Abstract
WO3:Eu3+ powder was prepared by microemulsion method with cetyltrimethyl ammonium bromide (CTAB) as a surfactant. The prepared WO3:Eu3+ samples were wrapped with SiO2 by a coating method. The WO3:Eu3+@ SiO2 nanocomposites with the core-shell structure were prepared by controlling tetraethyl orthosilicate (TEOS) content (1, 3, 5 and 7%) and water–alcohol ratio (1:3, 1:1.5, 1:1 and 2:1). The structure, morphology and luminescence properties of the prepared WO3:Eu3+@ SiO2 samples were characterized by XRD, SEM, TEM and fluorescence spectra. The results show that the prepared WO3:Eu3+@SiO2 nanocomposites are spherical and granular, and the average particle size is below 100 nm. Under the condition of different TEOS contents, the strongest characteristic peak is located at 554 nm in the green light band at the excitation wavelength of 328 nm. Among the different water–alcohol ratio conditions, the prepared WO3:Eu3+@SiO2 nanocomposite has the strongest luminescence intensity at the water–alcohol ratio of 1:3, and the excitation wavelength is 328 nm. The excitation spectra measured at 612 nm have prominent characteristic peaks at 362, 394, 422, 465 and 499 nm, which correspond to the transition of 7F0→5D4, 7F0→5L6, 7F0→5D3, 7F0→5D2 and 7F0→5D1, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the past few years, more and more attention has been paid to the achievements of designing catalysts on the nanoscale. Continuous research by scientists has succeeded in producing an ability to synthesize new materials and perform new functions, and some important achievements have been made. For example, sol-based units of palladium cores and ornamentals assemble nanoparticles Pd@CeO2 [1]. A unique methane combustion catalyst is produced when they are uniformly deposited on a functionalized alumina carrier [2]. Core-shell structures such as precious metals (silver, gold, palladium and platinum) particles in carbon spheres [3], Pt@CoO yolk/shell nanoparticles [4], Pt@mSiO2 [5] and Au nanoparticles in hollow ZrO2 and hollow SiO2 spheres constitute a broad class of nanoengineered catalysts. In cases where the solid metal core is encapsulated in an oxide shell, the goal is usually to isolate the metal particles from each other to avoid sintering while maintaining the accessibility of the active point. In the past decade, significant efforts have been made to prepare mesoporous core-shell nanomaterials for their potential applications in drug delivery vectors [6], optical bioprobes, sensors [7] and biomedicine. Dou et al [8] synthesized a new structure of silver shell gold nanorods modified by BaGdF5:Yb3+ and Er3+ nanoparticles through a simple method. Coating silver shells increased the biological stability of AuNRs, and AuNR@Ag nanoparticles could be used as excellent surface-enhanced Raman scattering probes. Lin et al [9] studied and synthesized new core-shell MOFs/CDs@OCMC nanoparticles with fluorescence characteristics, and evaluated the diagnosis and treatment of cancer.
