Abstract
Self-powered broadband photodetector was fabricated using PbS/CdS-decorated TiO2 nanorods (NRs). TiO2 NRs were grown using hydrothermal method and CdS/PbS layers were deposited by chemical bath deposition process. Metal-free top electrode of the photodetector was prepared using chemically reduced graphene oxide (rGO). PbS/CdS-decorated TiO2 NRs based photodetector showed significant photocurrent over broad spectral range. The maximum responsivity \((R_{\lambda } ) \) and detectivity (\(D_{\lambda }\)) of the photodetector at zero bias was obtained, ~0.34 A W–1 and ~162742.42 Hz1/2 W–1, respectively, at 400 nm. TiO2/CdS/PbS/rGO device showed sharp rise and decline of photocurrent under ON/OFF of white light. This device also demonstrated excellent self-powered nature with open circuit voltage ~0.40 V, short circuit current ~0.077 mA and power conversion efficiency ~0.16%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
One-dimensional (1D) TiO2 nanostructured materials such as nanorods (NRs), nanowires and nanotubes have received a great deal of attention during the last decade because of their unique physical, chemical and optical properties, and utilization in a variety of applications, such as photocatalysis, dye-sensitized solar cells, and sensors [1,2,3,4]. TiO2 is a large bandgap semiconductor with bandgap ~3.2 eV and particularly important in optoelectronic devices such as UV detectors and solar cells [5,6,7,8]. Since TiO2 have limited photo-absorption in visible range, it is difficult to fabricate broadband photodetectors using pristine NRs. To achieve photoabsorption in wide spectral range, wide bandgap metal oxides are often sensitized with low bandgap chalcogenide semiconductors, such as PbS, PbSe, CdS, CdSe, etc. [9,10,11,12,13,14,15]. Most of these semiconductors exhibit excellent size tuneable optical absorption in wide spectral region. Nowadays, several research works have been focused on the fabrication of self-powered photodetectors, which can be operated without the applied external bias [16,17,18,19]. Self-powered broadband photodetectors are particularly attractive for applications in next-generation nanodevices, which can be operated wirelessly and independently [20,21,22]. Gao et al [5] reported self-powered UV photodetector using NiO/TiO2 heterojunction grown on fluorine-doped tin oxide (FTO) substrate. They obtained responsivity ~1.34 and ~5.66 mA W–1 in case of NiO/TiO2 NR and NiO/TiO2 NRs/TiOx samples respectively, with rise time less than 0.1 s. Xie and co-workers [23] studied photoelectrochemical-based self-powered photodetector using SnO2/TiO2 NR arrays. The photodetector parameters such as responsivity, rise time and decay time were found to be ~145 A W–1, ~0.037 and 0.015 s, respectively. Although, TiO2-based UV photodetectors were studied by various researchers, however, there is no report available on self-powered broadband photodetector using 1D TiO2 nanostructures.
In this research, we have studied self-powered broadband photodetector using CdS/PbS nanoparticle-decorated TiO2 NRs. TiO2 NRs were synthesized on FTO-coated glass substrates. Top electrical contacts of the devices were made using chemically grown reduced graphene oxide (rGO) layers instead of conventional metal electrodes. Self-powered photosensing nature of TiO2 NRs/CdS/PbS device has been demonstrated in our present study. The photodetector parameters of the fabricated device have been investigated in detail.
