Abstract
Homodinuclear cobalt(II) complexes of the tetraiminediphenol macrocycle were synthesized by employing the process of Schiff base condensation reaction of 2,6-diformyl-4-methylphenol and 4-substituted-o-phenylenediamines in the presence of cobalt(II) template. The spectroscopic techniques and mass spectrometry were utilized so as to carry out the characterization for the obtained complexes. On implementing thermal analysis, the complexes were found to exhibit high thermal stability such that they form Co3O4 nanoparticles on thermal decomposition. The diffraction peaks appearing in the X-ray diffraction (XRD) spectra indicated that the nanoparticles were crystalline in nature. Scanning electron microscopy (SEM) was used to identify the surface morphology of Co3O4 nanoparticles. Energy dispersive X-ray spectroscopy (EDS) was undertaken so as to check the chemical purity and stoichiometry of Co3O4 nanoparticles. The size of Co3O4 nanoparticles of all the four complexes was found to be 20–40 nm resulting out of transmission electron microscopy (TEM) which also showed that the nanoparticles were spherical in shape. It has been authenticated with experimental evidence that the substituent present on the macrocycles has made substantial impact on the surface morphology, particle size and band gap of Co3O4. UV–Vis spectroscopic technique clearly reveals the quantum confinement effects of the title material.
Graphic abstract
Synthesis of homogeneous, spherical shaped nanocrystalline Co3O4 using homodinuclear Co(II)complexes of tetraiminediphenol macrocycle as precursor for the first time by thermal decomposition.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over the last few decades, nanoparticles have witnessed tremendous amount of attraction such that extensive research interest could be noticed among the researchers of multiple branches of science, wherein multitude of materials have been identified which is primarily because of their ability to tune size-dependent properties for the required specific purposes. These materials are in great demand because of possessing the inheritance of enormous potential for the extended applications, such as antibacterial drugs, heterogeneous photo-catalysis, catalysts, antioxidants, ion transporters, non-linear optics, radiopharmaceuticals, photoelectrochemical cells, MRI scanning agents, single electron transistors, optical switching, etc. [1,2,3,4]. The morphology of the nanoparticles i.e., either rod- or spherical-shaped as well as hexagonal- or tetrapod-shaped, ought to have an influence and impact over the properties in such a way that its predominance is the key for self-assembly process [5,6,7,8,9,10].
Spinel-type cobalt oxide (Co3O4) has attained the position of prominence such that it has consistently occupied the standpoint of being the prominent subject of specific scientific as well as technological innovation due to its ever-expanding array of applications. Co3O4 structures with the measure of nanoscale exhibit quite interesting as well as technological oriented properties such as optical, magnetic, electrochemical and field emission which are typically not observed, while they are in their bulk form. Being a p-type semiconducting material, it has variety of applications i.e., it extends its contribution in solid-state sensors, as heterogeneous catalysis, as anode materials in lithium-ion batteries, as magnetic materials in electrochromic devices and energy storage [11,12,13,14,15,16,17,18,19,20]. The panorama of its prospective applications that could be expanded to various fields makes Co3O4 as the potential candidate for intensive research so that scintillating efforts are on to develop new synthetic routes which would enable to achieve various types of nanostructures with different types of characteristic nature. On this footing, spinel-type Co3O4 possessed with different morphologies have been synthesized which are of the type of nanoparticles, nanoboxes, nanocubes, hollow nanospheres, nanowires, nanofibres, nanowalls, nanotubes, nanorods, nanosheets and mesoporous structures that have been already reported in the literature [21,22,23,24,25,26,27,28,29]. The techniques, such as chemical vapour deposition [30], microwave-assisted [31], sol–gel [32], hydrothermal [33], mechanochemical [34], ionic liquid-assisted [35], thermal decomposition [36], ultrasonic-assisted [37], electrostatic spray pyrolysis [38] and co-precipitation [39], which have been reported for the preparation of Co3O4 nanoparticles, have mostly employed reagents of toxic nature that are expensive and require long reaction times and also complex instruments are essential to be used. Henceforth, developing an inexpensive, simple and non-toxic route for the synthesis of Co3O4 nanoparticles is very much desirable. From the available report, it is identified that the thermal decomposition implemented on metal complexes is proven to be the simplest, innovative, fast developing and inexpensive techniques which could be utilized for synthesizing nanoscale metal oxides possessing comparatively high-specific surface areas [40,41].
Recently, metal complexes as new precursors have been utilized in the process of solid-state thermal decomposition which is predominantly used now as compared to other conventional methods because it is simple, much faster, economical and cleaner [42]. Even though various methods are adopted so as to prepare metal oxides, it remains to be a daunting task for developing an energy efficient method which is simple, rapid and easy to control and also acquiring the facilities to prepare metal oxide nanostructures for a large scale with the tailoring ability so as to obtain designable morphology. By selecting a suitable precursor, such as Schiff base complexes coupled with a sensible calcination procedure, nanoparticles with distinctive sizes and shapes could be obtained. This method also has a few probable advantages which include high yield of purity, low energy consumption, high functional efficiency and operational simplicity that have even the exemption of the need for special equipment and also the absence of solvent. Hence, the present research has been focussed on the possible changes on the structure and grain morphology of Co3O4 nanoparticles impacted by the substituents like methyl, chloro and nitro present on the macrocyclic moiety.
