Abstract
In this study, the spinel copper manganese oxide (CMO) nanocrystals were synthesized by simple chemical co-precipitation method and was characterized by Fourier transform infrared spectroscopy, X-ray diffraction, X-ray photoelectron spectroscopy, scanning electron microscopy and transmission electron microscopy (TEM). The morphology of the material found from TEM is to be spherical in shape with the crystallite size of 3.51 nm. The electrochemical characterizations of the CMO were performed by cyclic voltammetry, galvanostatic charge/discharge and electrochemical impedance spectroscopy. The specific capacitance of CMO was found to be 280 F g−1 at a current density of 1 A g−1. To enhance its capacitance value, it was doped with multi-walled carbon nanotube and poly(3,4-ethylenedioxythiophene) polystyrene sulphonate (PEDOT:PSS) and their electrochemical characterizations were recorded and compared with CMO. Doped material (CMO/MWCNT/PEDOT:PSS) exhibits a high specific capacitance of 1087 F g−1 at current density of 1 A g−1 with a good rate performance and retained an excellent electrochemical stability of 104% capacitance retention and coulombic efficiency of 101% over 10,000 cycles at a current density of 5 A g−1. This work recommends an idea for such advanced electrode material for efficient supercapacitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The requirement for energy storage devices is becoming high due to the depletion of our natural resources, increase in population and also high usage of electronic devices like mobile phones, laptops, etc. [1]. The energy storage devices like battery due to poor conductivity cannot attain high energy storage capacity. Though conventional capacitors provide high energy storage capacity, the energy and power densities are lower than batteries due to their low surface area. Hence, supercapacitors lead a role to bridge the gap between batteries and conventional capacitors. They are promising devices for energy storage applications as they exhibit high surface area and long cycle life resulting in higher energy density than capacitors and greater power density than batteries [2]. They are being considered for various applications as power sources for pulsed light generators, lasers, camera flash equipment and backup power source for computer memory [3, 4]. They are categorized into three types depending on the nature of electrode materials and mechanism involved for the storage of charge, namely electrical double-layer capacitor (EDLC), pseudocapacitor and hybrid capacitor. EDLC stores charge electrostatically (non-Faradaically) and there is no transfer of charge between electrode and electrolyte [5]. For example, different forms of carbon materials like graphene [6] and CNT [7] can be used to store charge in EDLC electrodes. In pseudocapacitors, charge storage arises from the fast reversible surface faradic reactions happening at electrode/electrolyte interface. Metal oxides/hydroxides/sulphides [8,9,10] and conducting polymers like polyaniline [11], PEDOT:PSS [12], polypyrrole [13] are good examples of pseudocapacitors. In hybrid capacitor, the charge storage arises from the combination of both faradaic and non-faradaic processes and it has the ability to achieve longer cycle life, greater energy and power densities than EDLC [14]. In recent times, nanomaterial-based supercapacitors play a vital role in the energy storage applications [15]. Researchers are searching for the energy storage device which is eco-friendly, reusable, conversion efficiency and long cycle life with high energy density and great power density [16].
Due to high storage capacity and earth abundance, transition metal oxides (TMOs) with spinel-type nanomaterials have attracted the attention of researchers worldwide recently [17, 18]. Many TMOs with properties of supercapacitors such as NiCo2O4 [19], MnCo2O4 [20], CuCo2O4 [21], CoFe2O4 [22], NiFe2O4 [23] and CuFe2O4 [24] have been reported. Among the TMOs, copper and manganese are abundant, eco-friendly and of low cost compared to other metals such as nickel and cobalt. Copper oxide [25] and manganese oxide [26] are among the most investigated metal oxides for supercapacitor applications. Though having large working potential window with low electrical conductivity [27], manganese oxide could not serve as a good electrode in supercapacitor. Moreover, the chemical instability of copper oxide [28] also limits its usage as an efficient electrode. Whereas, when manganese oxide combines with copper oxide as copper manganese oxide (CMO), it serves as capable electrode material. In recent times, many researchers concentrated on preparing CMO by different synthetic methods, such as hydrothermal [29], sol–gel [30], ceramic [31], co-precipitation [32, 33], micro-emulsion [34], novel combustion [35], mechano-chemical [36], solid-state reaction [37] and thermal oxidation method [38].