Tungsten oxide (WO3) is an important semiconductor functional material. Nanoscale WO3 has a unique role because of its large specific surface areas, such as catalysis, stealth properties and other applications [10,11,12]. Gorobtsov et al [13] studied the synthesis of nanoscale WO3 by oxalic acid chemical precipitation. Deepa and Rajendran [14] prepared tungsten trioxide nanopowders using CTAB/n-cloth/octane/H2O microemulsion system, Na2WO4 and hydrochloric acid as raw materials. The structure, size and morphology of tungsten trioxide nanoparticles were characterized by an X-ray diffractometer (XRD) and transmission electron microscope (TEM). Nano-SiO2 has a large specific surface area, a small size effect and excellent optical and electrical properties, so it is widely used in rubber products, colours, catalysts, medical carriers and other fields [15]. Khosravi et al [16] oxidized cyclohexane to cyclohexanone (Ke) and cyclohexanol (Al) using a new type of highly efficient magnetic ZrFe2O4@SiO2-TCPP nanocatalyst. Mesoporous ZrFe2O4 nanoparticles with nanometre structure were synthesized by solvothermal method, and ZrFe2O4@SiO2 core-shell was prepared by wrapping silica with TEOS. There are two popular methods for the preparation of mNPs(metal oxide)/SiO2 materials: the coating method and the deposition method [17,18,19,20,21]. mNPs are firstly prepared by the coating method, and then the surface of metal oxide is chemically modified to inhibit its agglomeration and improve the compatibility with SiO2. Finally, the modified metal oxide is used as a template to catalyse the hydrogen condensation of ethyl TEOS with an alkaline catalyst to form the Si–O–Si network to realize the metal oxide coating. That is the mNPs@SiO2 materials. However, the photocatalytic performance of traditional WO3 materials is not excellent, so it is necessary to modify the surface of WO3 to improve the photocatalytic activity. SiO2 is a good coating material. SiO2 is used as composite shell material because of the high stability of silica gel in a liquid medium, nanoshell structure and controllable shell thickness in the deposition process. As a new type of functional material, nanocomposite materials show excellent application potential in various fields and have become a hot topic in the research of nanocomposite materials. Based on the advantages of SiO2 and WO3 nanoparticles, this study intends to prepare WO3:Eu3+@SiO2 nanoparticles to improve the performance of WO3.
In this study, using europium nitrate and ammonium tungstate as precursors, WO3:Eu3+ powder was prepared by microemulsion method with cetyltrimethyl ammonium bromide (CTAB) as the surfactant. The prepared WO3:Eu3+ samples were wrapped with SiO2 by a coating method, and nanocomposites were prepared by controlling TEOS content at 1, 3, 5 and 7%. The WO3:Eu3+@SiO2 core-shell structure was prepared by controlling the water–alcohol ratio at 1:3, 1:1.5, 1:1 and 2:1. The structure, morphology and luminescence properties of the prepared WO3:Eu3+@SiO2 samples were characterized by XRD, SEM (scanning electron microscope), TEM and fluorescence spectra. The optimum TEOS content and the ratio of water to alcohol were analysed synthetically.
2 Experimental
2.1 Raw materials
Europium nitrate (Eu(NO3)3·6H2O]) produced by Shanghai Aladdin Biochemical Technology Co., LTD was used. Ammonium paratungstate ((NH4)10H2(W2O7)6·xH2O) produced by Shanghai Aladdin Biochemical Technology Co., LTD was used. Hexadecyl trimethyl ammonium bromide (CTAB, C12H42NBr) as surfactant and n-butyl alcohol (C4H10O) produced by Nanjing Chemical Reagent Co. LTD were used. Tetraethyl orthosilicate (TEOS, C8H20O4Si) produced by Shanghai Maclin Biochemical Technology Co., LTD was used. Absolute ethyl alcohol (C2H6O) and ammonium hydroxide (NH4OH) produced by Shanghai Jiuyi Chemical Reagent Co., LTD were used. All reagents were analytically pure.
2.2 Preparation of nano-samples
2.2a Preparation steps of WO3: Eu3+ nanoparticles: Figure 1 shows the preparation flowchart of WO3:Eu3+ nanoparticles. A quantity of 1.144 g CTAB was dissolved in 50 ml n-butanol solution. Europium nitrate, 0.288 g, powder was dissolved in 10 ml distilled water and stirred until dissolved. The above solutions are mixed to obtain a solution of europium nitrate, and solution A was obtained. Ammonium paratungstate particles, 4.8 g, were dissolved in 50 ml distilled water and stirred until dissolved to make a clarified aqueous solution. Then slowly dropped into europium nitrate solution, placed in a 90°C water bath, heating and stirring continuously for 4 h, and solution B was obtained. The remaining solution was poured into the crucible and dried at 100°C for 4 h. The white powder was obtained. Then place it into a muffle furnace to dry and heat it up to 600°C. After drying for 4 h, it was cooled to room temperature with furnace temperature to obtain yellow-green tungsten oxide (WO3:Eu3+) doped with Eu ion powder and then ground and collected.