2 Experimental
2.1 Materials
The literature procedure was followed to synthesize 2,6-diformyl-4-methylphenol [43]. o-phenylenediamine, 4-methyl-o-phenylenediamine, 4-chloro-o-phenylenediamine, 4-nitro-o-phenylenediamine (Sigma-Aldrich) and cobalt(II) nitrate hexahydrate (Merck, India) were procured and used as is. All solvents used for the experiment were of the standard of analytical grade and those materials were purified accordingly before subjecting to the process of synthesis [43].
2.2 Analytical and physical measurements
Microanalytical (C, H, N) data were acquired utilizing a FLASH EA 1112 Series CHNS analyzer. The IR spectra were obtained utilizing the range between 400 and 4000 cm−1 making use of a JASCO FT/IR-5300 FTIR spectrometer using KBr pellets. A UV-3600 Shimadzu UV–Vis–NIR spectrophotometer was utilized to record both diffuse reflectance and near-IR absorption spectra. ESI mass spectra were enabled with the use of a LCMS-2010A Shimadzu spectrometer. A TA Q600 SDT instrument was put on use to carry out the TG-DTA analyses which were conducted at the standardized heating rate of 10°C min−1 starting from the room temperature (30°C) to 1000°C under a vibrant nitrogen atmosphere. The typical powder X-ray diffraction (XRD) patterns were obtained on a Bruker D8-advance diffractometer supplemented with graphite monochromated radiations arising out of CuKα1 (1.5406 Å) and Kα2 (1.5444 Å). The scanning electron microscopy (SEM) investigations were accomplished utilizing Philips XL-30 SEM operating typically at 20 kV. SEM and energy dispersive X-ray spectrometry (EDS) analyses were performed for the prepared specimens after subjecting the compounds with dusting on carbon tape. TEM analyses were conducted on FEI technai G2 20 STEM keeping the acceleration voltage 200 kV. TEM analyses were performed for the specimens such that they were prepared on carbon-coated copper grids consisting of 200 meshes. The samples were suspended accordingly in suitable solvents which were also ultra-sonicated for 1–2 min.
2.3 Synthesis of homodinuclear cobalt(II) complexes of tetraiminediphenol macrocycle
2.3.1 Synthesis of (Co 2 L 1 (NO 3 ) 2 )·2H 2 O
4-methyl-o-phenylenediamine (0.122 g, 1 mmol) was dissolved in methanol (30 ml) and added dropwise to the refluxing solution of 2,6-diformyl-4-methylphenol (0.164 g, 1 mmol) and cobalt(II) nitrate hexahydrate (0.291 g, 1 mmol) for 10 min. The resultant solution with the mixture was refluxed for 3 h by subjecting to continuous stirring. Thereafter, the formed dark brown solid was filtered and washed repeatedly with methanol. Subsequently, the obtained solid was chloroformed and dried under vacuum.
(Co2L1(NO3)2)·2H2O: Yield: 45%. Anal. Calcd for C32H30Co2N6O10: C, 49.50; H, 3.89; N, 10.82; Co, 15.18. Found: C, 49.65; H, 3.81; N, 10.91; Co, 15.22%. UV–Vis (DMSO), λmax: 290 (π → π*), 341 (n → π*), 387 (charge transfer), 453 (d–d) nm (transition). FTIR (KBr): 1600 (νC=N), 1532 (νC=C), 1343 (νC–OPh), 2831 (νC–H), 551 (νCo–O), 485 (cm−1) (νCo–N). ΛM (DMSO): 12.25 ohm−1 cm−2 mol−1. µeff: 4.65 BM. ESI MS (m/z): 776 (Co2L1(NO3)2·2H2O)+, 774 (Co2L1(NO3)2·2H2O-2H)+, 758 (Co2L1(NO3)2·H2O)+, 678 (Co2L1(NO3)2)+.
2.3.2 Synthesis of (Co 2 L 2 (NO 3 ) 2 )·2H 2 O
The solution of 2,6-diformyl-4-methylphenol (0.164 g, 1 mmol) and cobalt(II) nitrate hexahydrate (0.291 g, 1 mmol) in methanol (50 ml) was refluxed at about 30 min. Then, another solution of 4-chloro-o-phenylenediamine (0.143 g, 1 mmol) was added dropwise in methanol (30 ml) to the above-mentioned hot solution for 15 min. The resultant solution comprising the mixture was refluxed for 3 h with the continuous process of stirring. The resultant dark brown solid was separated by the filtering process and washed repeatedly with methanol. Thereafter, the cleansed solid was further cleansed by chloroform as well as drying under the condition of vacuum.