2 Experimental
TiO2 NRs were synthesized on FTO-coated glass substrates using hydrothermal technique [7]. As-grown NRs were annealed at 500°C for 1 h to improve the crystallinity. Nanocrystalline CdS layers were deposited on TiO2 NRs using chemical bath deposition process (CBD). In this method, 0.025 M of cadmium acetate, 0.1 M of ethylenediamine and 0.1 M thiourea were mixed in 100 ml de-ionized (DI) water and the solution was heated at 60°C. During CdS attachment, TiO2 NRs were dipped in the prepared solution for 10 min at 60°C. Afterwards, lead sulphide layers was deposited on CdS-decorated TiO2 NRs using similar CBD process. At first, an aqueous solution was prepared by dissolving 0.05 M lead nitrate (PbNO3)2, 0.04 M triethanolamine and 0.02 M NaOH in a beaker of 100 ml DI water. On the other hand, 0.06 M thiourea [CS(NH2)2] was dissolved in 100 ml DI water in another beaker. The above two solutions were mixed thoroughly and the solution temperature was maintained at 40°C. Subsequently, CdS-decorated TiO2 NRs were dipped slowly in the solution for 1 h to deposit PbS layer. The control sample TiO2 NRs/PbS was also prepared using similar CBD process as described above. The TiO2 NRs on FTO substrates were dipped in the precursor solution for 1 h at 40°C during CBD growth. The pH of the solution was maintained at ~10 throughout the growth process. After PbS layer growth, all samples were thoroughly washed with DI and dried in air.
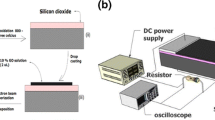
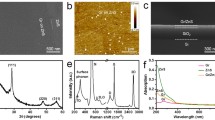
In this present study, photodetector properties of TiO2/CdS/PbS NRs were analysed using FTO as a bottom electrode and rGO as the top conducting electrode. Graphene oxide (GO) flakes were grown by using well-known modified Hummer’s method, and the detail growth process has been reported elsewhere [24,25,26]. Conductive-reduced GO flakes were prepared by a low-cost chemical method using ascorbic acid as a reducing agent [27]. To adjust the pH of the exfoliated GO solution (pH~6), NaOH pellets (98%, Sigma Aldrich) were added followed by ultrasonication for 1 h. Then, another solution prepared by adding 10 g L-ascorbic acid (99%, Sigma Aldrich) in 100 ml DI water was poured slowly to the previous GO solution. The reduction process was conducted at 95°C for 1 h. The resultant black precipitates were filtered and washed with 1 M HCl solution followed by DI water to achieve neutral pH. Finally, the filtrate was freeze-dried to obtain rGO powder for further applications. To investigate the electrical conductivity of as-prepared rGO powder, small pellets (area A = 1 cm2 thickness t = 0.535 mm) were made by laboratory hot press without any heat treatment. The electrical conductivity of rGO was calculated to be 3.93 S m–1. For deposition of top rGO electrodes, at first rGO powders were dissolved thoroughly in N, N-dimethyl formamide solution. Afterwards, this rGO solution was spin coated on TiO2/CdS/PbS NRs and dried in air to evaporate the solvent. Illustrative representation of synthesis of GO and rGO are presented in figure 1a. The scanning electron micrographs (SEM) of as-grown GO and rGO powders are presented in figure 1b and c, respectively. The schematic representation of the growth of TiO2 NRs, CdS-coated TiO2 NRs and TiO2 NRs/CdS/PbS are presented in figure 2a, b and c, respectively. The final device structure after deposition of rGO on the NRs is illustrated in figure 2d.
The surface morphology of TiO2 NRs, CdS deposited on TiO2 NRs, and PbS-deposited TiO2 NRs and TiO2 NRs/CdS/PbS were studied using Zeiss Sigma 300 scanning electron microscope (SEM). The crystalline nature and structural information of the samples were studied by using a Bruker D8 Advanced X-ray diffractometer. Optical absorption spectra of all samples were acquired by using a Shimadzu UV3600 plus UV-VIS-NIR spectrometer. Current–voltage (I–V) characteristics and photocurrents of all devices were studied using a dual channel Agilent, B2912A source measuring unit. Transient photoresponse results were obtained upon illumination of white light of intensity 100 mW cm–2 from solar a simulator (Enlitech, Taiwan). Spectral dependence of photocurrent of the samples were studied under incident of various wavelength of light (300–700 nm) from a monochromator attached with a white light source (Science Tech, Canada).