2.2b Preparation of WO3:Eu3+@SiO2 nanocomposite: Figure 2 shows the preparation flowchart of WO3:Eu3+@SiO2 nanoparticles. TEOS was dissolved in 36 ml of anhydrous ethanol, and the content of TEOS was 1, 3, 5 and 7% under alkaline conditions, respectively. A quantity of 0.3 g WO3:Eu3+ powder was dissolved in 72 ml deionized water, and TEOS solution was added to WO3 solution drop by drop according to different contents. Then the pH value was adjusted to 10 under the alkaline condition of NH3·H2O. The stirring rate was 1000 r min–1 at room temperature and pressure, and the reaction lasted for 12 h. To obtain pure WO3:Eu3+@SiO2 nanocomposites, ultrasonic was used to clean them with anhydrous ethanol twice, followed by centrifugation with a high-speed centrifuge. After centrifugation, the upper clear liquid was poured out, and the lower solid was taken out. The obtained solid was put into the crucible and dried at 90°C in the air-blast drying oven until it was solid. After 3 h, it was removed to be cooled and then ground in the mortar into dense powder; the WO3:Eu3+@SiO2 nanocomposite was obtained.
WO3:Eu3+, 0.3 g, samples were dissolved in 12, 24, 36 and 72 ml deionized water, respectively. A quantity of 9 ml TEOS was dissolved in 36 ml anhydrous ethanol. Then it was slowly added into WO3:Eu3+ solutions with different water contents, and the pH was adjusted to 10 with ammonia and stirred on a magnetic stirrer at 90°C for 4 h. To obtain pure WO3:Eu3+@SiO2 nanocomposites, ultrasonic was used to clean them with anhydrous ethanol twice, followed by centrifugation with a high-speed centrifuge. After centrifugation, the upper clear liquid was poured out, and the lower solid was taken out. The obtained solid was put into the crucible and dried at 90°C in the air-blast drying oven until it was solid. And then, ground in the mortar into dense powder, the prepared WO3:Eu3+@SiO2 nanocomposite was obtained.
2.3 Microstructure and morphology characterization of the prepared WO3:Eu3+@SiO2 nanocomposite
The microstructure of the prepared WO3:Eu3+@SiO2 nanocomposite sample was characterized by a D8 Advance X-ray diffractometer (XRD). According to the measured data, phase analysis was performed in Jade software, and the substances contained in the sample were determined using PDF cards. The instrument voltage was set to 20 kV, the current to 5 mA, the Angle range from 2° to 80°, the residence time was 0.1 s, the step length 0.02°, and the speed at 12° min–1.
Morphology of the prepared WO3:Eu3+ and WO3:Eu3+@SiO2 nanocomposite samples was observed by Merlin Compact scanning electron microscope (SEM). Nano Measurer software was used to measure the particle size of the SEM images. The instrument’s resolution was High Vacuum mode 1.0nm@15kV WD = 2 nm, the magnification ranged from 12 to 2 million times, the sample current ranged from 5 Pa to 20 nA, and the acceleration voltage ranged from 0.05 to 30 kV.
2.4 Fluorescence spectra of the prepared WO3:Eu3+@SiO2 nanocomposite
FS5 steady-state/unsteady fluorescence spectrometer was used to test the prepared samples’ luminescence under different TEOS and water content. The excitation light source was a deuterium lamp, the signal-to-noise ratio (SNR) was 600:1, the test step size was 1 nm, and the standing time was 0.5 s.
