% and decrease in activation energy for further increase of TeO2 until 15 mol%. In the concentration range of 15–25 mol% of TeO2, anomalous increasing behaviour was observed. The dc conductivity and activation energy variation with TeO2 were found to be opposite non-linear due to electronic and ionic conduction. If the electronic conduction alone existed, the dc conductivity could have increased with the increase of TeO2. On the other hand, the conduction mechanism in the present glasses switches over from electronic to ionic with TeO2 content. For 0–10 mol% of TeO2 the electronic (polaronic) paths will be blocked by Li2+ ions, resulting in a decrease in conductivity due to the addition of alkali ions to the network formers TeO2 or B2O3. This idea can be considered provided alkali ions are doped at the expense of glass network formers; TeO2 or B2O3. For 10–15 mol% of TeO2, ionic conduction dominates due to the drive of lithium ions in the glass network [42,43]. The present system contains network-modifier Li2O and conditional glass-former TeO2, they are the sources to break the basic structural units of BO3 groups and generate NBOs. The conductivity may also depend on the addition of a network-modifier Li2O and glass-former TeO2 to the basic structural units of the borate glass matrix. In boro-tellurite glasses, BO4 units and TeO4 units have a strong tendency to link with each other to form BTeO3 and BTeO5 units, which depicts higher connectivity in the glass network, as a result of which the conductivity is more at a lower percentage of TeO2. The electrical transport in the borate glasses decreases due to the change of BO4 into BO3 units, which interrupts the conduction pathway. Consequently, at the lower percentage of Li2O content, the creation of BO3 units (NBOs) is responsible for decreasing conductivity with the increase of conditional glass-former TeO2 [43,44]. The change in this type of conduction mechanism was found in various other glasses mixed with TeO2 content [40]. The values of activation energy (Ea) at various concentrations of TeO2 are presented in table 1 [45,46,47,48].
3.3 Electric modulus and relaxation mechanism formalism
The complex electric modulus, M* is the reciprocal of the permittivity comprising real M′ and imaginary M″, which are obtained using ε′ and ε″ values and can be represented as
where ε′, ε″and M′, M″ are the real and imaginary parts of dielectric constants and electric modulus, respectively. To describe the space charge relaxation mechanism, Macedo et al. [49] introduced the concept of electric modulus [50,51]. Figure 6, a and b shows the variation of M″ with frequency for LBT glasses at 483 K and the variation of M″ with frequency for LBT5 glass at different temperatures, respectively. It is observed that lower values of M″ corresponding to Ms, which are obtained at low frequencies, depict the negligible contribution of electrode polarization. The higher limit value of M″ corresponds to M∞ and its value decreases with increasing temperature. It is observed that as the TeO2 content increases in the glass matrix, the peak value of M″ starts decreasing. The peaks of M″ indicate dielectric relaxation dispersion beyond 103 Hz. The peak value of M″ shifts towards the lower frequency side with increasing TeO2 content. The various parameters were obtained by curve fitting of the Davidson–Cole model. The fitted solid lines show good agreement between theory and the experimental result of electric modulus and are shown in figure 6b.
But \(\varphi = a\tan \left( {\omega \tau } \right)\),
where M∞, MS, τ and β are the modulus at high frequency, modulus at low frequency, relaxation time and Kohlrausch exponent, respectively.
In figure 6b, the Davidson–Cole model is theoretically fitted to equation (6) for an LBT5 glass sample with different temperatures to get the parameters M∞, MS, τ and β. The peaks of M″ shifted to a high-frequency end with a rise in temperature due to temperature-dependent relaxation mechanisms in the glass system. The peak frequency of M″ indicates the change from the short range to the long range. The values of M∞, MS, β and τ from fitting are listed in table 2. The increased trend in β (<1) values with temperature represents the decrease in glass compactness, thereby impeding the interaction among polarons with the adjacent glass matrix [52]. The relaxation time values for LBT5 glass were found to be a decreasing trend with an increase in temperature, and hence, the conductivity increased. Figure 7 shows the variation of relaxation time (τ) and exponent (β) with inverse temperature.