(Co2L2(NO3)2)·2H2O: Yield: 45%. Anal. Calcd for C30H24Cl2Co2N6O10: C, 44.09; H, 2.96; N, 10.28; Co, 14.42. Found: C, 44.16; H, 2.91; N, 10.21; Co, 14.52%. UV–Vis (DMSO), λmax: 287 (π → π*), 340 (n → π*), 384 (charge transfer), 458 (d–d) nm (transition). FTIR (KBr): 1622 (νC=N), 1533 (νC=C), 1335 (νC–OPh), 2918 (νC–H), 568 (νCo–O), 468 (cm−1) (νCo–N). ΛM (DMSO): 10.82 ohm−1 cm−2 mol−1. µeff: 4.61 BM. ESI MS (m/z): 816 (Co2L2(NO3)2·2H2O)+, 818 (Co2L2(NO3)2·2H2O+2H)+, 819 (Co2L2(NO3)2·2H2O+3H)+, 656 (Co2L2)+.
2.3.3 Synthesis of (Co 2 L 3 (NO 3 ) 2 )·2H 2 O
To the refluxing solution of 2,6-diformyl-4-methylphenol (0.164 g, 1 mmol) and cobalt(II) nitrate hexahydrate (0.291 g, 1 mmol) in methanol (50 ml), the solution of 4-nitro-o-phenylenediamine (0.153 g, 1 mmol) was added drop by drop for 20 min. Continuous stirring of 3 h was enabled for the resultant dark solution, so that it was refluxed. The resulting dark brown product that was formed in the solution had to be filtered, washed a few times with methanol that was followed by washing with chloroform and drying was enabled under vacuum.
(Co2L3(NO3)2)·2H2O: Yield: 45%. Anal. Calcd for C30H24Co2N8O14: C, 42.98; H, 2.89; N, 13.36; Co, 14.06. Found: C, 42.79; H, 2.91; N, 13.42; Co, 14.56%. UV–Vis (DMSO), λmax: 274 (π → π*), 331 (n → π*), 388 (charge transfer), 420 (d–d) nm (transition). FTIR (KBr): 1600 (νC=N), 1531 (νC=C), 1333 (νC–OPh), 2919 (νC–H), 549 (νCo–O), 465 (cm−1) (νCo–N). ΛM (DMSO): 14.12 ohm−1 cm−2 mol−1. µeff: 4.59 BM. ESI MS (m/z): 838 (Co2L3(NO3)2·2H2O)+, 839 (Co2L3(NO3)2·2H2O+H)+, 339 (Co2L3)2+.
2.3.4 Synthesis of (Co 2 L 4 (NO 3 ) 2) )·2H 2 O
2,6-diformyl-4-methylphenol (0.164 g, 1 mmol) and cobalt(II) nitrate hexahydrate (0.291 g, 1 mmol) were added with methanol (50 ml) so as to obtain a solution which had to be refluxed for 30 min. Another solution of o-phenylenediamine (0.108 g, 1 mmol) in methanol (30 ml) was added to the above-mentioned hot solution dropwise for 15 min. The resulted solution mixture was to be refluxed for 3 h with continuous stirring. The dark brown solid, which was separated, so as to be filtered and washed a few times with methanol which was followed by washing with chloroform and finally, dried under the condition of vacuum.
(Co2L4(NO3)2)·2H2O: Yield: 45%. Anal. Calcd for C30H26Co2N6O10: C, 48.14; H, 3.50; N, 11.23; Co, 15.75. Found: C, 48.21; H, 3.41; N, 11.36; Co, 15.82%. UV–Vis (DMSO), λmax: 289 (π → π*), 333 (n → π*), 372 (charge transfer), 453 (d–d) nm (transition). FTIR (KBr): 1601 (νC=N), 1528 (νC=C), 1335 (νC–OPh), 2832 (νC–H), 559 (νCo–O), 487 (cm−1) (νCo–N). ΛM (DMSO): 15.08 ohm−1 cm−2 mol−1. µeff: 4.61 BM. ESI MS (m/z): 748 (Co2L4(NO3)2·2H2O)+, 712 (Co2L4(NO3)2)+, 650 (Co2L4(NO3))+, 588 (Co2L4)+.
2.4 Synthesis of Co3O4 nanoparticles
The homodinuclear cobalt(II) complexes of tetraiminediphenol macrocycle were charged into a crucible of porcelain that was heated by an electric furnace at the rate of 5°C min−1 from room temperature to 500°C in the air atmosphere which was maintained at 500°C for 2 h. The decomposed product generated from the macrocyclic complexes was cooled gently to room temperature and collected as the sample for characterization.