Chen et al [39] designed a unique cupric oxide–manganese dioxide on a copper grid for high-performance supercapacitors with a very high specific capacitance of 343.9 F g at a current density of 0.25 A/g along with a good rate capability. Similarly, Saravanakumar et al [40] reported CuMn2O4 electrode material prepared by solvothermal method with specific capacitance value of 571.6 F g−1 at 1 A g−1 [40]. Recently, Sheikhzadeh and Sanjabi [41] reported spinel CuMn2O4 electrode material prepared by electrodeposition method with the specific capacitance value of 744 F g−1 at 10 mV s−1 and the retention capacitance of 91% of 10,000 cycles. But Jingbin et al [42] reported Cu1.5Mn1.5O4 spinel oxide as a novel anode material for lithium-ion batteries that retain a specific capacity of 464 mAh g−1 at a current of 100 mA g after 60 cycles.
In view of all these reports, we aimed to synthesize copper manganese oxide, Cu1.5Mn1.5O4 (CMO), spinel nanocrystal by simple chemical co-precipitation method. The CMO was characterized for its functional group by Fourier transform infrared spectroscopy (FTIR), phase structure by X-ray diffraction (XRD) analysis, whereas the morphology and particle size were analysed by recording their scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Further its electrochemical properties like cyclic voltammetry (CV), galvanostatic charge/ discharge (GCD) and electrochemical impedance spectroscopy (EIS) were studied. The specific capacitance of CMO was found to be 280 F g−1 at a current density of 1 A g−1. Further to enhance its capacitance value, it was doped with a carbon material—multi-walled carbon nanotube (MWCNT), a conducting polymer—poly (3,4-ethylenedioxy thiophene) polystyrene sulphonate (PEDOT:PSS) separately and in a combination of MWCNT and PEDOT:PSS denoted as CMO/MWCNT/PEDOT:PSS and their electrochemical performance were recorded. By such doping, a high specific capacitance of 1087 F g−1 at a current density of 1 A g−1 was obtained bearing 104% capacitance retention with a coulombic efficiency of 101% over 10,000 cycles, and at a current density of 5 A g−1 was obtained for CMO/MWCNT/PEDOT:PSS.
2 Experimental
2.1 Materials
Copper acetate, manganese acetate, sodium hydroxide, 1-methyl-2-pyrrolidone (NMP) and ethanol (99.9%) were purchased from Merck specialities Private Limited, India. Multi-walled carbon nanotube (MWCNT) was purchased from Sisco Research Laboratory, India. Poly(3,4-ethylenedioxythiophene) polystyrene sulphonate (PEDOT:PSS), polyvinylidene difluoride (PVDF), acetylene black (AB) and nickel foam were purchased from Sigma Aldrich. All chemicals were of analytical grade and used as such without any further purification.
2.2 Synthesis of CMO
Copper manganese oxide, Cu1.5Mn1.5O4 (CMO), spinel nanocrystal was prepared by simple chemical co-precipitation method [43]. Copper acetate (0.05 M) and manganese acetate (0.1 M) were dissolved in deionized water (50 ml) and stirred for 15 min. The mixed solution was added to sodium hydroxide (0.05 M) dissolved in 50 ml deionised water under vigorous stirring at 25°C. After 10 min, the pH of the solution was adjusted to 11 by adding alkali solution. Further, the solution was stirred for 30 min in hot condition. The obtained precipitate was filtered, washed with water several times and once with ethanol to remove any other impurities present. It was dried in air for 24 h and calcinated at 300°C for 3 h to get the purified CMO. Figure 1 represents the schematic diagram for the synthesis of CMO.