To understand the shape parameters and relaxation mechanism, a typical plot of frequency-dependent electric modulus (M″) for LBT3, LBT4 and LBT5 samples shows well-defined peaks. It is also observed that f0 shifts towards the lower frequency side with TeO2 content due to electronic conduction. It can be seen from figure 8 that the deviation of the symmetric nature of the curves represents the relaxation of the non-Debye mechanism. The frequency-dependent (M″) is fitted to the modified KWW model and is shown in figure 8.
where a and b are shape parameters, they correspond to the low- and high-frequency sides of the relaxation peaks, respectively. The fitting parameters a and b using equation (7) are listed in table 3, which shows the non-Debye nature.
The imaginary part of the electric modulus denotes energy loss under an electric field. The modulus M″ can be represented as the Fourier transform of the relaxation function \(\emptyset \left( t \right)\) [53].
The function \(\emptyset \left( t \right)\) is the time function of the electric field in the glass sample and is given by the Kohlrausch–Williams–Watts (KWW) model [54].
where β is the Kohlrausch exponent representing the deviation from Debye relaxation and τm is the relaxation time. The parameters β and τm are obtained by curve fitting of the KWW model proposed by Bergman to understand non-Debye relaxation mechanism of the glass samples. Figure 9 shows the theoretical fitting of experimental data to equation (9).
where \(M_{0}^{{{\prime \prime }}}\) is the maximum peak value of the M′′ curve at f0.
The value of β is less than 1 for all LBT glass samples, which is consistent with that obtained by Davidson–Cole model. These values are presented in table 3.
3.4 Impedance analysis using Nyquist plots
Impedance spectroscopy has an advantage in identifying the charge transport mechanism in the grain of the LBT samples at different temperatures. Figure 10 shows the impedance Cole–Cole plots for LBT3 glass at different temperatures and the inset shows a magnification curve from 403 to 463 K. The analysis of impedance data required an equivalent circuit model, which gives information about the electrical properties of the glass samples in different regions. In the present samples, each semicircle is denoted by a parallel RC circuit [55] due to the decentralization of the impedance, the capacitor will be replaced by a constant-phase element (CPE). The total impedance of the circuit is given by [56,57].
where the impedance of CPE is \(Z_{{{\text{CPE}}}} = \frac{1}{{A_{0} \left( {j\omega } \right)^{n} }},\)
where ω is the angular frequency, j is the imaginary quantity, A0 is a constant of frequency independent in F cm–2 Sn–1 [58] and n is an exponent that lies in the range between 0 and 1 depicting the deviation from the exact semicircle [36]. The bulk resistance Rb of the impedance curve at the intersection of lower-end frequencies gives information about the degree of deviation from the exact semicircle. If the value of n = 1, the above impedance equation gives the impedance of a capacitor, A0 = C. The semicircles in figure 10 for the LBT3 sample were fitted using Z view software. A good agreement between theoretical and experimental curves was found. Semicircular arcs for all the LBT samples were depressed and their centres lie below the real impedance (Z′), which impedes relaxation in non-Debye nature [59]. The deviation of Debye’s nature for the present glass system could be due to the nonpolar cluster formation [54].
The real and imaginary impedance data were fitted to an equivalent circuit using the following relations.
and
The Cole–Cole plots for LBT samples and the equivalent circuit comprising the parallel combination of a single R-CPE circuit element are shown in figure 11. The fitting parameters obtained for the circuit elements are listed in table 4. Several other people have also discussed the equivalent circuit fitting for their samples in the same context [60,61]. The impedance of the imaginary part Z″ is shifted to the higher frequency side with an increase in temperature, where the resistance of the glass sample decreases and hence the electrical conductivity increases [62]. The real part Z′ decreases with temperature, which may be attributed to the hopping mechanism of the charge carriers. The fitted curves indicate that the peak value of Z″, i.e., \(Z_{\max }^{{{\prime \prime }}}\) shift towards higher frequencies with increase in temperature. The asymmetric variation in the width of the \(Z_{\max }^{\prime \prime }\) peak recommended an electrical process with an increase in relaxation time [63].
