Abstract
Medical attention is needed to overcome the haemocompatibility of Ti alloy implants, where nanotechnology is one of the concerned subjects. In the present study, a combined electrochemical approach was employed to access in-vitro studies on Ti6Al4V alloy (Ti64). A higher bath temperature with specific field potential was used for the formation of nanotubes. In-vitro haemocompatibility (platelet adhesion (PA) and staining) study had been conducted on TiO2 nanotube composite samples, which were derived by varying electrochemical parameters [51 nm (high pore density), 61 nm (moderate pore density), and 72 nm (lowest pore density)] with divergent topography in fresh electrolytes. Platelet adhesion and composition were observed under FE-SEM and EDS. Image J software was used to analyse the various topographies. Haemolysis rate (HR) and PA tests were conducted to evaluate biocompatibility. Topography of 61 and 72 nm TiO2 nanotube (TNT) had shown better HR. Additionally, the MTT assay had shown higher cell viability. Besides, fluorescence microscopy had revealed maximum staining of adhered platelets on 51 nm TNT, obtained with the highest pore density.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A prosthesis is a widely used material all across the globe and has a greater impact on day-to-day life. Therefore, they are high in demand due to medical surgery, old age problems, accidents or revision surgery. Stainless steels, Co-Cr alloys and Ni-Ti alloys have been commonly used metallic implant materials that took the attention of the field of medical sciences [1]. Metals and alloys based on Ti and its alloys are popularly known for their integrity towards biological fluids, resistance to corrosion, mechanical properties, etc. However, their direct use in a mammalian body is constrained by the stress-shielding effect and the release of nanoparticles and debris as a result of mechanical actuation [2]. In this connection, TiO2 coatings through anodization is a reliable and potential way to foster the Ti and their alloys and to improve the surface properties. However, in-situ metallic oxide formation attenuates the corrosion of metallic ions into the exposed body fluids for a prolonged period of implantation [3]. Researchers had synthesized TiO2 nanotubes (TNTs) in different electrolytes to understand the geometry of the tubes and surface wettability from a medical implant application point of view [4]. It has been studied that the pore diameter of the tubes could be responsible for wettability and roughness [5]. Metal oxide coatings of TiO2-based nanotube topography play a crucial role in implant applications. Previously, it had been reported that cell adhesion depends on the size of TNTs [6,7]. In the previous research by Junkar et al [8], it was demonstrated that a 20-nm tube diameter supports cell adhesion. This work had shown the interconnectivity of cellular behaviour and nano-topography of a substrate, showing that human mesenchymal stem cells prefer a 20-nm nanotube hole width for inducing osteogenic differentiation; whereas human osteoblasts prefer a 50-nm nanotubular pore for osteoblastic maturation [8]. Platelets adhesion differences could be affected by the diameter of nanotubes. However, nanotube floor surface conditions, like freshly prepared, cleaned nanotubes or oldy nanotubes, had a prominent role. In addition, platelet adhesion depends on the ageing of hydrophilic and hydrophilic surfaces [8,9]. However, the optimum size scale dimension of pore design on Ti6Al4V alloy is a point of discussion for both platelet adhesions along with tissue cells [10]. Wang et al [11] had found that TNTs with a size of 70 nm attached to the bone had shown a significant rise in bone implant contact, as well as higher gene expression levels [11]. In earlier research studies, 45 and 90 nm diameter TiO2 nanoscale size has the potential to enhance the interaction between cell and material surfaces. In addition to this, nano-modification based on TiO2 metal oxide compound in-situ or ex-situ with different length scales or TiO2 particles over the surface of titanium have a vital role concerning osseous tissue to improve the osseointegration properties [12,13,14,15,16]. Platelets adhesion behaviour and blood-contact reactions (haemolysis rate) are challenging concerns for metallic implants [17]. For extended long periods of time, haemocompatible material, Cassie–Baxter states are preferable in a different nanometric scale. The Cassie state’s stability plays a crucial role in the protein resistance of superhydrophobic surfaces. Therefore, compared to a flat surface