Abstract
The UV–visible studies of the hybrid tris(4-bromo-N,N-dimethylanilinium) hexachlorobismuthate (III) compound [(C8H11BrN)3BiCl6], prepared by slow evaporation at room temperature, were investigated in detail from 200–2400 nm. The absorption peaks show the presence of a peak in the UV–C region around \( \lambda_{\rm des} = 254 \) nm which has the property of disinfecting water. The optical bandgap Eg, determined by both absorbance and reflectance measurements, was estimated to be (3.390 ± 0.009) and (3.354 ± 0.009) eV, respectively. In addition, these measurements approve the direct behaviour of the allowed optical transitions. The low Eurbach energy (128 meV) confirms the high quality of the prepared sample. The dependence on the incident wavelength of the optical constants such as the extinction coefficient k and the refractive index n was discussed. The dispersion parameters E0 and Ed of this compound were calculated on the basis of the Wemple–Didomenico model. Furthermore, the dielectric studies show that the dissipation factor tan \( \delta \) has very low value. All the results demonstrate that this compound may be proposed as a good candidate for optical and optoelectronic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Despite the prohibition of materials having an adverse effect on the environment, the use of polluting materials continues to grow. These compounds which contain heavy metals such as lead, nickel or cadmium, find a multitude of applications in the domestic, medical, industrial, and even agricultural fields. Besides, they can have dangerous effects on health, medical and technological areas and can lead to harmful effects on the environment. In this context, we have focused on hybrid materials. In the last few years, the physical applications of organic–inorganic hybrid compounds, such as ferroelectricity [1,2,3,4] and ferroelasticity [5], have become a subject of great interest. These hybrid compounds can constitute a new generation of promising high-performance materials leading to various potential applications due to their particular structure, electric, dielectric and optical properties [6,7,8,9]. In addition, these hybrid materials (organic–inorganic) whose preparation is simple and inexpensive, have recently attracted the attention of scientists, leading to their applications in the field of the photovoltaic industry and light absorbers [10,11].
The physical properties of such compounds are strongly related to the properties of both the inorganic anion and the organic cation which can be tuned depending on the required applications [12,13]. An important challenge nowadays, through the use of hybrid materials, is to provide developed electrochemical storage devices such as batteries and super-capacitors which can give important energy values.
The field of this family of materials moving towards its use in the field of optics extends from bio-imaging to optoelectronic devices and even to the design of screens [14]. Indeed, the uniqueness of the optical responses of organic or organometallic molecular species lead to the development of innovative hybrid compounds, on a macro or even nanometric scale.
In this context, a new organic–inorganic metal halide (OIMHs) tris(4-bromo-N,N-dimethylanilinium) hexachlorobismuthate (III) (C8H11BrN)3BiCl6 has been synthesized by slow evaporation at room temperature. This sample is characterized by the existence of spaces between the inorganic entities which are filled by organic cations through hydrogen bonds in order to provide their connection.
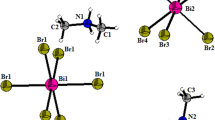
The crystal lattice of this compound is built up from the packing of distorted [BiCl6]3– octahedra surrounded by [C8H11BrN]+ organic cations. The cohesion of the structure is achieved by an extensive network of N–H…Cl and C–H…Cl hydrogen bonds. In the previous study [15], the Hirshfeld surface analysis associated with 2D fingerprint plots were used to visualize and explore the significant intermolecular interactions in the crystal structure quantitatively. The infrared and Raman spectra were recorded in the 4000–400 and 4000–50 cm−1 frequency regions, respectively, and they were compared with those found in homologous compounds in the literature. The 13C CP-MAS NMR spectrum is in full agreement with the crystallographic data determined by X-ray structure. At ambient temperature, optical absorption UV–visible and photoluminescence (PL) spectra of this material were interpreted and analysed by comparison with those of the homologous hybrids.
Particularly, PL and optical absorption measurements carried out at ambient temperature reveal the presence of a strong emission in the violet range of energy located at 2.92 eV (424 nm).