3 Results and discussion
Several attempts made to isolate the fully π-conjugated metal-free macrocyclic ligand derived from diamines and diformyls, were unsuccessful [43,44]. The experimental work that had been accounted earlier [45,46], it had dealt with the successful preparation of an array of tetraiminediphenol macrocycles accomplished by the Schiff base condensation reaction of diformyls with diamines in the presence of appropriate metal ions as their complexes enabled by template method and their thermal stability have also been reported. Recently, the syntheses have been reported for a series of macrocyclic ligand formed via templated Schiff base condensations around metal salts [47,48]. It has been observed that the stability of the complexes has been governed by the rigidity of the ligand backbone. The obtained results clearly disclose that tetraiminediphenol macrocyclic framework formed by the reaction of 2,6-diformyl-4-substituted phenols with o-phenylenediamine could endow with the required rigidity in such a way that it possesses a significant ability to make the metal ion at the centre to be stable. To sustain the consistent progress to prepare metal complexes of macrocyclic ligands, cobalt(II) complexes have been prepared making use of ligand framework of similar kind by the condensation of 2,6-diformyl-4-substituted phenols with 4-substituted-o-phenylenediamines in the presence of cobalt(II) template. It has been also made a successful execution to prepare the metal oxide nanoparticles making use of these metal complexes such that the work has been reported [49]. Consequently, the dinuclear cobalt(II) complexes of tetraiminediphenol macrocycle have been prepared by making use of the modified procedure of Garcia et al [50] in which the [2+2] template condensation has been established between 2,6-diformyl-4-methylphenol and 4-methyl-o-phenylenediamine, 4-chloro-o-phenylenediamine, 4-nitro-o-phenylenediamine and o-phenylenediamine in presence of cobalt (II) nitrate hexahydrate in methanol.
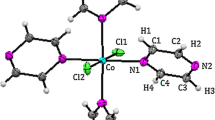
The stoichiometry of the complexes has been deduced as (Co2L(NO3)2)\(\cdot \)2H2O such that the attained complexes have been subjected to elemental analysis, spectroscopic studies, molar conductivity measurements, magnetic moments at room temperature and molecular optimization techniques. The IR spectra of the cobalt(II) complexes exhibit three NO stretching bands at 1203, 1055 and 813 cm−1. The absence of a broad band at 1384 cm−1 indicates the coordination of both nitrate groups as bidentate ligands [51,52]. The diffuse reflectance spectra of Co(II) complexes show three d–d transition bands at around 499–508, 673–687 and 904–1220 nm, which are due to 4T1g(F) → 4T1g(P), 4T1g(F) → 4A2g(F) and 4T1g(F) → 4T2g(F), respectively [53,54], and are of the type expected for distorted octahedral high spin Co(II) complexes. The Co(II) complexes show magnetic moment values around 4.59–4.65 BM which are greater than the respective spin only values and indicate weak antiferromagnetic coupling interaction between the metal ions which further confirms the dinuclear nature of the complexes [55,56]. The measured molar conductance values (10.82–15.08 Ω−1 cm−2 mol−1) of the cobalt(II) complexes are too low to account for any dissociation of the complexes in DMSO, indicating the non-electrolytic nature of the complexes and the nitrates are coordinated with cobalt ions. Hence, the molecular composition of Co(II) complexes are assigned as (Co2L1(NO3)2)·2H2O, (Co2L2(NO3)2)·2H2O, (Co2L3(NO3)2)·2H2O and (Co2L4(NO3)2)·2H2O. This macrocyclic entity possesses N4O2 donor framework arising out of four imine and two phenolate groups, so that it stabilizes the two cobalt ions effectively in their +2-oxidation state. The homodinuclear cobalt(II) complexes of the tetraiminediphenol macrocycle is shown in figure 1 along with their depicted structure.
3.1 Thermal analysis
The prepared complexes have been subjected to the study of thermal stabilities as a function of temperature. The results are found to be well corroborated with the formulae as suggested from the analytical data. The obtained thermogram (TG) patterns of all the dinuclear cobalt(II) complexes point out that they are thermally quite stable. The TG of all the dinuclear cobalt(II) complexes are portrayed in figure 2.
The TG of (Co2L1(NO3)2)·2H2O complex exhibits loss of two lattice water molecules along with a mass loss of 4.68% (calc. 4.64%) occurring at the temperature ranging between 95 and 120°C. The two ionic nitrates are removed in the form of N2O5 with a mass loss of 13.88% (calc. 13.92%) which occurs between 180 and 350°C that can be identified with an exothermic peak at 325°C on the DTA curve [42]. The macrocycle remains stable up to 350°C [57], whereas it is completely lost between the temperatures ranging between 350 and 470°C which is reflected by the corresponding exothermic peak at 450°C. While the final residue is analysed, it is identified to be Co3O4 which corresponds in its mass that is equivalent to the calculated value [58].