of the same material, the Cassie-to-Wenzel transition increases effective surface area, leading to higher protein adsorption [18,19]. From the past literature study, tissue formations with less blood clotting and cellular growth, hydrophobic surfaces with Cassie–Baxter states, partial Cassie states and Cassie–Wenzel transitions were found beneficial for concomitant biological activities [18,19,20]. Superhydrophobic surfaces (CA > 150°) are favoured by Cassie–Baxter equation. However, Wenzel had suggested that hydrophilic surfaces (CA < 90) become more hydrophilic, while hydrophobic surface becomes more hydrophobic (CA > 90 and CA > 150) [20]. Attraction intervened with the negatively charged Ti surface and a negatively charged osteoblast negotiated with the charged proteins with a distinctive transportation of quadrupolar internal charge [21]. The haemocompatibility of titanium and alloys could be possibly solved by providing biomimetic architectures in nanoscale length. Earlier it had been observed that TNT possesses good blood compatibility and had shown that it could be the best potential to enhance the surface for blood-contacting materials. In addition, existing literature support investigating the proper surface condition reaction to platelet structure in different surface topographies of TiO2, especially over Ti6Al4V alloy plate is one of the challenging concerns [22,23]. Regardless, it was believed that the instant initiation of clots has to be delayed after putting an implant. Surface refinement with TiO2 compound or its nanotubes dimension manifest compatibility of blood better in analogy to commercially pure titanium or titanium alloys. However, the best results require a balance of these two concomitantly exclusive properties, such as cell proliferation and the absorption of proteins [23,24]. Electrochemical modification to alter surface engineering is a key challenge for TiO2 anodic oxidation optimization. It is to hasten favourable tissue interaction in the host body and the platelet response mutually for a prolonged period of time with electro-processing parameters alterations [25].
In the previous literature, it was found that the electrochemical approach had influenced the pore diameter and pore density of pristine nanotubes. The electrochemical growth behaviour and surface oxide (TiO2) property relies on anodization parameters like voltage, current density, cathode and anode distance [26].
As such, specific field potential accompanied by bath temperature is the least studied parameter. Nevertheless, the influence of pore density, surface wettability and topography in distinct length TNTs designed over Ti6Al4V alloy in response to platelet adhesion and activation are not clearly understood from the past studies.
In this study, freshly prepared pristine nanotubes had been fabricated by applying anodization techniques on Ti64 alloy plates with specific field potential and bath temperature for concomitant in-vitro studies. Titania morphology over Ti6Al4V alloy has been chosen with high pore density, moderate pore density and lowest pore density with 51, 61 and 72 nm, respectively. However, three different electrochemical parameters have been utilized to fabricate this nano-morphology. Moreover, surface properties’ influence on the in-vitro application is explained with the partial Cassie–Baxter states and Cassie–Wenzel transition states. Moreover, surface chemistry via anodization with distinct nano-topography could be tailored for biological activity.
2 Experimental
A medical grade V Ti6Al4V (Sigma) alloy plate was polished with emery paper thoroughly, thereafter, it was degreased in a solution of acetone and ethanol. Furthermore, etching of the alloy plate in the solution mixture of nitric acid, hydrofluoric acid and water was conducted. Next, the fresh titania nanotube deposition was performed in unconnected electrochemical parameters. Nanotubes were obtained in the combination of electrochemical parameters including 20 V–30°C, 30 V–30°C and 20 V–55°C. Electrolytes were assorted in the mixture of de-ionized water and ethylene glycol with ammonium fluoride salt (0.6 wt%) into the solution. DC power supply instrument (KEYSIGHT E3632A) was used to facilitate the experiment.
2.1 X-ray diffraction description
XRD characterization was utilized to understand the structural properties of the material by X-ray (PANalytical 3 kW X’pert Powder XRD) with Cu Kα radiation (kα = 0.154 nm). XRD of the as-deposited TiO2 specimen was conducted in anode Cu Kα with 1.54 Å and 40 kV. In all the specimen, 2θ scan range was 20°–80° with scan step size 0.026261.