It is clear that the investigation of the UV–visible optical absorption, reported in ref. [15] seems to be incomplete and insufficient to give all optical characteristics of the proposed hybrid material which can be used for optical applications.
The main aim of this study is to investigate in detail the optical properties of the [(C8H11BrN)3BiCl6] hybrid compound through UV–visible measurements performed at room temperature. The determination of the optical parameters is decisive for the choice of a compound which may be used on the basis of an optoelectronic device.
2 Experimental
As reported in the previous study [15], the single crystal (C8H11BrN)3BiCl6 sample was successfully prepared by slow evaporation at room temperature. The obtained compound was dried and prepared in order to perform optical measurements. The X-ray diffraction investigation reveals that the compound crystallizes in the orthorhombic system with the non-centrosymmetric space group P212121. The obtained unit cell parameters are: a = 9.2820(3) Å, b = 13.7960(5) Å, c = 27.4298(12) Å, V = 3512.5(2) Å3 with Z = 4. The asymmetric unit of this sample is formed by the monomeric [BiCl6]3 anion and three protonated 4-bromo-N,N-dimethylanilinium cations.
The UV–visible measurements were obtained, at room temperature, by a UV-3101PC scanning spectrophotometer using a source emitting wavelength radiations which varies from 200–2400 nm.
3 Results and discussion
3.1 Determination of the optical bandgap E g
The objective of studying the optical measurements is to determine the principal properties of this compound such as the optical energy bandgap, the Urbach energy, the extinction coefficient, the refractive coefficient as well as the optical conductivity in order to select its appropriate area of application.
Figure 1 portrays the room temperature UV–visible absorption spectrum A(\( \lambda \)) of the synthesized (C8H11BrN)3BiCl6 compound as a function of the incident wavelength \( \lambda \). As shown, the absorption edges were found to be in the ultraviolet region, which means that the sample can be used in photovoltaic applications or light photocatalysis. The detected strong absorption in this region can be associated with the electronic transition from the valence band to the conduction band.
A first brief optical study of this compound shows that it exhibited two dominant bands located at 254 and 322 nm. Indeed, the first one around 254 nm is associated with the transitions of type π → π* characteristic of the organic [C8H11BrN]+ cations and the second one located at 322 nm can be assigned to Ligand to Metal Charge Transfer (LMCT) transition. A deeper investigation of the first absorption peak shows that this peak is located in the UV–C region (200 ≤ \( \lambda \) ≤ 280 nm) at \( \lambda \)des = 254 nm. This particular wavelength has the property of disinfecting water using the long ultraviolet radiation 254 nm wave. Indeed, the UV–C rays inactivates and/or destroys the DNA of bacteria and viruses harmless to human health. In addition, with this chemical disinfection method, not all micro-organisms react in the same way when exposed to radiation which gives the possibility of exploiting this compound in this application in the UV–C region. It should also be noted that some micro-organisms are made inactive with a relatively small amount of UV rays, but others need larger doses to get the desired activation percentages. It is worth noticing that the peak observed at 322 nm was assigned to the LMCTT from the np(t1gt2gt1ut2u) orbital of Cl to 6p(t1u) orbital of Bi3+, whereas the peak located at 262 nm (in the vicinity of \( \lambda \)des) is correlated with the 6s6p metal centred transition from the 6s2 to the T1u state which is related to the 3P1 atomic state in Bi3+ [15].