3 Results and discussion
Top view SEM micrographs of TiO2 NRs, CdS-deposited TiO2 NRs, PbS-deposited TiO2 NRs and TiO2 NRs/CdS/PbS are presented in figure 3a, b, c and d, respectively. Figure 3a represents the formation of vertically aligned TiO2 NRs on FTO substrate with square top and average diameter is 80 nm. Figure 3b, c and d depict the SEM micrographs of TiO2 NRs after deposition of CdS, PbS and CdS/PbS layers, respectively. The SEM images clearly show the deposition of CdS and PbS layers on NR surfaces. The presence of elements Cd, Pb, S at the surface of TiO2 NRs were confirmed by EDX study during SEM imaging, and presented in supplementary figure S1a–c. The length of the TiO2 NRs/CdS/PbS NRs has been estimated from cross-section SEM image, and presented in supplementary figure S2. The typical length of the NRs was found to be 1.23 μm. XRD spectra of pristine TiO2 NRs, CdS-deposited TiO2 NRs, PbS-deposited TiO2 NRs and PbS/CdS-deposited TiO2 NRs are shown in figure 4a, b, c and d, respectively. In figure 4a, the diffraction peaks at (101), (211), (002) and (221) planes represent the rutile phase (JCPDS#77-0440) [28] and the peak at (004) depicts the anatase phase of TiO2 NRs (JCPDS#84-1285) [29]. It can be observed that, after CdS deposition the two peaks appeared at (111) and (220) planes (figure 4b), which confirmed the formation of cubic phase of CdS on TiO2 NRs (JCPDS#75-1546) [30]. In figure 4c, all XRD peaks correspond to the crystal planes (111), (200), (220), which confirmed the growth of cubic structure of PbS on TiO2 NRs (JCPDS#78-1899) [31]. The presence of both CdS and PbS peaks were also observed in TiO2 NRs/CdS/PbS sample (figure 4d).
UV visible absorption spectra of TiO2 NRs, CdS-deposited TiO2 NRs, PbS-deposited TiO2 NRs and TiO2 NRs/CdS/PbS are plotted in figure 5a. TiO2 NR shows strong UV absorption with absorption edge at 400 nm. On the other hand, the absorption edges of CdS-deposited TiO2 NRs and PbS-deposited TiO2 NRs are red shifted to visible region compared to pristine TiO2 NRs. Interestingly, TiO2 NRs/CdS/PbS showed enhanced absorption in wide spectral range, which is useful for broadband photodetector application [32,33]. The optical bandgaps (\(E_{{\text{g}}}\)) of the samples were calculated by using well-known Tauc’s equation as shown below [34].
where \(E_{{\text{g}}}\) is the energy bandgap of the samples, \(C\) is a constant, \(\alpha\) the absorption coefficient and \(h\nu\) the incident photon energy. The value of exponent n denotes the nature of transition. For direct allowed transition \(n = \frac{1}{2}\) and for indirect allowed transition \(n = 2\). In case of direct bandgap semiconductors, the bandgaps were calculated by extrapolating the linear region of the plots \(\left( {\alpha h\nu } \right)^{2 }\) vs. (\(h\nu ) \) to the energy (\(h\nu )\) axis. Tauc’s plots of all samples are presented in figure 5b. The calculated absorption edges of TiO2 NRs, TiO2 NRs/CdS, TiO2 NRs/PbS were found to be 3.2, 2.4 and 1.9 eV, respectively. Interestingly, the Tauc’s plot of TiO2 NRs/CdS/PbS sample shows three distinct absorption edges. The absorption at 1.8 and 2.4 eV are accredited to the attachment of PbS and CdS on TiO2 NRs surfaces, respectively. On the other hand, the absorption beyond 3.0 eV is ascribed to TiO2 NRs.
(a) UV–visible absorption spectrum of TiO2 NRs, CdS-deposited TiO2 NRs, PbS-deposited TiO2 NRs and CdS/PbS-deposited TiO2 NRs recorded in the wavelength range 300–800 nm. (b) Tauc’s plots [\((\alpha h\nu)^{2}\) vs. \(h\nu\)] of TiO2 NRs, CdS-deposited TiO2 NRs, PbS-deposited TiO2 NRs and PbS/CdS-deposited TiO2 NRs.