2.2 Haemolysis rate test
Diluted blood was obtained by mixing 4 ml of anticoagulated blood with potassium oxalate with 5 ml saline (0.9% by weight). Each sample was incubated in 10 ml of saline water at 37°C for 30 min, and further 0.2 ml of diluted blood was added. Incubation was continued at 37°C for an additional 60 min. Centrifugation of the immersion liquid at 2500 rpm for 5 min was done to collect the erythrocytes for haemolysis assay. The obtained supernatant fluid optical density was measured with UV–Visible Spectrophotometer (UV-1800 Shimadzu corporation) at 545 nm wavelength. Finally, the haemolysis ratio (HR) evaluation was done in accordance with the given relationship formula for HR and optical density of the different groups, as shown in equation (1).
where Dt, Dnc and Dpc are the OD values of the sample group, negative reference and positive reference, respectively.
2.3 Platelet adhesion study through field-emission scanning electron microscopy
Blood was taken from healthy adult human volunteers into anticoagulant tubes, following the approval of the Institutional Ethical Committee. Platelet-rich plasma (PRP) was prepared by centrifuging fresh whole blood containing sodium citrate (3.8 wt%) for 10 min at a rate of 1000 rpm. Specimens were kept immersed in PRP and incubated at 37ºC, 5% CO2 for 60 min. In addition, specimens were rinsed with saline to get rid of unadhered platelets. Platelets that were adhered to the surface were fixed in 2.5% glutaraldehyde solution for 60 min at room temperature, followed by dehydration in a gradient mixture of ethanol/distilled water 50, 60, 70, 80, 90, 95 and 100% for 10 min each specimen, and then dried in the air.
2.4 Platelet adhesion staining study through fluorescence microscopy
After TiO2 surfaces were incubated in human blood plasma, PBS was used to gently discard the unadhered platelets. Platelets were fixed in a 3.7 wt% formaldehyde in PBS solution for around 25 min, and subsequently washed in a PBS. Cell membranes were permeabilized using 1% Triton-X in PBS for another 6 min. The specimens were then incubated in PBS solution followed by putting in 300 µl of Calcein-Am for 40 min. The sample was eventually rinsed with PBS and the images were taken through fluorescence microscopy (Carl Zeiss Axio VertA1). An ImageJ software tool was utilized for the calculation of the percentage of stain platelets area.
2.5 MTT assay to measure cell viability
In-vitro cell toxic activity of the oxide-coated metal drug was done using MTT Assay. In this study, MG-63 cell lines were used. The medium with cell components of oxide-metal samples was incubated for 24 h without fetal bovine serum. Furthermore, cells were incubated with a medium having test specimen components for 48 h at 37°C in a 5% CO2 incubator. Once the incubation period was done, the well plate from the incubator was taken and the spent media were removed. Finally, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) reagent (Sigma) was added to a final concentration of 0.5 mg ml–1 of a total volume. Though, after incubation for 180 min, 100 µl of dimethyl sulphoxide (DMSO) was added to 96-well plates. Reverse pipetting was done to dissolve the formazan crystal when a test was conducted in dense cultures. Finally, the absorbance value was noted at 570 nm with ELISA (ThermoFisher) Plate reader.
2.6 Statistical analysis
Experimental data were analysed for variance evaluation and statistical significance (considering a 5% significance level) with a P-value less than 5% significance level (P < 0.05). Three specimens of each composition were used in this work. The study was conducted in triplicates.