4 Conclusion
The impact of TeO2 addition in lithium borate glass containing tellurium oxide was investigated over a wide range of temperatures and frequencies owing to its vital role in the field of industrial applications. The ac conductivity was found to exhibit anomalous behaviour in addition to ionic and electronic dominant regions in the glass. In the lithium borate glasses with tellurite content, the hopping mechanism and nature of conductivity dispersion were analysed when Jonscher’s universal power law fitted to the experimental data. Further, it signifies the ac conduction mechanism of the CBH. The activation energy shows non-linear behaviour due to electronic and ionic dominance for the glass samples up to 10 mol% of TeO2. Beyond this composition shows increased anomalous behaviour. The values of β < 1 depict the non-Debye type of relaxation in the investigated samples. Various suitable theoretical models have been used to analyse the impedance, dielectric constant and electrical modulus of the investigated glass samples, which enable the tellurite-modified lithium borate glass with appropriate tellurite composition suitable for optical switching devices and in the field of lasers.
References
Singh S and Singh K 2015 J. Mol. Struct. 1081 211
Hivrekar M M, Sable D B, Solunke M B and Jadhav K M 2018 J. Non-Cryst. Solids 491 14
Gupta P K 1996 J. Non-Cryst. Solids 195 158
Lakshminarayana G 2017 J. Lumin. 186 283
Moustafa M G and Hassaam M Y 2017 J. Alloys Compd. 710 312
Azizan S A, Hashim S, Razak N A, Mhareb M H A, Alajerami Y S M and Tamchek N 2014 J. Mol. Struct. 1076 20
Rao K V, Babu S, Venkataiah G and Ratnakaram Y C 2015 J. Mol. Struct. 1094 274
Arya S, Danewalia S and Singh K 2016 J. Mater. Che. C 4 3328
Swapna K, Mahamuda S K, Srinivasa Rao A, Sasikala T and Rama Moorthy L 2014 J. Lumin. 146 288
Madhukar Reddy C, Deva Prasad Raju B, John Sushma N, Dhoble N S and Dhoble S J 2015 Renew. Sustain. Energy Rev. 51 566
Meera B N and Ramadrishna J 1993 J. Non-Cryst. Solids 159 1
Yano T, Kunimine N, Shibata S and Yamane M 2003 J. Non-Cryst. Solids 321 157
Rao K J 2002 Structural chemistry of glasses Elsevier. North Holland, p 481
Saddeek B, Shaaban E R and Moustafa H M 2008 Phys. B 403 2399
Kamitsos E I, Patsis A P, Karajassides M A and Chryssikos G D 1990 J. Non-Cryst. Solids 126 52
Gandhi Y, Mohan N K and Veeraiah N 2011 J. Non-Cryst. Solids 357 1193
Naresh Pallati 2020 J. Mol. Struct. 1213 128184
Naresh P, Kavitha B, Inamdar H K, Srinivasu D, Narsimlu N, Srinivas Ch et al 2019 J. Non-Cryst. Solids 514 35
Kims S H 1999 J. Mater. Res. 14 1074
Elkhoshkhany N, Eslam Syala and El Sayed Yousef 2020 Results Phys. 16 102876
Harish Bhat M, Kandavel M, Ganguli Munia and Rao K J 2004 Bull. Mater. Sci. 27 189
Qing-Hui Li Gu and Dong-Hong and Gan Fu-Xi, 2004 Chin. Phys. Lett. 21 320
Tanabe S, Hirao K and Soga N 1990 J. Non-Cryst. Solids 122 79
Sundarakannan B, Kakimoto K and Ohsato H 2003 J. Appl. Phys. 94 5182
Yang Y S, Cho J H, Kim S J, Kim J E, ChoiH W and Rim Y H 2004 J. Phys. Chem. B 108 16659
Hill R M and Jonscher A K 1979 J. Non-Cryst. Solids 32 53
Louati B, Hlel F and Guidara K 2009 J. Alloys Compd. 486 299
Ben Taher Y, Queslati A, Khirouni K and Gargouri M 2016 Mater. Res. Bull. 78 148
Vanitha T, Anupinder S, Punia R, Dahiya S and Lakhwant S 2017 J. Alloys Compd. 696 529
Barton J L 1966 Verres Refract. 20 328.