2.3 Characterization
FTIR spectra of the prepared CMO was recorded using Perkin-Elmer Spectrometer over the wavelength range of 400–4000 cm−1 using KBr as a reference to determine the functional group. The phase formation, crystalline structure and particle size were investigated by X-ray powder diffraction (X’pert PRO analytical diffractometer) using Cu Kα as radiation (α = 1.5418 Å) as X-ray source. The composition of the element and oxidation states were analysed using X-ray photoelectron spectroscopy (XPS) with Thermo Scientific MULTILAB 2000 using Al Kα as a radiation source. Surface morphology and elemental composition were analysed by SEM with energy-dispersive X-ray spectroscopy (EDAX) using TESCAN (Bruker) microscope. The microstructure was observed by TEM using FEI Tecnai 20 G2 microscope. The electrochemical performance was analysed by Biologic (SP-120) workstation.
2.4 Electrode preparation and electrochemical characterization
The active material was mixed with acetylene black (AB, conducting material) and polyvinylidene difluoride (PVDF, binder) in the ratio 80:15:5. The mixture was continuously ground in a mortar and the required amount of N-methyl-2-pyrrolidone (NMP) was used as solvent to make a homogenous slurry. Nickel foam was pretreated and washed in acetone, HCl, distilled water and anhydrous ethanol. After washing, it was dried in hot air oven at 80°C for 12 h. The homogenous slurry was coated on nickel foam, which acts as a current collector and then it was dried at 80°C for 12 h in an oven. After drying, it was uniaxially pressed. The weight of the coated material (~ 4 mg) was maintained by measuring the weight of the Ni foam before and after coating [44]. The percentage composition and the mixing procedure for the different electrode materials are given in table 1. The active material was mixed with AB, PVDF, NMP as mentioned in table 1. The prepared electrode is shown in figure 2.
The standard three-electrode cell configuration was used for electrochemical studies. The active material coated on nickel foam performs as working electrode, Ag/AgCl (3 M KCl) and Pt wire acts as reference and counter electrode, respectively. The electrolyte used was 5 M potassium hydroxide. The CV and GCD studies were carried out in the potential range between 0 and 0.5 V with different scan rates ranging from 5 to 50 mV s−1 and different current densities from 1 to 5 A g−1, respectively. EIS was also studied by applying an alternate current (AC) in the frequency range from 100 kHz to 10 mHz. The specific capacitance of the electrode material from the discharge curve was calculated by using equation (1) [45]
where C is the specific capacitance of the electrode material (F g−1), I the applied current for charge–discharge cycles (A), Δt the discharge time (s), m the mass of the working electrode (g) and ΔV the operating potential window (V). The columbic efficiency can be calculated by the following equation (2)
where, η is the columbic efficiency, tdischarge the discharging time and tcharge the charging time.
3 Results and discussions
3.1 FTIR spectroscopy
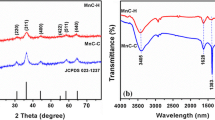
FTIR was accomplished to detect the functional groups of the CMO and is depicted in figure 3a. FTIR spectra in the region 400–700 cm−1 is related to the spinel structure. The sharp peak at 527 cm−1 corresponds to the stretching bond of Cu–O in the tetrahedral site. The band that appeared at 588 cm−1 associates with the stretching bond of Mn–O in octahedral site [46]. This agrees the formation of spinel Cu1.5Mn1.5O4. The broad band detected at 3421 cm−1 and a small band at 1603 cm−1 provide the stretching and bending vibrations of –OH group, arising due to the adsorption of water on the nanocrystal surface [47]. The stretching frequencies obtained in the FTIR spectra confirm the formation of CMO.