Similarly, the obtained TG of other cobalt(II) complexes exhibit a loss in weight corresponding to a lattice water molecule between the temperatures ranging from 90 to 110°C. The two ionic nitrates are removed in the form of N2O5 between 160 and 345°C which is identified by the corresponding exothermic peak on the DTA curve. The macrocycles remain stable up to the point before they are lost in the temperature range of 320–475°C as reflected by the exothermic peak found at about 410°C. After observing all the cases, the final residues are analysed to be identified as Co3O4 and its mass is found to be corroborated with the theoretical value. The thermal decomposition reaction of macrocyclic Co(II) complexes that have resulted in the formation of solid Co3O4 as well as gaseous products of N2O5 and H2O molecules can be described by the following sequence:
The decreasing order of thermal stability of the cobalt(II) complexes, (Co2L3(NO3)2)·2H2O > (Co2L1(NO3)2)·2H2O > (Co2L2(NO3)2]·2H2O > (Co2L4(NO3)2)·2H2O, exhibits that the complexes with electron-withdrawing substituents on the macrocycle are more stable than that of the complexes with electron-donating substituents. Considering all the above observations, the proposed general structure and geometry of the Co(II) are shown in figure 3.
3.2 Molecular optimization
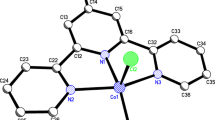
Based on the analysis performed for the physical as well as spectral data subjected to the complexes mentioned above, it is imperative to arrive at the generalization that the metal ions are strongly bonded to the Schiff bases via the deprotonated oxygen atoms and the imino nitrogen atoms. The optimized structures of the cobalt complexes, (Co2L1(NO3)2)·2H2O, (Co2L2(NO3)2)·2H2O, (Co2L3(NO3)2)·2H2O and (Co2L4(NO3)2)·2H2O are depicted in figure 4. The complexes comprise one dinucleating macrocycle, two cobalt(II) ions and two nitrate groups. Each cobalt ion is bridged by the two phenolate oxygen, two imino nitrogen and one nitrate ion, forming a dinuclear cobalt(II) macrocyclic complexes. The Co1···Co2 separation is found to be in the range between 2.8948 and 3.1927 Å.
3.3 Preparation of Co3O4 nanoparticles
As per the obtained results of thermal analysis, the temperature for the thermal conversion of the microcrystalline powder of the homodinuclear cobalt(II) Schiff base macrocyclic complexes (Co2L1(NO3)2).2H2O, (Co2L2(NO3)2)·2H2O, (Co2L3(NO3)2)·2H2O and (Co2L4(NO3)2)·2H2O which are to be formed into their respective Co3O4 nanoparticles of Co3O4-1, Co3O4-2, Co3O4-3 and Co3O4-4 was fixed at 500°C for 2 h so as to establish the decomposition of the entire precursor.
To prepare Co3O4 nanoparticles, the cobalt complex (Co2L1(NO3)2)·2H2O was charged into a porcelain crucible which was kept in an electric furnace so that the complex at the room temperature could be heated to 500°C keeping the rate of heating at 5°C min−1 in air and on reaching 500°C, the same temperature was maintained for 2 h. The same procedure was followed for each of the remaining samples of cobalt complexes (Co2L2(NO3)2)·2H2O, ([Co2L3(NO3)2)·2H2O and (Co2L4(NO3)2)·2H2O such that all the samples decompose at the same temperature of 500°C for 2 h. The products, which were derived after the decomposition from the complexes, were cooled back to room temperature so as to be collected for specific characterizations. The XRD analysis was performed to confirm the crystallographic phase of the prepared spinel cobalt oxides. The surface morphology of the Co3O4 nanoparticles was investigated by SEM. The composition of the nanoparticles was characterized by EDS analysis. The size and shape of the Co3O4 nanoparticles were investigated by high-resolution TEM (HRTEM) and selected area electron diffraction (SAED), respectively. The properties of optical absorbance of the Co3O4 nanoparticles were studied by UV–Vis spectroscopy keeping the temperature at 300 K.
3.4 Powder XRD studies of Co3O4 nanoparticles
After the cobalt complexes were allowed to undergo the calcination process, Co3O4 nanoparticles were obtained. The XRD patterns of the calcined samples are portrayed in figure 5. Diffraction peaks are observed at different positions of 2θ as 18.93, 31.22, 36.78, 44.79, 55.63, 59.63 and 65.19° and they could be assigned to the (111), (220), (311), (400), (422), (511) and (440) planes, respectively. It is worthwhile to obtain that the diffraction peaks corresponding to all the planes are very well corroborated with the pure cubic phase of spinel Co3O4 possessing the lattice constant, a = 8.083 as per the standard pattern (JCPDS no. 42-1467). No sign of any other peak that is either related to Co2O3 and CoO oxides or metallic cobalt, is found that authenticates the high measure of crystalline phase for the obtained spinel Co3O4. This is found to be very well-corroborated with the results of TG/DTA by which it could be confirmed that the complete decomposition of the cobalt complex has occurred at 500°C. Further observation reveals that Co3O4 nanoparticles possess sharp intense peaks of broad width which indicate high crystallinity possessing small-sized particle. Moreover, the XRD of Co3O4-3 nanoparticle is different from the others (Co3O4-1, Co3O4-2 and Co3O4-4) because of weak diffraction intensity due to its poor crystalline nature. The Scherrer’s formula can be utilized to calculate the average crystallite size of the particle [59]:
where D is the average crystallite size, β the full width at half maximum (FWHM), λ the wavelength of X-ray radiation (CuKα radiation, 0.15406 nm), θ the diffraction angle and K the Scherrer constant (0.9). Here, the (311), (511) and (440) are the reflection peaks of Co3O4 which are utilized for the calculation of size of the particle. With the obtained calculation, the Co3O4-1, Co3O4-2, Co3O4-3 and Co3O4-4 nanoparticles are found to be having the values within the limit of 20–23, 26–32, 25–38 and 35–38 nm, respectively.