Namikawa H 1975 J. Non-Cryst. Solids. 18 17
Sanjay, Kishore N and Agarwal A 2009 J. Alloys Compd. 487 52
Padmasree K P, Kanchan D K and Kulkarni A R 2006 Solid State Ion. 177 475
Jonscher A K 1977 Nature 267 673
Funke K 1997 Solid State Ion. 94 27
Sdiri N, Elhouichet H, Azeza B and Mokhtar F 2013 J. Non-Cryst. Solids 371 22
Elliott S R 1987 Adv. Phys. 36 135
Hanumantharaju N, Sriprakash G and Gowda V C V 2019 J. Non-Cryst. Solids 512 103
Shengelaya A, G-m Zhao, Keller H, Muller K A and Kochelaev B I 2000 Phys. Rev. B 61 5888
Eraiah B and Anavekar R V 2001 Phys. Chem. Glasses 42 121
Jourdaine L, Souquet J L, Delord V and Ribes M 1988 Solid State Ion. 28 1490
Selvaraju K and Marimuthu K 2012 Physica B 407 1086
Raguenet B, Tricot G, Silly G, Ribes M and Pradel A 2011 J. Mater. Chem. 21 17693
Ashwajeet J S and Sankarappa T 2017 Ionics 23 627
Sokolov I A, Tarlakov Yu P, Yu Ustinov N and Pronkin A A 2005 Russian. J. Appl. Chem. 78 741
Higazy L A 1995 Mater. Lett. 22 289
Graca M P F, Valente M A and Ferreira M G 2003 J. Non-Cryst. Solids 325 267
Khor S F, Talib Z A, Daud W M, Sidek H A A and Ng B H 2009 J. Non-Cryst. Solids 355 2533
Macedo P B, Moynihan C T and Bose R 1972 Phys. Chem. Glasses 13 171
Moguš-Milanković A, Šantić A, Ličina V and Day D E 2005 J. Non-Cryst. Solids 351 3235
Sankarappa T, Kumar M P, Devidas G B, Nagaraja N and Ramakrishnareddy R 2008 J. Mol. Struct. 889 308
Sidebottom D L, Green P F and Brow R K 1997 Phys. Rev. B 56 170
Bergman R 2000 J. Appl. Phys. 88 1356
MacDonald J R 1987 Impedance spectroscopy: emphasizing solid materials and systems Wiley and Sons, New York, p 13
Song S G, Ling Z and Placido F 2005 Mater. Res. Bull. 40 1081
Mulder W H, Sluyters J H, Pajkossey T and Nyikos L 1990 J. Electroanal. Chem. 285 103
Song G L 2000 Cem. Concr. Res. 30 1723
Jorcin Jean-Baptiste, Orazem Mark E, Pebere Nadine and Tribollet Bernard 2006 Electrochim. Acta 51 1473
Liu J, Duan C G, Yin W G, Mel W N, Smith R W and Hardy J R 2003 J. Chem. Phys. 119 2812
Zaafouri A, Megdiche M and Gargouri M 2014 J. Alloys Compd. 584 152
Langar A, Sdiri N, Elhouichet H and Ferid M 2014 J. Alloys Compd. 590 380
Dyre J C, Maass P, Roling B and Sidebottom D L 2009 Rep. Prog. Phys. 72 046501
Ghosh A and Pan A 2000 Phys. Rev. Lett. 84 2188
Acknowledgement
We are thankful to the Maharani Cluster University, Palace Road, Bengaluru, for providing an Impedance analyzer instrument facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
VEERANNA GOWDA, V.C., HANUMANTHARAJU, N., NAGENDRA, K. et al. Hopping mechanism and impedance spectroscopy study in tellurite-modified lithium borate glasses: electric modulus formalism. Bull Mater Sci 47, 178 (2024). https://doi.org/10.1007/s12034-024-03237-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-024-03237-w