3 Results and discussion
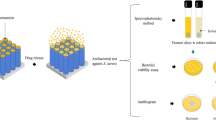
A brief illustration to understand the PRP interaction in electrochemically anodized dissimilar TNT topographies is made (see figure 1). XRD diffractogram of as-deposited anodized nanotubes fabricated on Ti6Al4V alloys at different electrochemical parameters had been found non-heat-treated as-deposited titania (as shown in XRD diffractogram, figure 2) on grade V Ti6Al4V alloy sheet. They had been considered in the influence of the surface chemistry of the nanotube topography. However, from XRD analysis, the XRD peak was not identified for the pristine TiO2 phase. XRD diffractogram for the alloy plate with as-deposited TiO2 had shown no extra peaks due to the absence of oxide layer, which got transformed into crystalline due to heat treatment. α-Ti present in the Ti64 alloys has the attributes of a hexagonal crystal system with space group P63/mmc, with space group number 194 and α-Ti with JCPDS file number 44-1294. On the other hand, β-Ti is the other entity present in the Ti64 alloy that has different attributes with cubic crystal system with space group Im-3m and space group number 229, and β-Ti diffraction pattern with JCPDS file number 44-1288. The synthesized pristine nanotubes are non-heat treated in this study. X-Ray diffractogram peak had revealed the intensity for the alloy plate explicitly, as shown in figure 2. Figure 3 illustrates the influential role of fluoride ions and a brief mechanism of TiO2 formation. The tube morphological characterization was done with field ion-scanning electron microscopy (see figure 4). Perhaps, in higher pore density nanotubes, tube surfaces were filled up and none of the tubes were visible when tested in PRP. From past literature, it was found that the surface had been encapsulated by protein [27]. Platelets along with leukocytes were attached to the surface, as observed from FE-SEM morphology. Nevertheless, figure 1 schematically signifies the tube spacing with different dimensions of nanotubes that signify different behaviour with the platelets that are being invited from PRP. However, the other two nanometre scales with moderate and lower pore densities and their interaction with PRP were not observed in FE-SEM morphological study. Moreover, FE-SEM confirms the morphology of the adhered platelets on three different scales of TiO2. Once TiO2 contact the rich plasma, its invitation towards those hydroxyl group present in the plasma might lead to the coverage of all the nanotubes with no nanotubes visible under FE-SEM image morphological analysis (see figure 5). The morphology of the platelets adherence in different length scale tubes with distinct tube pore density is shown in table 1. In high pore-density surfaces, EDAX results had shown a high nitrogen content (one of the sources of the amino acid chain). Thus, adhered platelets were seen more on 51 nm diameter (see figures 5–8). The titania nanotubes obtained in different electrochemical novel approaches are shown in figure 4. TNTs designed over Ti had been found to be a serious topic for blood-compatible studies in the field of implant application [28]. Thus, it is a major key criterion for understanding the physiological response between the blood and TNT for orthopaedic implant application [29]. In earlier studies, TNTs had been used in mesenchymal stem cell generation [30,31]. Huang et al [32] had synthesized TNT on SS 316L with superhydrophobic and superhydrophilic surface properties. The surface chemistry of the material influences platelet adhesion and platelet invitation on the exposed surface [33,34]. Surprisingly, the surface character may be altered using electrochemical parameters. The surface charge plays a major aspect in the adhesion and clotting of platelets [35]. With an increase in the nanotube pore density with a reduction in the inter-tube distance, the distributions of individual titania nanotubes over the substrates would be higher. Thus, the probability of more static surface charges had increased due to greater surface area. Therefore, conformational changes occur within the adsorbed protein, allowing subsequent biological interactions with the implant surface [36,37]. Hence, researchers emphasized on evaluation of the influence of specific decoration of nanotubes over an alloy on the haemocompatibility and its response to immunity [38,39,40]. The inter-tube separation is responsible for the pore density of the nanotubes. Thus, it is hypothesized that the surface dimension and topography of the nanotubes play a vital role while modulating haemocompatibility. Henceforth, further investigation was required while exploring other modifications of geometrical parameters with titania nanotube on grade V medical Ti6Al4V alloy, without affecting the bulk composition. As shown in figure 5a, the morphology of the platelets was circular in shape. On the other hand, figure 5b shows diffused circular platelets with fringes. However, it is observed from figure 5a that the tubes were filled when the surface was exposed to PRP in post-incubation. The angle of contact made by the liquid drops over the surface was observed on the apparent surface, and is given by equation (2):
FE-SEM image of TiO2 nanotubes on Ti alloy at three different electrochemical conditions. (a) 20 V, 55ºC, (b) 20 V, 30ºC and (c) 30 V, 30ºC. Figure 4b representing mapping and EDS for as-deposited TiO2 over Ti64 alloy, (d) contact angle hysteresis of as-deposited TiO2 on Ti alloy in 20 V, 55ºC electrochemical parameters.