To study the self-powered photodetection properties of TiO2 NRs/CdS/PbS sample, the following three samples were fabricated. The samples are (i) FTO/TiO2 NRs/CdS/rGO, (ii) FTO/TiO2 NRs/PbS/rGO and (iii) FTO/TiO2/CdS/PbS/rGO. The J–V characteristics of FTO/TiO2 NRs/CdS/rGO, FTO/TiO2 NRs/PbS/rGO and FTO/TiO2/CdS/PbS/rGO devices under dark condition are shown in figure 6a, b and c, respectively. The semi-logarithmic J–V plots under dark and illumination of broadband light of intensity 100 mW cm–2 for the above samples are presented in the insets of figure 6. Both the control devices presented in figure 6a and b show rectifying nature with rectification ratio ~20 and 3.4 at –1 V, respectively. On the other hand, FTO/TiO2/CdS/PbS/rGO device (figure 6c) shows better rectification behaviour with rectification ratio ~402 at –1 V bias, which is 20 times higher than FTO/TiO2 NRs/CdS/rGO and 118 times higher than FTO/TiO2 NRs/PbS/rGO samples. After illumination of white light, the dark current increases for all devices. The change in dark current for TiO2 NRs/CdS, TiO2 NRs/PbS and TiO2 NRs/CdS/PbS samples was observed to be 0.037, 0.28 and 0.31 mA cm–2, respectively. More interestingly, TiO2 NRs/PbS and TiO2 NRs/CdS/PbS devices demonstrated self-powered photodetection. The power conversion efficiency (PCE) of all self-powered devices was estimated using the following equation [35]:
where fill factor \({\text{(FF}}) = \frac{{V_{{\text{m}}} \times J_{{\text{m}}} }}{{V_{{{\text{OC}}}} \times J_{{{\text{SC}}}} }}\), Voc is open circuit voltage, JSC is short circuit current density, \(V_{{\text{m}}}\) and \(J_{{\text{m}}}\) are the maximum voltage and current density of J–V curve under illumination. \( P_{{{\text{in}}}}\) is the incident radiation of white light (100 mW cm–2). The open circuit voltage (Voc) and PCE of TiO2 NRs/PbS/rGO sample were found to be ~0.35 V and 0.07%, respectively. On the other hand, the parameters Voc, JSC and PCE for TiO2 NRs/CdS/PbS/rGO sample were observed to be ~0.40 V, 0.077 mA cm–2 and 0.16%, respectively. However, TiO2 NRs/CdS sample did not show any self-powered photodetection property under illumination of light. The self-powered photodetection or photovoltaic property has been observed in TiO2 NRs/PbS and TiO2 NRs/CdS/PbS devices due to the formation of n–p junction at the interface of TiO2/PbS and CdS/PbS, respectively. More importantly, the significant improvement of short circuit current (JSC), open circuit voltage (Voc) and PEC has been observed in TiO2 NRs/CdS/PbS device due to broad photoabsorption and favourable band alignment at CdS/PbS junction [32,36,37].
J–V characteristics of (a) TiO2 NRs/CdS/rGO, (b) TiO2 NRs/PbS/rGO and (c) TiO2 NRs/CdS/PbS/rGO samples under dark condition. Semilogarithmic J–V characteristics of TiO2 NRs/CdS/rGO, TiO2 NRs/PbS/rGO and TiO2 NRs/CdS/PbS/rGO samples under dark and broadband light source of intensity 100 mW cm–2 are presented in the insets.