3.2 XRD analysis
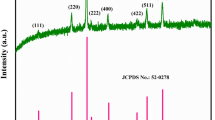
The XRD pattern of the CMO was recorded and depicted in figure 3b. The peaks observed at the 2θ values are 30.86°, 35.86°, 38.92°, 43.60°, 48.42°, 53.02°, 57.74° and 63.51° which correspond to the lattice planes (220), (311), (222), (400), (331), (422), (511) and (440), respectively. The diffraction peaks were matched with the standard reference JCPDS card no.70-0260. The CMO prepared by the simple co-precipitation method exhibits the cubic Cu1.5Mn1.5O4 (P4332 space group) with the lattice constant a = b = c = 8.2800 Å. The obtained diffraction pattern confirms the formation of CMO nanocrystal. No additional peaks appeared, indicating the high purity of the electrode material. The CMO belongs to inverse cubic spinel structure, where manganese ions are distributed among the tetrahedral and octahedral sites, while copper ions are located at the octahedral sites [48]. The average crystallite size was calculated from the high intensity peak using the Debye–Scherrer equation (3) [49]
where D (nm) is the crystalline size, K the diffraction constant (~ 0.9), λ the wavelength of the incident X-ray, β the full-width half-maximum multiplies and θ the angle of diffraction. The average crystallite size was found to be 1.38 nm using equation (3)
3.3 XPS analysis
Further the oxidation state with the elemental composition of the prepared CMO was analysed using XPS and is shown in figure 4a. XPS spectrum of Mn2p depicted in figure 4b shows two peaks at 641.6 and 653.45 eV, which are attributed to Mn2p3/2 and Mn2p1/2 levels, respectively. The spin energy separation of 11.6 eV is smaller than 11.7 eV of standard manganese oxide [50]. This confirms the doping of Cu in manganese oxide [51, 52]. The binding energy of Mn2p3/2 peaks at 641.6 eV shows the existence of Mn3+ [53, 54] and the satellite peak at 647.26–649.51 eV belongs to MnO satellite feature [55]. XPS spectrum of Cu 2p level is presented in figure 4c. The broad peak at 933.3 eV corresponds to Cu2+ and 930.73 eV corresponds to Cu+ [56]. The satellite peak at 941 and 943 eV belongs to Cu2+ contribution [56, 57] (Cu+ is diamagnetic and, consequently, does not show shake-up satellite structure). The peak at 953 eV indicates the presence of Cu in Cu2+ state [58]. The existence of redox equilibrium between Cu and Mn as Cu+ + Mn4+ ↔ Mn3+ + Cu2+ exists in Cu1.5Mn1.5O4 phase [59, 60]. Figure 4d shows the deconvoluted XPS spectrum of O1s. The two peaks located at 529.42 and 530.84 eV confirm that the oxygen in this sample is present in the form of oxide [61, 62].
3.4 Morphological analysis
The surface morphology and the composition of the CMO were analysed by SEM with EDAX analysis. Spherical-like nanocrystals are observed for CMO (figure 5a). The presence of predominant characteristic elemental peak in the EDAX spectra, namely copper, manganese and oxygen, are shown in figure 5b and confirmed the prepared CMO is in pure form.
The magnified surface morphology of the prepared CMO was further confirmed by TEM image and is shown in figure 6a. The average particle size was analysed quantitatively by fitting the histogram and ImageJ software (figure 6b) and is found to be the order of 3.51 nm. The grain size observed by the TEM is closer to the crystallite size obtained from the XRD pattern, confirming the formation of monocrystalline particles and the grain sizes of which are close to the values revealed by a study on similar spinel materials [63]. The selected area electron diffraction pattern shown in figure 6c indicates the presence of (222), (400), (331) and (422) planes, which are in good agreement with XRD pattern of the prepared CMO.
4 Electrochemical characterizations
4.1 Effect of MWCNT, PEDOT:PSS doping on the electrochemical performance of CMO electrode material
Figure 7a displays the CV curve of CMO at various scan rates. It is clearly seen from the graph that the CMO exhibits pseudocapacitance behaviour, as it has the redox peak with the potential window of 0 to 0.5 V. The redox peak may be due to the conversion of Cu2+/Cu+ and Mn4+/Mn3+. The area under the CV curve increases with increase in the scan rate. This may be due to the diffusion rate of electrolyte ions. Even at high scan rate of 50 mV s−1, the materials retain their shape in the CV curve indicating the good electrical conductivity of the electrode materials. Figure 7b depicts the GCD curve of CMO. The specific capacitance of the GCD curve was calculated using equation (1). The calculated specific capacitances of CMO are 280, 250, 186, 107, 70 F g−1 at current densities of 1, 2, 3, 4, 5 A g−1. The above results indicate that the specific capacitance decreases with increase in current density. This may be due to the diffusion of ions at different current rates. At a lower current density, the electrolyte ions have enough time to enter into the inner pores of the electrode surface to attain the maximum utilization of the active surface areas of the electrode materials [64, 65], whereas at high current densities, only the surface of the electrode materials is accessed by the electrolyte ions. The electrolyte ions could not find sufficient time to intercalate the inner pores of the electrode surfaces to obtain the maximum utilization of the active surface areas of the electrode materials.