3 Results and discussion
3.1 Microstructure analysis of the prepared WO3:Eu3+@SiO2 nanocomposite
Figure 3a shows XRD images of the prepared WO3:Eu3+@SiO2 nanocomposite with different contents of Si. Compared with the standard JCPDS card, it can be seen that the diffraction peaks of the prepared samples at 22.9°, 23.5°, 24.1°, 28.6° and 33.9° correspond to (002), (020), (200), (111) and (202), respectively. These diffraction peaks correspond to the same peaks on PDF#72-1465 standard card of monocline WO3, indicating that the prepared sample is WO3 powder, the space group is P21/n(14), and its cell parameters are a = 7.301, b = 7.530, c = 7.680, α = β = γ = 90°. The other phases of the samples are basically consistent with the PDF-43-1008 standard card of Eu2O3 in the cubic crystal system. The parameters are a = b = c = 10.868 Å, α = β = γ = 90°, which belong to the space group Ia3(206). As can be seen from figure 3a, the diffraction peak crystal and microstructure of the prepared samples do not change before and after the encapsulation of SiO2. With the increase of TEOS content, the XRD pattern keeps increasing, so it can be inferred that SiO2 exists in the form of amorphous. In addition, the diffraction peaks of the nanopowders were almost the same before and after being coated with SiO2, indicating that changing the content of TEOS has little effect on the intensity of the diffraction peaks.
The full-width at half-maximum (FWHM) and grain size of the prepared samples were obtained by the Scherrer formula and shown in figure 3b. It shows that the average FWHM is inversely proportional to the average grain size. The larger the half-width is, the smaller the grain size is. With the increase of TEOS content, the grain size first decreases, then increases and then decreases. The average particle sizes of the prepared nanocomposites with 1, 3, 5 and 7%TEOS content are 11.6, 13.7, 10.9 and 13.5 nm, respectively. The grain size is the smallest when TEOS content is 5%, and the grain size is the largest when TEOS content is 3%.
Figure 4a shows XRD patterns of the prepared WO3:Eu3+@SiO2 nanocomposite under alkaline conditions with different water–alcohol ratios (H2O: C2H6O). After phase retrieval, diffraction peaks were found on the (002), (020), (200), (120) and (202) crystal planes corresponding to 23.2°, 23.7°, 24.4°, 26.7° and 34.2°, respectively. It is consistent with the peak on PDF#72-1465 standard card of monoclinic WO3, indicating that the sample is WO3 nanopowder. The other phases of the samples are basically consistent with the PDF-43-1008 standard card of Eu2O3 in the cubic crystal system. The parameters are a = b = c = 10.868 Å, α = β = γ = 90°, which belongs to the space group Ia3(206). Meanwhile, the doped TEOS is not evident in the diffraction peak, which also indicates that SiO2 wraps WO3:Eu3+ in an amorphous form. The spatial group of the sample is P21/N (14), and its cell parameters are a = 7.306, b = 7.540, c = 7.692, α = β = γ = 90°. It can be seen from figure 4a that the water–alcohol ratio has a significant influence on the diffraction peak intensity. When the water–alcohol ratio is 2:1, the diffraction peak intensity is close to that of pure WO3:Eu3+ nanopowder; when the water–alcohol ratio is 1:1.5, the diffraction peak intensity is weaker than that of pure WO3:Eu3+ powder.
The grain size and FWHM of the prepared WO3:Eu3+@SiO2 nanocomposites with different water–alcohol ratios were calculated by the Scherrer formula and given in figure 4b. It can be found that the grain size of the prepared samples is inversely proportional to FWHM. With the increase of water content, the grain size of the sample decreases first, then increases and then decreases. Under the condition that the TEOS content remains unchanged, the grain size reaches its maximum when the water–alcohol ratio is 1:3, and the SiO2 shell coated with prepared WO3 becomes thicker. The intensity of the prepared WO3 diffraction peak is weakened. This corresponds to the weakening of the prepared WO3 diffraction peak of the prepared WO3:Eu3+@SiO2 nanocomposite with the water–alcohol ratio of 1:3 in figure 3b.