The transient photoresponse of TiO2 NRs/CdS, TiO2 NRs/PbS and TiO2 NRs/CdS/PbS samples are presented in figure 7a, b and c, respectively. The transient response of TiO2 NRs/PbS and TiO2 NRs/CdS/PbS devices were studied under ON/OFF of white light of intensity ~100 mW cm–2 at zero bias. However, in case of TiO2 NRs/CdS sample, transient photocurrent response was measured at –1 V bias. It can be mentioned that, TiO2 NRs/CdS sample did not show any self-powered photodetection and no photocurrent was detected at zero bias. From transient photoresponse, it is obvious that, the rise and fall of photocurrent of TiO2 NRs/PbS junction is quite faster compared to TiO2/CdS junction. More importantly, the ON/OFF ratio and rise/fall time significantly improves in case of TiO2 NRs/CdS/PbS device. The ON/OFF ratio for TiO2/PbS and TiO2 NRs/CdS/PbS devices were found to be 2.21 and 30.17, respectively. The response and recovery time of TiO2 NRs/CdS/PbS device was estimated from transient photoresponse results by using the following equations [7]:
The symbols JR and JD are photocurrent densities at the time of rise and decay. \({\mathcal{A}}\) and \({\mathcal{B}}\) are constants, t is the ON/OFF time of light, τ1 and τ2 are time constants. The experimental data and fitted curve during rise and fall of photocurrent of TiO2 NRs/CdS/PbS device are shown in figure 8. The calculated time constants during the rise of photocurrent are found to be τ1~0.051 s and τ2~0.097 s. Similarly, the photocurrent decay time constants are found to be τ1~0.034 s and τ2~0.050 s. The transient photoresponse of TiO2 NRs/PbS device was also fitted with the above equations (supplementary figure S3). The extracted time constants during rise are τ1~0.08 s; τ2~3.61 s, while during decay are τ1~0.22 s and τ2~25.38 s, respectively. Obviously, TiO2 NRs/CdS/PbS sample demonstrated fast rise and fall time compared to control samples.
To determine the ability to generate photocurrent under various wavelength of light, external quantum efficiency (EQE) measurement of TiO2 NRs/CdS/PbS device was carried out at 0 V bias. EQE (%) has been estimated using the following equation [7].
where \(J_{\uplambda }\) is photocurrent density (mA cm–2), λ the wavelength of monochromatic light (nm) and \(P_{\lambda }\) the monochromatic light intensity (mW cm–2). Figure 9a shows the EQE (%) vs. λ plot of TiO2 NRs/CdS/PbS device studied in the wavelength range 300–700 nm at 0 V bias condition. The EQE spectrum shows photocurrent response in UV-visible broad spectral range. Interestingly, two distinct peaks in EQE spectrum have been observed. The peak at 400 nm is attributed to UV photoabsorption for TiO2, whereas the peak in visible region at 500 nm is ascribed to the photoabsorption from CdS and PbS layers [38,39]. The maximum EQE obtained was 105% at 400 nm. The EQE more than 100% may be accredited to multi-phonon generation in PbS layers [40].
The schematic band diagram and carrier transport mechanism is illustrated in figure 9b. The photovoltaic performance in TiO2 NRs/CdS/PbS device is higher due to the favourable band alignment and cascade band formation [32]. When CdS layer was introduced in between TiO2 and PbS, the energy gap of conduction band and valance band levels were reduced due to band cascade formation. Under illumination, electrons and holes are efficiently transported to the electrodes due to such stepwise structure. More importantly, strong visible photoabsorption of CdS enhances photocurrent of the TiO2 NRs/CdS/PbS device. The responsivity (\(R_{\lambda } )\) and detectivity \((D_{\lambda }\)) of the self-powered photosensing device were calculated using the following relations [41]:
\(J_{{{\text{light}} }}\) and \(J_{{{\text{dark}} }}\) denote the current density at light and dark, respectively. \( P_{\lambda }\) is the incident light power density. Here, detectivity \(D_{\lambda }\) is measured in \({\text{Hz}}^{1/2}\) W–1 (or Jones) and q is the charge of electron. The responsivity (\(R_{\lambda }\)) and detectivity (\(D_{\lambda } )\) plots of TiO2 NRs/CdS/PbS sample is shown in figure 10a and b, respectively. Similar to EQE spectrum, \(R_{\lambda }\) and \(D_{\lambda }\) also showed broad spectral response from 450 to 700 nm. The maximum responsivity and detectivity were observed at 400 nm and estimated to be 0.34 A W–1 and 162742.42 \({\text{Hz}}^{1/2}\) W–1, respectively at 0 V bias.