Figure 8 shows the individual CV and GCD curves of CMO/MWCNT, CMO/PEDOT:PSS and CMO/MWCNT/PEDOT:PSS at different scan rates and current densities. The calculated specific capacitances of the electrode materials are given in table 2. The increase in specific capacitance value can be clearly seen from CMO to CMO/MWCNT/PEDOT:PSS electrode material. This may be due to the addition of minimum amount of dopant to the CMO, which increases the electroactivity of the electrode. The specific capacitance of the CMO/MWCNT and CMO/PEDOT:PSS are nearly equal, but doping of both MWCNT and PEDOT:PSS on CMO (CMO/MWCNT/PEDOT:PSS) shows the highest specific capacitance value.
Figure 9a and b shows the CV and GCD curves of bare nickel foam, CMO, CMO/MWCNT, CMO/PEDOT:PSS, CMO/MWCNT/PEDOT:PSS electrodes at a scan rate of 10 mV s−1 and at a current density of 1 A g−1, respectively. It shows the influence of MWCNT, PEDOT and the nature of dopants on the electrochemical behaviour of CMO electrode. The CV curve exhibits the redox behaviour proving the presence of faradaic charge storage mechanism. CMO/MWCNT/PEDOT:PSS shows better performance and high electrochemical activity compared to all other composites electrode. Nickel foam shows negligible electrochemical activity.
In addition, the non-linear charge/discharge curve confirms the faradaic nature of the electrode material. Further, the CMO/MWCNT/PEDOT:PSS has a higher discharge time, thereby shows higher specific capacitance compared to CMO at a current density of 1 A g−1. The calculated specific capacitance of CMO/MWCNT/PEDOT:PSS at a current density of 1 A g−1 is 1087 F g−1, which is significantly higher than the CMO of 280 F g−1 at 1 A g−1. The specific capacitance of all the electrode materials as a function of different current densities is displayed in figure 10a. This demonstrates that the doping enhances the specific capacitance of CMO for the supercapacitor application.
(a) Specific capacitance as a function of current density for CMO, CMO/MWCNT, CMO/PEDOT:PSS and CMO/MWCNT/PEDOT:PSS electrode materials. (b) Nyquist plot for CMO, CMO/MWCNT, CMO/PEDOT:PSS and CMO/MWCNT/PEDOT:PSS electrodes (insets are the magnified view of Nyquist plots at high frequency region and the equivalent circuit). (c) Cyclic stability of CMO/MWCNT/PEDOT:PSS for 10,000 cycles at 5 A g−1.
4.2 EIS analysis
The impedance spectra was recorded for the four electrodes (CMO, CMO/MWCNT, CMO/PEDOT:PSS and CMO/MWCNT/PEDOT:PSS) and presented as Nyquist plot in figure 10b. At low frequency region, a vertical line along the y-axis more than 45° is observed. This indicates the ideal capacitive behaviour of the electrode. The equivalent circuit model which fits for all the electrodes is shown as an inset (top) in figure 10b and the parameters are tabulated in table 3. The solution resistance (Rs) of the CMO, CMO/MWCNT, CMO/PEDOT:PSS and CMO/MWCNT/PEDOT:PSS electrode obtained from the Nyquist plot is 0.530 Ω, 0.520 Ω, 0.524 Ω and 0.471 Ω, respectively. It is observed that CMO/MWCNT/PEDOT:PSS electrode has low Rs value compared to other electrode materials.