The inset of figure 1 shows the variation of dA/d\( \lambda \) with \( \lambda \). The optical bandgap Eg of this studied compound can be determined from the minimum of the dA/d\( \lambda \) curve in the high energy side. The obtained value is (3.390 ± 0.009) eV characterizing (C8H11BrN)3BiCl6 as a wide bandgap material. This method does not make it possible to distinguish whether the gap is direct or indirect. For this, we used the Tauc’s method based on the following relation [16]:
where hν is the energy of the incident photon, B is a parameter characterizing the degree of disorder which may occur in the studied compound and the value of n provides information on the nature of the transition: n = 0.5 for an allowed direct transition and n = 2 for an allowed indirect bandgap [17]. The plot of (Ahν)0.5 and (Ahν)2 vs. hν is shown in figure 2. The bandgap Eg (Egd and Egi are respectively the direct and the indirect bandgap) can be evaluated through the extrapolation of the linear part of each curve [18]. The bandgap is estimated to be (3.351 ± 0.168) eV for n = 0.5 and (4.150 ± 0.151) eV for n = 2.
In order to verify the n value, we have plotted in figure 3 the evolution of Ln(Ahν) against Ln(hν – Eg) used as trial values for Eg, those obtained previously according to the expression:
The fit of the linear part of each curve gives n = 0.505 for Egd = 4.150 eV and n = 0.551 for Egi = 3.351 eV confirming the direct bandgap of the studied compound. This value (3.351 eV) is higher than those obtained for other hybrid materials. Indeed, the optical bandgap Eg is evaluated at 3.11, 3.04 and 2.4 eV for (C2H5NH3)2CdCl4, (C2H5NH3)2Cd0.5Cu0.5Cl4 and (C2H5NH3)2CuCl4 compounds, respectively [19]. On the other hand, some hybrid compounds show high bandgap values such as [(CH3)2NH2]2ZnCl4 (Eg = 4.26 eV) [20].
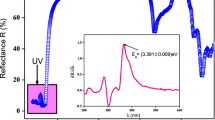
Figure 4 displays the evolution of the reflectance R relative to the (C8H11BrN)3BiCl6 compound against wavelength. As illustrated, this material shows importance of reflectance in the visible and IR domains. This selective property confirms that the studied compound may be used in applications in the ultraviolet domain. In the inset of figure 4a, we have plotted the variation of dR/d\( \lambda \) vs. \( \lambda \). The optical bandgap Eg relative to this compound determined from the maximum of the dR/d\( \lambda \) curve in the high-energy side [21] is (3.354 ± 0.009) eV. We can notice that this method proposed by Marotti et al [22] confirms the direct bandgap of the studied compound.
3.2 Determination of the Urbach energy E u
For the purpose of the determination of the pre-absorption edge, we have determined the Urbach tail Eu characterizing disordered and amorphous materials. Indeed, Eu, which depends weakly on temperature, is often interpreted as the width of the tail relative to localized states located between valence and conduction bands. According to Urbach model [23], Eu can be calculated using the following expression where C is a constant:
Using this exponential relation, the Urbach energy Eu can be easily determined from the slope of Ln(A) as a function of energy hν shown in figure 5a. The calculated value of Eu is (128 ± 2) meV, proves the presence of a small disorder in this hybrid compound. In addition, Eu represents only 3.7% of the bandgap value confirming the good quality of this compound.
On the other hand, the Urbach energy (Eu) can be related to the steepness parameter S by the expression [24]:
The calculated value of the steepness parameter is 202 × 10−3.
Another parameter used to evaluate the importance of this compound in optoelectronic devices is the maximum wavelength \( \lambda \)T, of incident radiation, currently defined as threshold wavelength, which is required to eject charge carriers. The value of \( \lambda \)T can be determined using the following expression [25,26]:
In this relation, D is a constant. So, we have plotted the (A/\( \lambda \))2 curve vs. 1/\( \lambda \) to find out the threshold wavelength \( \lambda \)T as shown in figure 5b. The obtained value of \( \lambda \)T = 402 nm.