As per suggestions proposed by Wenzel, θt is the fluid angle of contact over a rough geometric textured surface or apparent surface, and θef is the equilibrium contact angle over a non-apparent surface of the same solid material with no texture design over a surface. The fluid flow through the uneven rough protrusions might be due to the capillary action or Wenzel states [41]. Similarly, figure 5b and c with moderate density shows the platelet morphology of small dendritic extension with spreadness over the surface. The lowest pore density (in figure 5c) indicates the unclear morphology of the platelets with dendrite extension when observed closely under FE-SEM. Such phenomena are modelled by the assumptions of intermediate Cassie−Baxter states. Though Cassie and Baxter both had made an assumption that the material surface consisted of lower surface energy, the fluid did not fully drench the solid rough surface, hence was not permeable through the protrusions of the pores of the tubes with nano-structural surfaces. Due to the surface’s apparent fluid-repelling characteristics at the nanoscale, the surface was not entirely saturated with the drop of fluid. So, under the multiphase fluid mixing over the composite surface as per the Cassie–Baxter model, there was the presence of air pockets in the tube pores [42,43]. In the present state, partial Cassie–Baxter states should be more favourable for a lesser amount of platelet adhesion in these two pore sizes configurations with 61 and 72 nm diameters. Additionally, fsl for TiO2 had been obtained in different electrochemical parameters with different surface states (table 2). The exposed apparent surface was not completely wet by the fluid medium and the surface was not fully intact with the fluid droplet.
Here, fsl is signified with solid/liquid for the contact area fraction parameter. The model envisages the increment of hydrophobic criteria only, not dependent on the value of the initial equilibrium angle of contact θef. When TNTs are in the state of hydrophobic nature, it might be attributed to lower surface-free energy as well as a more surface area fraction of fluid (vapour/air) [44].
Theoretical (or geometrical) liquid/solid contact area fraction fsl value can be obtained from equation (3) [42,43,44].
where w denotes the wall thickness of a tube, R the outer radius and I the inter-tube spacing.
In this particular surface topography, the hydrophobicity of the surface material of pores of the titania nanotubes has a significant influence over the material–cell interaction. Bathing medium-induced anodization derived 51 nm tube diameter (with high pore densities and inter-tube distance) was supported by Wenzel states with 96% HR, while 72 nm tube diameter (with lowest pore densities) had shown 45% HR in the hydrophobic regime with haemolytic. However, 61 nm tube diameter with partial Cassie state in the near to superhydrophobic state had shown 3.8% HR (see figure 7), with non-haemolytic. It had been observed that partial Cassie–Baxter states in 61 nm hydrophobic surface offer better haemocompatibility, as shown in figure 5. However, the less lysis of blood cells might be due to the nearly superhydrophobic surface chemistry, which prevents surface interaction of blood cells. Due to the smaller diameter and high pore density, the number of individual TNTs anodized at electrolytic bath temperature was highest. In this condition, the surface had shown hydrophilicity that was predicted to be more water adsorption and content over the surface. It had been observed that the hydrophilic nanotubes with high pore density made a hydration layer in the presence of PRP naturally. High pore density nanotubes were more wettable compared to other tube densities. It is crucial to understand the influence of surface modification on cell adhesion since platelet adhesion is one of the key factors in the activation of coagulation factors during haemostasis. Images taken by fluorescence microscopy had proven that the TiO2 NT with the lowest diameter, and highest tube density had a higher affinity to the adhesion of platelets in comparison to all of the other surfaces (figures 6 and 8). ImageJ tool was utilized for the calculations of the adhered cell platelets and live platelet staining, as shown in figures 6 and 8. The observed results signify a significant decline in cell (platelets) adhesion on the modified surfaces. Cell staining with the help of a fluorescence study (as shown in figure 6) determined the percentage of staining. In the present study, the morphology of different nanotubes is shown in figure 4. The percentage of adhered stained images on high tube density was nearly 31% due to the Wenzel states surfaces. However, the percentage adherence of stained platelets as shown in figure 6b and c are nearly 22 and 15%, respectively. Percentage platelet staining shown in figure 6b and c was lesser in amount when compared to high pore density