3.2 Morphology of the prepared WO3:Eu3+@SiO2 nanocomposite
Figure 5 shows SEM images of the prepared WO3:Eu3+ sample (a), WO3:Eu3+@SiO2 nanocomposites with TEOS content of 1 (b), 3 (c), 5 (d) and 7% (e). The above XRD analysis shows that the prepared WO3:Eu3+@SiO2 nanocomposites are monoclinic crystal phases. Figure 5a shows the SEM image of pure WO3:Eu3+ nanopowder, and it can be seen that many particles are connected to each other and are roughly spherical. Due to tungsten oxide after heat treatment, it is the process of organic decomposition and water loss. The loss of water leads to the bonding of surface-active groups of different particles, which leads to the agglomeration of tungsten oxide particles. Figure 5b, c, d and e shows that grain size increases significantly with the increase of TEOS content. Many nanoparticles of varying sizes are piled on top of each other irregularly. Using Nano Measurer software, the grain size of the samples is calculated. The average grain size of the prepared samples is 71.5 nm of the prepared WO3:Eu3+ sample, 91.6 nm of the prepared WO3:Eu3+@SiO2 nanocomposites with 1% TEOS, 89.8 nm of 3% TEOS, 95.5 nm of 5 % TEOS and 105.3 nm of 7% TEOS, respectively. The sample size with 7%TEOS content is the largest, followed by 5%TEOS. The prepared WO3:Eu3+ sample without SiO2 modification has the smallest size. Figure 5f–j shows that grain size increases significantly with the increase of water–alcohol ratios. The results show that the prepared WO3:Eu3+@SiO2 nanocomposites are spherical and granular, and the average particle size is below 100 nm.
Figure 6 shows TEM image of the synthesized WO3:Eu3+@SiO2 nanocomposite, the diffraction pattern of WO3, and the high-resolution TEM image of WO3:Eu3+@SiO2 nanocomposite taken along [312] direction. The high-resolution TEM image shows the lattice fringes of WO3 as 0.31 and 0.35 nm in the (112) and (021) planes, respectively. It is found that the black part is WO3 and the other part is inorganic SiO2.
3.3 Luminescence properties of the prepared WO3:Eu3+@SiO2 nanocomposites
3.3a Emission spectra: Figure 7a shows the emission spectra of the prepared WO3:Eu3+ sample and WO3:Eu3+@SiO2 nanocomposites with different TEOS contents under alkaline conditions excited at 328 nm. When the excitation wavelength is 328 nm, the highest emission peak of WO3:Eu3+@SiO2 nanocomposite is at 554 nm, which is in the range of green light band originating from the 5D1→7F1. The luminescence intensity of the sample with 5%TEOS content is the highest, which is 526% higher than that of the WO3:Eu3+ powder before modification. The luminescence intensity of the sample with 3%TEOS content is the lowest.
Figure 7b shows the emission spectra of the prepared WO3:Eu3+ sample and WO3:Eu3+@SiO2 nanocomposites with different TEOS contents under alkaline conditions excited at 394 nm. There are three obvious emission peaks at 394 nm, a red emission peak at 612 nm, an orange emission peak at 571 nm, and a blue emission peak at 482 nm. The emission peak at 612 nm is the strongest, and the emission intensity of the prepared WO3:Eu3+@SiO2 nanocomposites with 5%TEOS content is 652% higher than that of the prepared WO3:Eu3+ sample. When the excitation wavelength is 571 nm, the luminescence intensity of the prepared nano-WO3:Eu3+@SiO2 composites with 5%TEOS content is 697% higher than that of the prepared WO3:Eu3+ sample. The main reasons are two aspects. First, the fluorescence enhancement of WO3 particles coated by SiO2 shell is mainly due to the localized surface plasmon resonance effect of metal oxide nanoparticles; second, the enhancement of fluorescence by shell thickness is not linear. With the increase of amorphous SiO2 thickness, the scattering and absorption of light by the shell increase. Therefore, the fluorescence intensity of 7% TEOS is less than that of 5% TEOS [22,23,24].
Under the excitation of different wavelengths, the strongest emission peak is at 554 nm at the wavelength of 328 nm, and the emission peak is in the green-light band. In the emission spectrum excited at 394 nm, the peak is at 612 nm, which is in the red-light band originating from the 5D0→7F2 belonging to an electric dipole transition. The emission peak intensity and luminescence intensity of the prepared WO3:Eu3+@SiO2 nanocomposites with 5%TEOS content are the strongest both at 328 and 394 nm.