3.3 The penetration depth and optical extinction
The penetration depth \( \delta \) is defined as the measure of how electromagnetic radiation or a deep light can penetrate into a compound. Theoretically, from an intensity point of view, \( \delta \) is the depth at which the incident intensity of the radiation falls to 1/e as compared to its original value. Using the absorption data, the penetration depth (or skin depth) can be evaluated using the expression [24]:
Figure 6 portrays the evolution of the penetration depth \( \delta \) against radiation wavelength relative to the (C8H11BrN)3BiCl6 material. As illustrated, the curve of δ(\( \lambda \)) shows three regions. In region I, this sample acts as a screen effect for wavelengths 200 ≤ \( \lambda \) ≤ 365 nm. Such a result is important since this sample can eliminate ultraviolet radiation. In region II, a small penetration may be observed and it is almost constant for 365 ≤ \( \lambda \) ≤ 1355 nm. In region III, a particular peak was observed for \( \lambda \) = 1470 nm which belongs to the domain of X-rays.
Furthermore, the optical extinction k vs. wavelength \( \lambda \) can be calculated using the expression [27]:
Figure 7 displays the variation of the optical extinction k as a function of \( \lambda \) for this prepared sample. As clearly shown, the k curve shows two particular maximums located in the UV domain at 266 and 325 nm.
3.4 The refractive index of the (C8H11BrN)3BiCl6 compound
In the visible domain, the optical refractive index n can be calculated by using the following expression [28]:
Figure 8a displays the evolution of the optical refractive index as a function of the wavelength \( \lambda \) at room temperature for the (C8H11BrN)3BiCl6 compound. As illustrated, n depends strongly on the wavelength. The n curve shows the highest value in the UV region, then it decreases with an increase in the wavelength. Beyond 400 nm, the value of n is around 1.5. Such remarkable transparency may be attributed to low scattering and absorption losses. A deeper examination of the evolution of n in the visible spectrum is presented in figure 8b.
The refractive index n can be expressed as a function of the wavelength \( \lambda \) using the well-known Cauchy’s relation given by:
The fit of our experimental data with relation (9), illustrated in figure 8b, gives the values of the Cauchy’s constants: n0 = 1.533, n1 = –99.455 (nm)2 and n2 = 4.625×108 (nm)4.
3.5 Evolution of the optical conductivity and dispersion energy parameters
Theoretically, the optical conductivity σop depends on optical parameters such as the absorption coefficient A, the refractive index n and the extinction coefficient k. This parameter can be calculated by using the following relation [24]:
where c denotes the speed of light in free space (or air).
The evolution of the optical conductivity σop with wavelength for the (C8H11BrN)3BiCl6 compound is illustrated in figure 9. As illustrated, three peaks corresponding to a maximum of conductivity can be detected at \( \lambda \)1 = 210, \( \lambda \)2 = 254 and \( \lambda \)3 = 323 nm located in the UV domain. This result shows that the incident photon energy induces more excited electrons at these selective wavelengths, in particular \( \lambda \)2 = \( \lambda \)des which can be used for disinfecting water as proposed previously. In addition, this compound can be used as an optical filter in the UV region.
The energy of the effective single oscillator E0 and the dispersion energy Ed characterizing the intensity of the inter-band optical transitions can be computed through the relation proposed by Wemple–Didomenico [29]:
Thus, the E0 and Ed values can be determined by fitting the linear part of the 1/(n2 – 1) curve vs. (hν)2 as seen in figure 10. The slope of this curve gives 1/(E0Ed) and its intercept with the vertical axis gives E0/Ed. The obtained values are E0 = 4.715 eV and Ed = 6.233 eV. We can remark particularly that E0 = 1.39Eg (Eg = 3.390 eV). This result agrees approximately with the relation E0 = 1.376Eg obtained using the single oscillator model [30].
The E0 and Ed values can be used to evaluate the zero frequency dielectric constant εop and the static refractive index n′ defined for \( \left( {h\nu } \right)^{2} = 0 \) in relation (11) by:
The obtained values of εop and n′ are 2.322 and 1.524, respectively. The value of n′ is close to that obtained from the fit by Cauchy’s law.