The specific capacitance, Cdm, can be calculated from the low frequency value of Nyquist plot using equation (4) [66, 67]
where Cdm is specific capacitance, Z″ is imaginary part of the impedance, f the subsequent frequency and m the mass of the electrode material. The Cdm value is used to calculate the electrochemical surface area (ECSA) by using equation (5) [67, 68]
where Cdm is specific capacitance obtained from Nyquist plot and Cd a constant value of 20 μF cm−2. The calculated values of Cdm and ECSA are tabulated in table 3. The calculated ECSA values are in good agreement with the specific capacitance values. CMO/MWCNT/PEDOT:PSS shows the highest ECSA, which provides the highest specific capacitance value among all the electrodes. It may be due to the pore structure of MWCNT together with the large electroactive conducting polymer and pseudocapacitive nature of CMO. CMO/MWCNT/PEDOT:PSS shows the most capacitive behaviour when compared to all the other electrodes.
4.3 Electrochemical stability
Further, to check the electrochemical stability of the electrode materials, GCD was performed for 10,000 cycles at current density of 5 A g−1. Figure 10c shows the cyclic stability of CMO/MWCNT/PEDOT:PSS for 10,000 cycles at 5 A g−1. It can be noticed that 104% of initial capacitance retention was retained with high coulombic efficiency of 101% over 10,000 cycles. This indicates the high stability of the CMO/MWCNT/PEDOT:PSS electrode. To compare our results with reported values, a comparative table is provided in table 4 with specific capacitance and cycle life of several metal oxide-based electrodes in different electrolytes.
5 Conclusions
This work involves the synthesis of spinel CMO nanocrystals by a simple chemical co-precipitation method. XRD pattern reveals the formation of cubic with inverse spinel structure. SEM and TEM analyses show the crystallite particle size to be 3.51 nm, and the elemental composition confirms the presence of Cu, Mn and O. Further, the oxidation state and elemental composition was analysed by XPS and confirms the presence of Cu1.5Mn1.5O4 nanocrystals. The electrochemical performance of CMO shows a specific capacitance of 280 F g−1 at the current density of 5 A g−1. To enhance its electrochemical performance, it was doped with MWCNT and PEDOT:PSS. The specific capacitance values of CMO/MWCNT and CMO/PEDOT:PSS are 645 and 604 F g−1 at the current density of 1 A g−1, respectively. CMO/MWCNT/PEDOT:PSS electrode shows a very high specific capacitance value of 1087 F g−1 at a current density of 1 A g−1, with good rate performance retained with an excellent electrochemical stability of 104% capacitance retention, having coulombic efficiency of 101% over 10,000 cycles at a current density of 5 A g−1. This study shows the excellent performance of CMO/MWCNT/PEDOT:PSS suitable for supercapacitor applications.
Change history
05 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12034-023-03001-6
References
Burke A 2000 J. Power Sources 91 37
Zhao X, Sánchez B M, Dobson P J and Grant P S 2011 Nanoscale 3 839
Zhang L L and Zhao X S 2009 Chem. Soc. Rev. 38 2520
Wang G, Zhang L and Zhang J 2012 Chem. Soc. Rev. 41 797
Bose S, Kuila T, Mishra A K, Rajasekar R, Kim N H and Lee J H 2012 J. Mater. Chem. 22 767
El-Kady M F, Strong V, Dubin S and Kaner R B 2012 Science 335 1326