topography, which might be due to the metastable Cassie–Baxter states (or intermediate Wenzel to Cassie–Baxter transition states) for fluid droplets on the roughened textured apparent surface. In addition, the surface was found in partial Cassie–Baxter states. The higher percentage of the un-adhered area, as shown in figure 6, indicated reduced platelet adhesion and activation that was more favourable for long-term haemocompatibility. Platelet staining with pore size 51 nm tube diameter with the highest tube density had shown uniform staining due to more isotropic individual alloy sheets. The stained platelet was more uniform. Results highlighted that some of the platelets are intact with the other platelets with no dendritic arms and filopodia (figure 5a). In addition, a greater number of nanotubes with hydrophilic surfaces provides a higher affinity for platelet adhesion over the surface. Staining had shown the distribution of adhered platelets was more uniform. On the other hand, pore size with 61-nm tube diameter, with hydrophobic surface had favoured the spreading with some filopodia, resulting in a change in the size of the stained platelets. However, in the case of 72-nm tube diameter, the exact mechanism for change in the stained platelet was not clearly understood. Regarding the morphology, round and oval morphological signatures were observed on highly dense nanotube surfaces and somewhat spindle shape with spreading morphology on a hydrophobic surface with moderate density. As observed in the FE-SEM image, the platelet’s attachment to the nanotubes without pseudopods was found in highly dense and moderate-density tubes. The early reaction of platelets to the titanium alloy adorned with nanotube morphology would significantly actuate bio-active repair by re-shaping the tissue [45]. FE-SEM image analysis had shown that the high pore density with hydrophilic surface tended to induce leukocyte and platelet adhesion at 60 min of study. In comparison to high pore density, moderate tube density with hydrophobic surfaces revealed a lower number of platelets adhesion on the exposed surfaces. It was observed that tightly packed nanotubes could influence platelets growth and their movement. However, surface wettability plays an influential role to judge cellular events. It was observed that the pore density of the tubes could actuate the overall surface charge, as well as surface wettability. The nitrogen concentration anticipated by FESEM-EDS data had shown a larger amount of nitrogen on smaller nanotubular shapes in maximum pore density tubes. The calculated HR for different conditions as well as standard deviation is plotted in figure 7. Additionally, adhered platelets percentage over the various dimensions had been plotted with standard deviation. One-way analysis of variance was conducted for the experimental data shown in figures 7 and figure 8. Data computes for all the groups and the results were applied for statistical significance (considering 5% significance level) with a P-value less than 5% significant level (P < 0.05). Different scales of nanotubes had shown different haemocompatible results. TiO2 in various length scales with surface properties had a significant role in implant application. Moreover, fluorescence microscopy staining analysis gives the confirmation of the percentage adhesion and activation. Non-heat-treated nanotube with 51-nm inner diameter with higher pore density had revealed round morphology. Most of the exposed surfaces were covered with the platelets and most of the tubes were covered with the PRP. However, 61-nm pore diameter with moderate pore density; spindle-shaped platelets with spreadness had been observed. In this dimension, some of the TiO2 tubes were visible due to the hydrophobicity of the surface material over Ti64 alloy. The model suggested by Cassie–Baxter and Wenzel could be utilized to understand the platelet behaviour morphology according to its surface wettability in order to concord the hydrophobic or hydrophilic surfaces. However, surface properties with different dimensions also play a critical role to define the behaviour of platelets. In this study, TiO2 was fabricated over medical grade V Ti6Al4V alloy in different electrochemical processing parameters. Cultured cells in the liquid extracts of synthesized materials: in different electrochemical anodization parameters are shown in figure 9. Cell toxicity assay study (see figure 10) had shown that an increase in nanometric tube inner diameter had shown a change in the viability (as computed using equation (4)) of cells on different topographies. Higher pore density with hydrophilic part had shown less viability of cells compared to distinct electrochemical conditions. On the other hand, titania tubes with low and moderate pore densities demonstrated greater viability. Exceptionally in hydrophobic regime, the chances of cell interaction with the material might be slower.