On the basis of the same model, we have also evaluated the oscillator wavelength \( \lambda \)0 and the average oscillator strength S0 relative to the (C8H11BrN)3BiCl6 compound using the following relation [31]:
We have reported in figure 11 the plot of (n2 – 1)−1 vs. (\( \lambda \))−2. From the linear fitting, we have noticed that the obtained values (S0 = 7.454×10−13 m−2 and \( \lambda \)0 = 262.5 nm) are in the same order of those obtained from other compounds such as [(CH3)3NH]CdCl3 [32], Cd50S50–xSex [33] and Ge20Se60Sb20–xAgx (x = 0, 5, 10, 15 and 20 at%) [34].
On the other hand, we have calculated the moments of the optical spectrum M–1 and M–3, which determine the average bond strength from the relations [34]:
The obtained values are M–1 = 1.322 and M–3 = 0.059 (eV)−2.
3.6 Dielectric studies
The real and imaginary parts of the complex dielectric constant ε*(\( \lambda \)) relative to the (C8H11BrN)3BiCl6 compound were computed using the refractive index n(\( \lambda \)) and the extinction coefficient k(\( \lambda \)) based on the following relation [35]:
Thus, the real part (ε1) and the imaginary part (ε2) of dielectric constant can be given by:
and
The plots of the real part ε1 and the imaginary part ε2 (in the inset) of the dielectric permittivity vs. \( \lambda \) for the (C8H11BrN)3BiCl6 compound were illustrated in figure 12. As expected, ε1 and ε2 show the same trend as the refractive index due to the smaller values of k(\( \lambda \)). On the other hand, the dissipation factor tan \( \delta \) (or tangent loss) was calculated using the formula [36]:
The variation of tan \( \delta \) against \( \lambda \), illustrated in figure 13, shows that with increasing the wavelength, the dissipation factor decreases strongly beyond 360 nm. From 400 nm, it becomes almost zero.
In the near infrared domain, the real and the imaginary parts of the dielectric permittivity ε*(\( \lambda \)) can be expressed by [27]:
and
In these relations, M1, M2 and M3 are constants. The evolution of the real part ε1 of the dielectric permittivity vs. \( \lambda \)2 and the imaginary part ε2 vs. \( \lambda \)3 are illustrated in figure 14a and b. The obtained values are M1 = 1.525, M2 = 8.656×10−9 and M3 = 2.653×10−13 nm−3.
4 Conclusion
The (C8H11BrN)3BiCl6 compound was prepared by slow evaporation at room temperature. The optical parameters for this sample were determined on the basis of UV–visible measurements. An analysis of the absorption peaks shows the presence of a particular peak at \( \lambda \)des = 254 nm located in the UV–C region which has the property of disinfecting water. The optical bandgap was determined by two methods, was evaluated at 3.390 and 3.354 eV, and was confirmed to have a direct behaviour. The allowed direct electronic transitions were at the origin of the photon absorption in the investigated sample. The obtained value of Eg characterizes this compound as a wide bandgap material. The Urbach energy, which characterizes disordered and amorphous materials, shows a low value (128 meV) confirming the good quality of this compound. The evolution of the skin depth \( \delta \), the dispersion parameters E0 and Ed, and optical conductivity σop with the incident wavelength were studied in detail. In addition, by evaluating the real and imaginary parts of the complex dielectric constant, we have demonstrated that the dissipation factor tan \( \delta \) has very low values. The refractive index n has important values in the UV region but it falls around unity in the Vis–IR domain. These relevant results show that the (C8H11BrN)3BiCl6 compound may be a promoter material for applications in the field of optoelectronic devices.