Pan H, Li J Y and Feng Y P 2010 Nanoscale Res. Lett. 5 654
Tang W, Liu L, Tian S, Li L, Yue Y, Wu Y et al 2011 Chem. Commun. 47 10058
Yan J, Fan Z, Sun W, Ning G, Wei T, Zhang Q et al 2012 Adv. Funct. Mater. 22 2632
Hu H, Guan B Y and David Lou X W 2016 Chem. 1 102
Eftekhari A, Li L and Yang Y 2017 J. Power Sources 347 86
Lei C, Wilson P and Lekakou C 2011 J. Power Sources 196 7823
Huang Y, Tao J, Meng W, Zhu M, Huang Y, Fu Y et al 2015 Nano Energy 11 518
Min L, Ahmed A, Pascal R, Sabine S and Rabah B 2020 J. Colloid Interface Sci. 579 520
Mohapatra S, Acharya A and Roy G S 2012 Lat. Am. J. Phys. Educ. 6 380
Xu J and Wang L 2019 Sci. Rep. 9 12557
Wu J, Mi R, Li S, Guo P, Mei J, Liu H et al 2015 RSC Adv. 5 25304
Vignesh V, Subramani K, Sathish M and Navamathava R 2018 Colloid Surf. A 538 668
Yang T, Xia D, Wang Z and Chen Y 2009 Mater. Lett. 63 5
Lavela P, Tirado J L and Vidal-ABarca C 2007 Electrochim. Acta 52 7986
Sharma Y, Sharma N, Subba Rao G V and Chowdari B V R 2007 J. Power Sources 173 495
Lavela P and Tirado J L 2007J Power Sources 172 379
Bomio M, Lavela P and Tirado J L 2008 J. Solid State Electrochem. 12 729
Zhou L, Zhao D and Lou X W 2012 Adv. Mater. 24 745
Saravanakumar B, Radhakrishnan C, Ramasamy M, Kaliaperumal R, Britten A J and Mkandawire M 2019 Results Phys. 13 102185
Bui P T M, Song J, Li Z, Akhtar M S and Yang O 2017 J. Alloys Compd. 694 560
Kang J, Chen L, Hou Y, Li C, Fujita T, Lang X et al 2013 Adv. Energy Mater. 3 857
Zhang Y X, Huang M, Kuang M, Liu C P, Tan J L and Dong M 2013 Int. J. Electrochem. Sci. 8 1366
Chen S, Tang W, He J, Miao R and Lin H 2018 J. Mater. Chem. A 6 9047
Batoo K M and Abd El-sadek M S 2013 J. Alloys Compd. 566 112
Hilczer A, Kowalska K, Markiewicz E, Pietraszko A and Andrzejewski B 2016 Mater. Sci. Eng. B 207 47
Albuquerque A S, Tolentino M V C, Ardisson J D, Moura F C C, de Mendonça R and Macedo W A A 2012 Ceram. Int. 38 2225
Wright P A, Natarajan S, Thomas J M and Gai-Boyes P L 1992 Chem. Mater. 4 1053
Ali R, Mahmood A, Khan M A, Chughtai A H, Shahid M, Shakir I et al 2014 J. Alloys Compd. 584 363
Priyadharsini P, Pradeep A, Rao P S and Chandrasekaran G 2009 Mater. Chem. Phys. 116 207
Yang H, Zhang X, Ao W and Qiu G 2004 Mater. Res. Bull. 39 833
Shoemaker D P, Li J and Seshadri R 2009 J. Am. Chem. Soc. 131 11450
Ranjbar-Nouri Z, Soltanieh M and Rastegari S 2018 Surf. Coat. Technol. 334 365
Chen H, Zhou M, Wang T, Li F and Zhang Y X 2016 J. Mater. Chem. A 4 10786
Saravanakumar B, Muthu Lakshmi S, Ravi G, Ganesh V, Sakunthala A and Yuvakkumar R 2017 J. Alloys Compd. 723 115
Sheikhzadeh M and Sanjabi S 2021 Synth. Met. 278 116802
Jingbin Q, Lin M, Zheng M, Jichun H and Decheng L 2016 RSC Adv. 6 55786
Li-Na C, Yue G, An-Hui L, Branton P and Li W-C 2012 J. Mol. Catal. A Chem. 360 35
Zhao X, Hou Y, Wang Y, Yang L, Zhu L, Caoc R et al 2017 RSC Adv. 7 40286
Xiong P, Hu C, Fan Y, Zhang W, Zhu J and Wang X 2014 J. Power Sources 266 384
Mendez-Martinez F, Gonzalez F, Lima E, Bosch P and Pfeiffer H 2010 J. Mex. Chem. Soc. 54 2
Rahman A, Ismail A, Jumbianti D, Magdalena S and Sudrajat H 2009 Indo. J. Chem. 9 355
Al-Senani G M, Abd-Elkader O H and Deraz N M 2021 Appl. Sci. 11 2034
Murty B S, Shankar P, Raj Baldev, Rath B B and Murday J 2013 Textbook of nanoscience and nanotechnology 1st edn. (Heidelberg: Springer Berlin), co-published with Universities Press (India) Pvt. Ltd.