From the previous study by Saharudin et al [46], the interface with the surface and tissue interaction was greatly interrupted by the topography with nano-regime. However, the nano surfaces gave higher specific sites to encourage cell receptors on the surface. In this study, the viability of cells based on the dilution factor study and optical density of the absorbance value indicated that the best non-toxicity of the cell–materials interaction was achieved in the anodized sample electrochemically modified at 30 V, 30°C with 72-nm inner diameter and 20-nm wall thickness. As Ti6Al4V is a mixture of α-phase and β-phase and known to be α + β alloy, the leaching out of aluminium and vanadium into the media can affect and regulate the healthiness of the cells. Leaching of the metal ions from an interface between TiO2 and Ti6Al4V alloy can affect the morphology of the cells, as well as the lactate dehydrogenase. Topography with different pore density of the tubes could tune the cell morphology and its viability [47].
4 Conclusions
-
1.
The highest levels of platelet adhesion and aggregation are seen in high pore densities with average hole sizes of 51 nm. Hydrophilic surfaces are found on high pore density nanoscale geometries and are in the Wenzel regime. Yet, the surface might encourage the wounds to heal by increasing the likelihood of the first blood clotting.
-
2.
Lowest fsl value obtained in average 61-nm inner tubes pore diameter shows the solid–liquid fraction interaction is less compared to average 72-nm pore diameter tubes. Both the nanoscale lengths fell under the hydrophobic regime. From the current investigation, surface wettability is governed by the assumptions suggested by the partial Cassie–Baxter or Wenzel–Cassie transitions model, which favours good haemocompatibility in 61-nm nanotubes. However, the surface has shown better cell viability at 61 and 72 nm.
-
3.
Two hydrophobic surfaces with 61 and 72 nm shows less platelet adhesion and activation that might be helpful for long-term implant life. However, 72 nm shows a high haemolytic value in comparison to 61-nm tube diameter that inhibits long-term haemocompatible materials.
-
4.
MG-63 cell lines study on different scales have shown an increase in the viability with nanotube size fabricated in different anodization parameters. From the results, 61 and 72 nm will be more appropriate due to their low toxicity nature.
-
5.
Drug dilution study of cell components on 61 and 72 nm have shown the highest cell viability compared to 51 nm. Next, low HR and lowest adhere platelets are found in hydrophobic surfaces compared to 51-nm hydrophilic surface.
References
Guillemot F 2005 Exp.Rev. Med. Dev. 2 741
Long M and Rack H J 1988 Biomaterials 19 1621
Sansone V, Pagani D and Melato M 2013 Clin. Cases Miner. Bone Metab. 10 34
Rafieerad A R, Zalnezhad E, Bushroa A R, Hamouda A M S, Sarraf M and Nasiri-Tabrizi B 2015 Surf. Coat. Tech. 265 24
Anitha V C, Lee J H, Lee J, Banerjee A N, Joo S W and Min B K 2015 Nanotechnology 26 065102
Brammer K S, Oh S, Frandsen C J and Jin S 2010 JoM 62 50
Mansoorianfar M, Tavoosi M, Mozafarinia R, Ghasemi A and Doostmohammadi A 2017 Surf. Coat. Tech. 321 409
Junkar I, Kulkarni M, Benčina M, Kovač J, Mrak-Poljšak K, Lakota K et al 2020 ACS Omega 5 7280
Khaw J S, Bowen C R and Cartmell S H 2020 Nanomaterials 10 2117