3.5 SEM studies of Co3O4 nanoparticles
SEM characterization was performed on the Co3O4 nanoparticles so as to evaluate the surface morphology. Figure 6a, b, c and d showcases the respective morphologies of nanoparticles Co3O4-1, Co3O4-2, Co3O4-3 and Co3O4-4, which are different from each other, obtained from the cobalt complexes of (Co2L1(H2O)2(NO3)2), (Co2L2(H2O)2(NO3)2), (Co2L3(H2O)2(NO3)2) and (Co2L4(H2O)2(NO3)2), respectively.
Figure 6a depicts the SEM image of Co3O4-1 nanoparticles which clearly shows the formation of spherical-shaped morphology that are very uniformly distributed and loosely aggregated in nature as it appears. The SEM image of the Co3O4-2 portrays the well-defined nanoparticles such that the influence of macrocycles on the morphology and size of nanoparticles needs to be studied further. As exhibited in SEM images, nanoparticles of Co3O4-1 and Co3O4-2 have the regular and uniform shapes, whereas Co3O4-3 and Co3O4-4 nanoparticles have the irregular and non-uniform shapes. These results authenticate that the morphology and particle size of Co3O4 nanoparticles are very closely dependent on the substituent which is present in the macrocyclic moiety.
3.6 EDS studies of Co3O4 nanoparticles
The EDS spectra of the cobalt oxide nanoparticles are portrayed in figure 7a, b, c and d. The EDS analyses of the Co3O4 nanoparticles conducted on each sample confirm that they contain Co and O only. The signals of Au appearing are originated from the Au sprayed during the preparation of samples [35].
The experimentally observed atomic percentages corresponding to Co and O are known to be 43.18 and 56.82%, respectively, which is common for all the synthesized cobalt oxide nanoparticles. The approximate atomic ratio of Co to O is found to be 3:3.96 such that it substantiates the final product to be composed of only Co3O4 nanocrystals [60].
3.7 HRTEM studies of cobalt oxide nanoparticles
The size as well as shape of the Co3O4-1 nanoparticles obtained by employing the thermal decomposition of the cobalt complexes (Co2L1(H2O)2(NO3)2) at 500°C were put under investigation enabled by HRTEM analysis.
The nature of very fine, loosely aggregated particles could be identified from TEM analysis that are uniform in size and appearing with a homogeneous sphere-like morphology represented by a narrow size distribution. As shown in figure 8a, the Co3O4-1 nanoparticles exhibiting the narrow size distribution have the mean diameter of ~ 20–25 nm, which corroborates with the particle size calculated from the XRD data. The TEM image (figure 8b) of the Co3O4-2 showcases the well-defined spherical nanoparticles of size within the region of 25–30 nm, which very much resembles the size of particle’s average value as computed by the Scherrer’s formula using XRD data. The TEM image of Co3O4-3 is depicted in figure 8c which exhibits the slightly aggregated nanoparticles possessing particle size of 25–35 nm. The sizes of the calculated average particles have a good agreement with that of the calculated values obtained from XRD. The Co3O4-4 portrays the well-defined spherical nanoparticles possessing the size of 35–40 nm as shown in figure 8d. The nanoparticles’ size as obtained from the TEM micrograph very well agrees with the results obtained through XRD.
The insets of TEM images are the respective SAED analysis by which it is evidenced that the nanoparticles are single crystalline in nature. As observed from the TEM images, it is authenticated that this specific method of preparation could successfully overcome the problem of agglomeration which is found to be suitable for the preparation of cobalt oxide nanoparticles.
3.8 Optical properties of cobalt oxide nanoparticles
Optical properties of the cobalt oxide nanoparticles were experimented at room temperature by utilizing UV–visible diffuse reflectance spectroscopy. Figure 9a–d portrays the absorbance spectra of the Co3O4 samples wherein there found to be two absorption bands i.e., with the wavelength ranges of 200–350 and 400–580 nm. The assignment for the first band can be ascribed to the O2− → Co2+ charge-transfer process. While the second band is to the O2− → Co3+ charge-transfer [61]. Cobalt oxide is a commonly known p-type semiconductor.