Audi A A and Sherwood P M A 2002 Surf. Interface Anal. 33 274
Li J, Xiong S, Li X and Qian Y 2012 J. Mater. Chem. 22 23254
Yang Z, Zhang W, Wang Q, Song X and Qian Y 2006 Chem. Phys. Lett. 418 46
Gautier J L, Rios E, Gracia M, Marco J F and Gancedo J R 1997 Thin Solid Films 311 51
Liu H Y, Zhu X F, Li M R, Tang Q W, Sun G Q and Yang W S 2014 Electrochim. Acta 144 31
Sun R, Wang L, Yu H, Zain ul A, Chen Y, Khalid H et al 2016 J. Inorg. Organomet. Polym. 26 545
Marco J F, Gancedo J R, Nguyen Cong H, del Canto M and Gautier J L 2006 Solid State Ionics 177 1381
Stewart S J, Multigner M, Marco J F, Berry F J, Hernando A and Gonzalez J M 2004 Solid State Commun. 130 247
Einaga H, Kiya A, Yoshioka S and Teraoka Y 2014 Catal. Sci. Technol. 4 3713
Vandenberghe R, Legrand E, Scheerlinck D and Brabers V 1976 Acta Crystallogr. Sect. B: Struct. Crystallogr. Cryst. Chem. 32 2796
Bayon R, Vicente G S, Maffiotte C and Morales A 2008 Sol. Energy Mater. Sol. Cells 92 1211
Xie S, Liu Y, Deng J, Zhao X, Yang J, Zhang K et al 2016 J. Catal. 342 17
Sakunthala A, Reddy M V, Selvasekarapandian S, Chowdari B V R and Selvin P C 2011 Energy Environ. Sci. 4 1712
Zákutná D, Vlček J, Fitl P, Nemkovski K, Honecker D, Nižňanský D et al 2018 Phys. Rev. B 98 064407
Heydari H, Moosavifard S E, Shahraki M and Elyasi S 2017 J. Energy Chem. 26 762
Vijayakumar S, Nagamuthu S and Muralidharan G 2013 ACS Appl. Mater. Interfaces 5 2188
Zhang J and Zhao X S 2012 ChemSusChem 5 818
Joshi A, Sahu V, Singh G and Sharma R K 2019 Sustain. Energy Fuels 3 1248
Ali G A M, Yusoff M M, Shaaban E R and Chong K F 2017 Ceram. Int. 43 8440
Gnana B, Raj S, Asiri A M, Wu J J and Anandan S 2015 J. Alloys Compd. 636 234
Dung Dang T, Thu Hang Le T, Bich Thuy Hoang T and Tung Mai T 2015 Adv. Nat. Sci. Nanosci. Nanotechnol. 6 025011
Fan Z, Chen J, Wang M, Cui K, Zhou H and Kuang Y 2006 Diam. Relat. Mater. 15 1478
Sena P, De Amitabha, Dutta Chowdhury A, Bandyopadhyaya S K, Agnihotri N and Mukherjee M 2013 Electrochim. Acta 108 265
Tang P, Han L and Zhang L 2014 ACS Appl. Mater. Interfaces 6 10506
Paulose R and Mohan R 2019 J. Nanosci. Nanotechnol. 19 8151
Ates M, Akif Serin M, Ekmen I and Ertas Yavuz N 2015 Polym. Bull. 72 2573
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kiruthika, K., Renuga, V. & Suryanarayanan, V. Fabrication and electrochemical performance of spinel copper manganese oxide nanocomposites for supercapacitor application. Bull Mater Sci 46, 98 (2023). https://doi.org/10.1007/s12034-023-02935-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-023-02935-1