Park J Y, Gemmell C H and Davies J E 2001 Biomaterials 22 2671
Wang N, Li H, Lü W, Li J, Wang J, Zhang Z et al 2011 Biomaterials 32 6900
Cooper L F, Zhou Y, Takebe J, Guo J, Abron A and Holmén A 2006 Biomaterials 27 926
Indira K, Mudali U K and Rajendran N 2013 Ceram. Int. 39 959
Dumitriu C, Stoian A B, Titorencu I, Pruna V, Jinga V V, Latonen R M et al 2014 Mater. Sci. Eng. C 45 56
Peng L, Eltgroth M L, La Tempa T J, Grimes C A and Desai T A 2009 Biomaterials 30 1268
Xu K, Shen X, Chen W, Mu C, Jiang C, Zhao Y et al 2016 J. Mater. Chem. B 4 1797
Xu L C, Bauer J W and Siedlecki C A 2014 Colloids Surf. B 124 49
Shen L and Zhu J 2016 Adv. Colloid Interface Sci. 228 40
Gao L, Zhou W, Wang Y, Wang S, Bai C, Li S et al 2016 Optik 127 5211
Kumar R and Sahani A K 2021 Mater. Today: Proc. 45 5655
Gongadze E, Kabaso D, Bauer S, Slivnik T, Schmuki P, van Rienen U et al 2011 Int. J. Nanomed. 11 1801
Huang H H, Chen J Y, Lin M C, Wang Y T, Lee T L and Chen L K 2012 Clin. Oral Implants Res. 23 379
Puckett S, Pareta R and Webster T J 2008 Int. J. Nanomed. 3 229
Vishnu J, Manivasagam V K, Gopal V, Garcia C B, Hameed P, Manivasagam G et al 2019 Nanomedicine 20 102016
Sivaprakash V, Natrayan L, Suryanarayanan R, Narayanan R and Paramasivam P 2021 J. Nanomater 2021 1
Poddar S, Bit A and Sinha S K 2020 Mater. Chem. Phys. 254 123457
Cai K, Bossert J and Jandt K D 2006 Colloids Surf. B Biointerfaces 49 136
Smith B S and Popat K C 2012 J. Biomed. Nanotechnol. 8 642
Yang H, Yu M, Wang R, Li B, Zhao X, Hao Y et al 2020 Acta Biomater. 116 400
Wendel H P and Ziemer G 1999 Eur. J. Cardiothorac. Surg. 16 342
Kopf B S, Schipanski A, Rottmar M, Berner S and Maniura-Weber K 2015 Acta Biomater. 19 180
Huang Q, Yang Y, Hu R, Lin C, Sun L and Vogler E A 2015 Colloids Surf. B Biointerfaces 125 134
Li J, Qin W, Zhang K, Wu F, Yang P, He Z et al 2016 Colloids Surf. B Biointerfaces 145 410
Takemoto S, Yamamoto T, Tsuru K, Hayakawa S, Osaka A and Takashima S 2004 Biomaterials 25 3485
Fröhlich E 2016 Curr. Med. Chem. 23 408
Brash J L, Horbett T A, Latour R A and Tengvall P 2019 Acta Biomater. 94 11
Vanzillotta P S, Sader M S, Bastos I N and de Almeida Soares G 2006 Dent. Mater. 22 275
Balan V and Verestiuc V 2014 Eur. Polym. J. 53 171
Wilson C J, Clegg R E, Leavesley D I and Pearcy M J 2005 Tissue Eng. 11 1
Kulkarni M, Mazare A, Park J, Gongadze E, Killian M S, Kralj S et al 2016 Acta Biomater. 45 357
Wenzel R N 1936 Ind. Eng. Chem. 28 988
Cassie A B D and Baxter S 1944 Trans. Faraday Soc. 40 546
Myint M T Z, Kumar N S, Hornyak G L and Dutta J 2013 Appl. Surf. Sci. 264 344
Farsinezhad S, Waghmare P R, Wiltshire B D, Sharma H, Amiri S, Mitra S K et al 2014 RSC Adv. 63 33587
Chen C Y, Kim D, Lee C, Da Silva J, Nagai S, Nojiri T et al 2020 Int. J. Oral Sci. 12 1
Saharudin K A, Sreekantan S, Aziz S N Q A A, Hazan R, Lai C W, Mydin R B S et al 2013 J. Nanosci. Nanotechnol. 13 1696
Koju N, Sikder P, Ren Y, Zhou H and Bhaduri S B 2017 Curr. Opin. Chem. Eng. 15 49
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poddar, S., Bit, A. & Sinha, S.K. Evaluation of different topography of TiO2 nanotube morphologies on Ti6Al4V alloy plate for in-vitro studies in orthopaedic application. Bull Mater Sci 46, 179 (2023). https://doi.org/10.1007/s12034-023-03019-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-023-03019-w