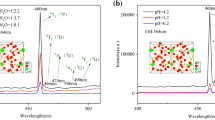
Figure 8a shows emission spectra of the prepared WO3:Eu3+ sample and WO3:Eu3+@SiO2 nanocomposites with different water–alcohol ratios at 328 nm excitation wavelength. There are three obvious emission peaks at 401, 554 and 655 nm, among which the highest emission peak is located at 401 nm. The emission peak intensity of the prepared sample with the water–alcohol ratio of 1:3 is the strongest, and the emission intensity is 669% higher than that of the prepared sample with the water–alcohol ratio of 2:1. The emission peak intensity of the sample with water–alcohol ratio of 1:1.5 is the next, and the emission peak intensity of the sample with water alcohol ratio of 2:1 is the worst.
Figure 8b shows emission spectra of the prepared WO3:Eu3+ sample and WO3:Eu3+@SiO2 nanocomposites with different water–alcohol ratios at 394 nm excitation wavelength. There are three obvious emission peaks at 483, 572 and 611 nm, among which the highest emission peak is located at 611 nm. At this time, the emission peak intensity of the prepared sample with the water–alcohol ratio of 1:3 is 138% higher than that of the prepared sample with the water–alcohol ratio of 2:1. The emission peak is located at 572 nm, and the emission peak intensity of the prepared sample with water–alcohol ratio of 1:3 is 146% higher than that of the prepared sample with water–alcohol ratio of 2:1. The sample with the same water–alcohol ratio of 1:3 has the strongest emission peak intensity, followed by the sample with the water–alcohol ratio of 1:1.5, and the sample with the water–alcohol ratio of 2:1 has the worst emission peak intensity.
3.3b Excitation spectra: Figure 9 shows excitation spectra of nanoparticles with different TEOS contents in alkaline conditions (a) and water-alcohol ratios (b) at 612 nm monitoring wavelength. It can be seen that there is a relatively weak peak of 328 nm in the excitation spectrum, which is mainly due to the charge transfer between W–O and Eu–O [25]. Several characteristic peaks between 350 and 550 nm belong to the transition of 7F0→5D1 of Eu3+. Peaks mainly located at 362, 394, 422, 465 and 499 nm, each corresponding to the transition of 7F0→5D4, 7F0→5L6, 7F0→5D3, 7F0→5D2 and 7F0→5D1, respectively. The maximum peak was at 499 nm, belonging to the transition of 7F0→5D1. Figure 8a shows that the luminescence intensity increases significantly after TEOS is added. The luminescence intensity of the sample is the strongest when TEOS is 5%, and the sample’s strongest characteristic peak is located at 499 nm. Figure 8b shows that the luminescence intensity is the strongest when the water–alcohol (H2O: C2H5OH) ratio is 1:3, and the strongest characteristic peak is also located at 499 nm of the sample.
4 Conclusion
WO3:Eu3+ powder was prepared by microemulsion method with cetyltrimethyl ammonium bromide (CTAB) as the surfactant. The prepared WO3:Eu3+ samples were wrapped with SiO2 by a coating method. The WO3:Eu3+@SiO2 nanocomposites with the core-shell structure were prepared by controlling TEOS content (1, 3, 5 and 7%) and water–alcohol ratio (1:3, 1:1.5, 1:1 and 2:1). The structure, morphology and luminescence properties of the prepared WO3:Eu3+@SiO2 samples were characterized by XRD, SEM, TEM and fluorescence spectra. The results show that the prepared WO3:Eu3+@SiO2 nanocomposites are spherical and granular, and the average particle size is below 100 nm. Under the condition of different TEOS contents, the strongest characteristic peak is located at 554 nm in the green-light band at the excitation wavelength of 328 nm. Under the condition of 5%TEOS content, the luminescence intensity of the prepared WO3:Eu3+@SiO2 composites is the highest, which increases by 526%. Under different water–alcohol ratio conditions, the prepared WO3:Eu3+@SiO2 nanocomposite has the strongest luminescence intensity at the water–alcohol ratio of 1:3, and the excitation wavelength is 328 nm. The emission peak intensity of the prepared WO3:Eu3+@SiO2 sample with the water–alcohol ratio of 1:3 is the strongest, and the intensity is 669% higher than that of the water–alcohol ratio of 2:1.