To study the dependency of the device with intensity of light, photocurrents of the samples were measured under illumination of various intensity of white light (5–100 mW cm–2) at zero bias. Figure 11 shows the change of photocurrent with optical power density. The photocurrent of the device varies in linear range with intensity of white light, which is attractive for broadband photosensing application. The photodetector properties of various TiO2-based device are compared in table 1. From the table we observe that our device TiO2 NRs/CdS/PbS/rGO shows better responsivity at 0 V bias compared to the other results.
4 Conclusion
In summary, self-powered broadband photodetection was studied using PbS/CdS-decorated TiO2 NRs grown on FTO-coated glass substrates. Growth, structural characterization and optical properties of PbS/CdS co-sensitized TiO2 NRs have been studied in detail. Top electrode of the photodetector was made from rGO using ascorbic acid as reducing agent. TiO2 NRs/CdS/PbS demonstrated broadband photosensing properties (350–700 nm) under zero bias. The major photodetector parameters such as EQE, \(R_{\lambda }\) and \(D_{\lambda }\) were studied without any external bias. The maximum responsivity \((R_{\lambda } ) \) and detectivity (\(D_{\lambda }\)) was obtained at 400 nm and estimated to be ~0.34 A W–1 and ~162742.42 Hz1/2 W–1, respectively. TiO2 NRs /CdS/PbS/rGO device showed sharp rise and decay of photocurrent during ON/OFF of white light. The time constants during rise and decay of photocurrent were estimated to be τ1~0.051 s, τ2~0.097 s and τ1~0.034 s, τ2~0.050 s, respectively. The photodetector also demonstrated excellent self-powered nature with \(V_{{{\text{oc}}}}\) ~0.40 V, JSC ~0.077 mA cm–2 and PCE ~0.16%. This study indicated the potential use of CdS and PbS co-sensitized TiO2 NRs for self-powered broadband photosensing applications.
References
Caratto V, Aliakbarian B, Casazza A A, Setti L, Bernini C, Perego P et al 2013 Mater. Res. Bull. 48 2095
Jagadale T C, Takale S P, Sonawane R S, Joshi H M, Patil S I, Kale B B et al 2008 J. Phys. Chem. C 112 14595
Bang J H and Kamat P V 2010 Adv. Funct. Mater. 20 1970
Jeng M-J, Wung Y-L, Chang L-B and Chow L 2013 Int. J. Photoenergy 2013 1
Gao Y, Xu J, Shi S, Dong H, Cheng Y, Wei C et al 2018 ACS Appl. Mater. Interfaces 10 11269
Wu S, Chen C, Wang J, Xiao J and Peng T 2018 ACS Appl. Energy Mater. 1 1649
Dhar S, Chakraborty P, Majumder T and Mondal S P 2018 ACS Appl. Mater. Interfaces 10 41618
Zheng W, Li X, He G, Yan X, Zhao R and Dong C 2014 RSC Adv. 4 21340
Lv P, Fu W, Mu Y, Sun H, Su S, Chen Y et al 2015 J. Alloys Compd. 621 30
Abbas M A, Basit M A, Park T J and Bang J H 2015 Phys. Chem. Chem. Phys. 17 9752
Yu L, Li Z, Liu Y, Cheng F and Sun S 2015 J. Mater. Sci. Mater. Electron. 26 2286
Guijarro N, Lana-Villarreal T, Lutz T, Haque S A and Gómez R 2012 J. Phys. Chem. Lett. 3 3367