The optical band gap of cobalt oxide nanoparticles could be approximated by the extrapolation obtained from the linear relationship between (αhν)2 and hν which is adopted according to the Tauc’s equation [62].
where α is the absorption coefficient, hν the photon energy, Eg the optical band gap, A the constant and n the dependent on the type of transition involved. For the case of Co3O4, n = ½ is considered as there are direct allowed transitions so that the observed band gap value is well matched with the literature reports [63,64]. The band gap can be approximated by the extrapolation observed from the linear region in the plot of (αhν)2 vs. hν.
As reflected in figure 10a, b, c and d, two absorption peaks give rise to two Eg values for each sample. The empirical values of Eg observed for the Co3O4 nanoparticles intended for this work are greater than the bulk values [65]. This suggests that the nanoparticles of Co3O4-1 to Co3O4-4 obtained via the method followed, are very much contingent within the regime of quantum confinement so that fine tuning is possible in terms of material properties. The results obtained are found to be considerably falling in line with the available literature data of Co3O4 nanoparticles [66,67]. The observations obtained confirm that there is an increase in optical band gap energy and a decrease in crystallite size.
4 Conclusion
Nanoparticles of Co3O4 were synthesized by an efficient as well as simple method of thermal decomposition with homodinuclear Co(II) complexes of tetraiminediphenol macrocycle. As the comparative analysis is drawn between this method and that of other methods, these macrocyclic complexes are faster, pollution-free and economically viable tools for the preparation of metal oxide nanomaterials. This method could be followed as it has the prominent advantages which include high yield of pure products, operational simplicity, low energy consumption, absence of solvent, exempting the need for special equipment and functional efficiency. From the obtained results of XRD, SEM, EDS and TEM, the Co3O4 nanoparticles are identified to be single crystalline of spherical shape with the approximate range of 20–40 nm. The surface morphology, particle size and band gap of Co3O4 are found to be affected by the substituent present on the macrocyclic complexes. The blue shift found in the absorption spectrum is because of the quantum confinement effect of these nanosized Co3O4 nanoparticles. These investigations reflect that the macrocyclic complex could be used as appropriate precursors for the preparation of nanomaterials. Moreover, neither catalysts nor surfactants are required for this method so that it can also be followed as a general method so as to prepare other transition metal oxide nanoparticles.
References
Lekhetho S M, Kutloano E S and Tebello N 2020 Inorg. Chim. Acta 509 119661
Zhao H and Douglas E P 2002 Chem. Mater. 14 1418
Kim J Y, Kim H M, Shin D H and Ihn K 2006 J. Macromol. Chem. Phys. 207 925
Ryan E M and Stephen J A 2010 Coord. Chem. Rev. 254 1686
Manna L, Scher E C, Li L and Alivisatos A P 2002 J. Am. Chem. Soc. 124 7136
Hu J, Li L S, Wang L W, Manna L and Alivisatos A 2001 Science 292 2060
Peng X 2003 Adv. Mater. 15 459
Peng Z A and Peng X G 2002 J. Am. Chem. Soc. 124 3343
Lee S M, Jun Y W, Cho S N and Cheon J 2002 J. Am. Chem. Soc. 124 11244
Jun Y W, Jung Y Y and Cheon J 2002 J. Am. Chem. Soc. 124 615
Kai Bo Z, Pei Yi G, Min Z, Yu Qing Z, Yuan B and Jing L 2020 Mater. Lett. 280 128558
Majid K K, Ahmad A D, Hamid O, Mehran J, Zahed A, Jean J D et al 2019 J. Alloys Compd. 805 924
Muhammad A, Sonia Z, Muhammad F W, Philips O A and Imran S 2020 J. Mater. Res. Technol. 9 12697
Li Y G, Tan B and Wu Y Y 2008 Nano Lett. 8 265
Farhadi S, Safabakhsh J and Zaringhadam P 2013 J. Nanostruct. Chem. 3 69
Mate V R, Shirai M and Rode C V 2013 Catal. Commun. 3 66
Li T, Yang S G, Huang L S, Gu B X and Du Y W 2004 Nanotechnology 15 1479
Casas-Cabanas M, Binotto G, Larcher D, Lecup A, Giordani V and Tarascon J M 2009 Chem. Mater. 21 1939