References
Cargnello M, Wieder N L, Montini T, Gorte J R and Fornasiero P 2010 J. Am. Chem. Soc. 132 1402
Cargnello M, Jaén J D, Garrido J C H and Fornasiero P 2012 Science 337 713
Sun X and Li Y 2004 Angew. Chem. 116 607
Yin Y, Rioux R M, Erdonmez C K, Hughes S, Somorjai G A and Alivisatos A P 2004 Science 304 711
Joo S H, Park J Y, Tsung C K, Yamada Y, Peidong Y and Somorjai G A 2009 Nat. Mater. 8 126
Sivakumar S, Diamente P R and van Veggel F C J M 2006 Chem. – Eur. J. 12 5878
Li S, Zhang M, Zhan Z, Liu R and Xiong X 2020 J. Magn. Mag. Mater. 500 166321
Dou J, Chen B, Liu G, Dong X and Zhu J 2022 J. Rare Earth 40 193
Lin C, Sun K, Zhang C, Tan T and Whittaker A 2020 Microporous Mesoporous Mater. 293 109775
Kulp C, Chen X, Puschhof A, Schwamborn S, Somsmen S, Schumann et al 2010 ChemPhysChem 11 2854
Stöber W, Fink A and Bohn E 1968 J. Colloid Interface Sci. 26 62
Rao K S, El-Hami K, Kodaki T, Matsushige K and Makino K 2005 J. Colloid Interface Sci. 289 125
Gorobtsov P, Simonenko T L and Simonenko N P 2021 Russ. J. Inorg. Chem. 66 1811
Deepa B and Rajendran V 2018 Nano-struct. Nano-Object 16 185
Martínez D S, Martínez-De La Cruz A and Cuéllar E L 2011 Appl. Catal. A Gen. 398 179
Khosravi H B, Rahimi R and Rabbani M 2021 Silicon 13 451
Dutov V V, Mamontov G V, Zaikovskii V I, Liotta L F and Vodyankinaa O V 2018 Appl. Catal. B Environ. 221 598
Areerob Y, Cho J Y, Jang W K and Oh W C 2018 Ultrason. Sonochem. 41 267
Khalid B, Rahim A, Gilani M A, Younus A R, Tabassum S, Iqbal J et al 2018 J. Mol. Liq. 4 450
Bian X 2018 J. Supercond. Nov. Magn. 31 3685
Khoshnam M and Salimijazi H 2021 Surf. Interfaces 26 101322
Xu D D, Deng Y L, Li C Y, Lin Y and Tang H W 2017 Biosens. Bioelectron. 87 881
Ghosh P, Oliva J, Rosa E D L, Haldar K K, Solis D and Patra A 2008 J. Phys. Chem. C 112 9650
Pushpal G, Arik K and Amitava P 2010 Nanoscale 2 1196
Feng W, Jin Y, Wu Y, Li D F and Cai A K 2013 J. Lumin. 134 614
Acknowledgements
We are supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China, Grant/Award Number: 22KJD430007; the Opening Project of Jiangsu Key Laboratory of Advanced Structural Materials and Application Technology, Grant/Award Number: ASMA202202; Jiangsu Province Industry-University-research cooperation Project, Grant/Award Number: BY2022095; Jinling University of Science and Technology, Grant/Award Number: jit-b-201824; Jinling Institute of Science and Technology Incentive Fund, Grant/Award Number: Jit-fhxm-201921.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, D., Xiong, S., Zhang, X. et al. Preparation and luminescence properties of WO3:Eu3+@SiO2 nanocomposites. Bull Mater Sci 46, 71 (2023). https://doi.org/10.1007/s12034-023-02917-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-023-02917-3