Lee S, Flanagan J C, Kang J, Kim J, Shim M and Park B 2015 Sci. Rep. 5 17472
Pawar S B, Shaikh J S, Devan R S, Ma Y R, Haranath D, Bhosale P N et al 2011 Appl. Surf. Sci. 258 1869
Rekemeyer P H, Chang S, Chuang C-HM, Hwang G W, Bawendi M G and Gradečak S 2016 Adv. Energy Mater. 6 1600848
You D, Xu C, Zhang W, Zhao J, Qin F and Shi Z 2019 Nano Energy 62 310
Tian W, Wang Y, Chen L and Li L 2017 Small 13 1701848
Bai Z, Fu M and Zhang Y 2017 J. Mater. Sci. 52 1308
Hatch S M, Briscoe J and Dunn S 2013 Adv. Mater. 25 867
Peng L, Hu L and Fang X 2014 Adv. Funct. Mater. 24 2591
Wang Z L 2013 ACS Nano 7 9533
Wang Z L 2008 Adv. Funct. Mater. 18 3553
Xie Y, Wei L, Wei G, Li Q, Wang D, Chen Y et al 2013 Nanoscale Res. Lett. 8 188
Abdolhosseinzadeh S, Asgharzadeh H and Seop Kim H 2015 Sci. Rep. 5 10160
Wang Y, Chen Y, Lacey S D, Xu L, Xie H, Li T et al 2018 Mater. Today 21 186
Deka N, Chakraborty P, Chandra Patra D, Dhar S and Mondal S P 2020 Mater. Sci. Semicond. Process. 118 105165
De Silva K K H, Huang H-H and Yoshimura M 2018 Appl. Surf. Sci. 447 338
Wang Y, Li L, Huang X, Li Q and Li G 2015 RSC Adv. 5 34302
Sankapal B R, Salunkhe D B, Majumder S and Dubal D P 2016 RSC Adv. 6 83175
Yang X, Yang Y, Wang B, Wang T, Wang Y and Meng D 2019 Solid State Sci. 92 31
Mamiyev Z Q and Balayeva N O 2015 Opt. Mater. 46 522
Jiao J, Zhou Z-J, Zhou W-H and Wu S-X 2013 Mater. Sci. Semicond. Process. 16 435
Wang P, Zhang Z, Wang H, Zhang T, Cui H, Yang Y et al 2019 J. Nanomater. 2019 1
Viezbicke B D, Patel S, Davis B E and Birnie D P 2015 Phys. Status Solidi B 252 1700
Xu T and Yu L 2014 Mater. Today 17 11
Zhu Y, Wang R, Zhang W, Ge H and Li L 2014 Appl. Surf. Sci. 315 149
Li Y, Wei L, Chen X, Zhang R, Sui X, Chen Y et al 2013 Nanoscale Res. Lett. 8 67
da Silva Filho J M C, Ermakov V A and Marques F C 2018 Sci. Rep. 8 1563
Fu Y, Cao F, Wu F, Diao Z, Chen J, Shen S et al 2018 Adv. Funct. Mater. 28 1706785
Davis N J L K, Böhm M L, Tabachnyk M, Wisnivesky-Rocca-Rivarola F, Jellicoe T C, Ducati C et al 2015 Nat. Commun. 6 8259
Dhar S, Majumder T and Mondal S P 2017 Mater. Res. Bull. 95 198
Huang Y, Yu Q, Wang J, Li X, Yan Y, Gao S et al 2015 Electron. Mater. Lett. 11 1059
Chen D, Wei L, Meng L, Wang D, Chen Y, Tian Y et al 2018 Nanoscale Res. Lett. 13 92
Ni S, Yu Q, Huang Y, Wang J, Li L, Yu C et al 2016 RSC Adv. 6 85951
Acknowledgements
We acknowledge the Central Research Facility (CRF) of NIT Agartala for XRD characterizations. We also acknowledge the DST-FIST project, Department of Physics, for UV-VIS-NIR spectroscopy measurements.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deka, N., Chakraborty, P., Patra, D.C. et al. Study of self-powered and broadband photosensing properties of CdS/PbS-decorated TiO2 nanorods/reduced graphene oxide junction. Bull Mater Sci 44, 289 (2021). https://doi.org/10.1007/s12034-021-02574-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-021-02574-4