References
Pietraszko A, Bednarska-Bolek B, Jakubas R and Zielinski P 2001 J. Phys.: Condens. Matter 13 6471
Jakubas R and Sobczyk L 1990 Phase Trans. 20 163
Uchino K 2000 Ferroelectric devices (New York: Marcel Dekker)
Zaleski J, Jakubas R, Sobczyk L and Mroz J 1990 Ferroelectrics 103 83
Tu Z and Wu M 2019 Sci. Bull. 65 147
Kulicka B, Jakubas R and Ciunik Z 2004 J. Phys. Chem. Sol. 65 871
Mostafa M F and Hassen A 2006 Phase Trans. 80 302
Zhang S and Lanty G 2009 J. Acta Mater. 57 3301
Ionescu D, Ciobanu B and Radinschi I 2007 J. Optoelectron. Adv. Mater. 9 2608
Kojima A, Teshima K, Shirai Y and Miyasaka T 2009 J. Am. Chem. Soc. 131 6050
Kim H-S, Lee C-R, Im J-H, Lee K-B, Moehl T, Marchioro A et al 2012 J. Sci. Rep. 2 591
Mitzi D 1999 in Progress in inorganic chemistry K Karlin (ed), vol 48 (New York: John Wiley)
Mitzi D 2001 J. Chem. Soc. Dalton Trans. 1 12
Parola S, Julián-López B, Carlos L D and Sanchez C 2016 Adv. Funct. Mater. 26 6506
Kahouli K, Kahouli A, Khirouni K and Chaabouni S 2020 J. Mol. Struct. 1199 126944
Nagabhushana H, Nagabhushana B M, Kumar M, Premkumar H B, Shivakumara C and Chakradhar R P S 2010 Phil. Mag. 26 3567
Gagandeep S, Kulwant B S L and Sahota H S 2000 Nucl. Sci. Eng. 134 208
Chawla A K, Kaur D and Chandra R 2007 Opt. Mater. 29 995
Lefi R, Ben Naser F and Guermazi H 2017 J. Alloys Compd. 696 1244
Mahfoudh N, Karoui K, Khirouni K and Ben Rhaiem A 2019 Physica B: Condens. Matter 554 126
Mguedla R, Ben Jazia Kharrat A, Saadi M, Khirouni K, Chniba-Boudjada N and Boujelben W 2020 J. Alloys Compd. 812 152130
Marotti R E, Guerra D N, Bello C, Machado G and Dalchiele E A 2004 Sol. Energy Mater. Sol. Cells 82 85
Demichelis F, Minetti-Mezzetti E, Tagliaferro A and Tresso E 1986 J. Appl. Phys. 59 611
Husain S, Keelani A O A and Khan W 2018 Nano-Structures & Nano-Objects 15 17
Shakur H R 2011 Physica E 44 641
Caponetti E, Pedone L, Chillura Martino D, Panto V and Turco Liveri V 2003 Mater. Sci. Eng. C 23 531
Mguedla R, Ben Jazia Kharrat A, Taktak O, Souissi H, Kammoun S, Khirouni K et al 2020 Opt. Mater. 101 109742
Ouni B, Boukhachem A, Dabbous S, Amlouk A, Boubaker K and Amlouk M 2010 Mater. Sci. Semicond. Process. 13 281
Wemple S H and Didomenico M 1971 Phys. Rev. B 3 1338
Moss T S 1959 Optical properties of semiconductors (London: Butter Worths Scientific Publication LTD)
Sharma P, Dahshan A and Aly K A 2014 J. Alloys Compd. 616 323
Kchaou H, Karoui K, Khirouni K and Ben Rhaiem A 2017 J. Alloys Compd. 728 936
Hassanien A S and Akl Alaa A 2015 J. Alloys Compd. 648 280
Abdel Rafeaa M, Farag A A M and Roushdy N 2009 J. Alloys Compd. 485 660
Omar M A 1987 Elementary solid physics (Massachusetts: Addison-Wesley Publishing Company)
Chalana S R, Ganesan V and Mahadevan Pillai V P 2015 AIP Adv. 5 107207
Acknowledgement
This work was supported by the Tunisian Ministry of Higher Education and Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kharrat, A.B.J., Kahouli, K. & Chaabouni, S. Detailed investigation of the optical properties of the (C8H11BrN)3BiCl6 compound by UV–visible measurements. Bull Mater Sci 43, 275 (2020). https://doi.org/10.1007/s12034-020-02248-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-020-02248-7