Li W-Y, Xu L-N and Chen J 2005 Adv. Funct. Mater. 15 851
Zhuo L H, Ge J C, Cao L H and Tang B 2009 Cryst. Growth Des. 9 1
Sun L, Li H, Ren L and Hu C 2009 Solid State Sci. 11 108
Yang L, Kuikun G, Tianye Y and Mingzhe Z 2019 J. Energy Storage 21 362
Li L, Chu Y, Liu Y, Song J L, Wang D and Du X W 2008 Mater. Lett. 62 1507
Jing W, Xiaoyu H, Da L, Bruce E L, Jia L, Zhaohan Z et al 2020 Appl. Catal. B: Environ. 276 119102
Du J, Chai L, Wang G, Li K and Qian Y 2008 Aust. J. Chem. 61 153
Jixing W, Cheng W and Mengmeng Z 2019 Chem. Eng. Trans. 356 1
Li Y, Zhao J, Dan Y, Ma D, Zhao Y, Hou S et al 2011 Chem. Eng. J. 166 428
Sun H, Ahmad M and Zhu J 2013 Electrochim. Acta 89 199
Ren M, Yuan S, Su L and Zhou Z 2012 Solid State Sci. 14 451
Marcel M, Charan K N, Colin G, Heinrich L and Stefan E S 2017 RSC Adv. 7 50269
Shmatok Y V, Globa N I and Kirillov S A 2017 Electrochim. Acta 245 88
Ramakrishna I and Tapan K S 2019 Mater. Today 9 458
Piyali B, Sachin S, Yusuke Y, Zeid A A, Zhong-Li W and Rajaram B 2021 J. Colloid Interface Sci. 582 322
Brayan M, Maria G V F, Josue M G, Fabricio M B, Fabio A P S, Murilo P M et al 2019 Mater. Chem. Phys. 226 318
Meenatchi B, Sathiya Lakshmi V, Manikandan A, Renuga V, Sharmila A, Nandhine Deve K R et al 2017 J. Inorg. Organomet. Polym. Mater. 27 446
Kavitha T, Haider S, Kamal T and Ul-Islam M 2017 J. Alloys Compd. 704 296
Bolong J, Jiaojing Z, Yanguang C, Hua S, Tianzhen H and Junhu K 2020 RSC Adv. 10 30214
Tariq A H A, Lary H S, Hersh A K and Shaida A K 2017 J. Mater. Sci: Mater. Electron. 28 1951
Muhammad R S A J 2019 Open Chem. 17 865
Meghdadi S, Amirnasr M, Zhiani M, Jallili F, Jari M and Kiani M 2017 Electrocatalysis 8 122
Masoud S N and Afsaneh K 2014 C. R. Chim. 17 352
Kavitha T, Parameswari K, Kuppusamy K and Yuvaraj H 2011 Mater. Lett. 65 1482
Kumar D S and Alexander V 1999 Polyhedron 18 1561
Kumar D S and Alexander V 1995 Inorg. Chim. Acta 238 63
Kumar D S, Aruna V A J and Alexander V 1999 Polyhedron 18 3123
James S, Kumar D S and Alexander V 1999 J. Chem. Soc. Dalton Trans. 11 1773
Janusz G and Katarzyna S 2020 Polyhedron 181 114433
Laura M T, Qiuran W, Sam H B, Peng C, Jia Q, Michael R G et al 2021 Polyhedron 198 115044
Pushpanathan V and Kumar D S 2014 J. Nanostruct. Chem. 4 95
Garcia V P, Yazigi D V, Cabrera A, Galvez P V, Arriagada M, Leon D R et al 2009 Polyhedron 28 2335
Nakamoto K 1978 Infrared and Raman spectra inorganic and coordination compounds (New York: Wiley Interscience) 3rd edn
Guerriero P, Casellato U, Tamburini S, Vigato P A and Graziani R 1987 Inorg. Chim. Acta 129 127
Lever A B P 1984 Inorganic electronic spectroscopy (Amsterdam: Elsevier) 2nd edn
Mohamed G G and El-Gamel N E A 2004 Spectrochim. Acta A 60 3141
Lloret F, Julve M, Cano J, Garcia R R and Pardo E 2008 Inorg. Chim. Acta 361 3432
Omar M and Mohamed G 2005 Spectrochim. Acta: Part A 61 929
Aruna V A J and Alexander V 1996 Inorg. Chim. Acta 249 93
Khalaji A D, Nikookar M and Das D 2014 J. Therm. Anal. Calorim. 115 409
Scherrer P and Gottingen N G W 1918 Math. Phys. 2 98
Farhadi S and Safabakhsh J 2012 J. Alloys Compd. 515 180
He T, Chen D R, Jiao X L, Wang Y L and Duan Y Z 2005 Chem. Mater. 17 4023
Tauc J, Grigorovici R and Vancu A 1966 Phys. Status Solidi 15 627
Kandula S and Jeevanandam P 2015 RSC Adv. 5 5295
Mostafa Y N 2013 Mater. Lett. 94 112
Yang L, Guan W, Bai B, Xu Q and Xiang Y 2010 J. Alloys Compd. 504 L10
Gu F, Li C, Hu Y and Zhang L 2007 J. Cryst. Growth 304 369
Mostafa Y N and Ibrahim S A 2012 Mater. Res. Bull. 47 2638
Acknowledgements
VP is grateful to Dr Samar Kumar Das as well as UGC-NRC, School of Chemistry, University of Hyderabad, for having given the facility to do the required characterizations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pushpanathan, V., Dhas, S.S.J. & Kumar, D.S. Investigation on the substituent effects of homodinuclear cobalt(II) complexes of tetraiminediphenol macrocycle on the synthesis of pure Co3O4 nanoparticles. Bull Mater Sci 44, 279 (2021). https://doi.org/10.1007/s12034-021-02562-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-021-02562-8