Abstract
Epoxy/nanoclay composites based on diglycidyl ether of bisphenol-A was cured by isophoronediamine and different weight percents of organically modified montmorillonite (O-MMT) clay (3, 5 and 7 wt%). Epoxy/nanoclay composites were characterized by different techniques such as Fourier transform infrared spectroscopy, scanning electron microscopy and X-ray diffraction. The compressive strength of the epoxy/nanoclay composite with a 3 wt% of O-MMT was higher than that of pure epoxy and other composites. Thermogravimetric analysis showed no change in the thermal behaviour of the cured epoxy after incorporation of O-MMT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Epoxy/nanoclay composites have received great attention from both academia as well as industrial domains due to their remarkable improvement in mechanical and thermal properties of pristine epoxy [1, 2]. The most important factors that affect the final properties of epoxy/nanoclay composites are type of curing agent, nanoclay and epoxy used. Different epoxy/nanoclay composites are prepared based on diglycidyl ether of bisphenol-A (DGEBA) [3,4,5,6,7]. The most widely used type of nanoclay is organically modified montmorillonite (O-MMT) because of its easy expansion or swelling of interlayer distance (d spacing) [8,9,10]. Recently, synthesis of epoxy cured by fatty acid amido amine reinforced with \(\hbox {SiO}_{{2}}\) nanoparticles has been studied [11]. Epoxy resins have good chemical and corrosion resistance, but poor impact properties due to their rigid and brittle structures [12]. To improve the impact properties of epoxy, epoxy/nanoclay composites were fabricated. The common clay modified or unmodified montmorillonite (MMT) was used in the synthesis of polymer composites. There are a numerous epoxy/clay composites which were prepared using different curing agents and their thermal and mechanical properties were tested [13,14,15,16]. DGEBA (epoxy) resin was cured by a cycloaliphatic polyamine with different weight percents of O-MMT [17]. Cured epoxy reinforced with 7 wt% of O-MMT exhibited a compressive strength of up to 25.6%. In another study, discussion of the compressive strength of cured epoxy by 1-methyl-5-norbornene-2,3-dicarboxylic anhydride was reported and the authors observed that there was a reduced compressive strength of cured epoxy [18]. The objective of the current study was to investigate the effect of incorporation of O-MMT clay into epoxy resin cured by isophoronediamine (IPD) on compressive strength. According to the literature, study on the preparation of epoxy/nanoclay composites based on DGEBA resin with IPD has not yet been investigated [19]. Epoxy/nanoclay composites have been prepared using the in-situ curing method which enhances the formation of exfoliated composites [9, 20].
2 Experimental
2.1 Materials
DGEBA with a trade name of D.E.R.™ 331™ Liquid Epoxy Resin was purchased from DOW Chemicals Company. IPD was obtained from Huntsman Company, Germany, with a trade name of ARADURE 42 BD with a amine hydrogen equivalent weight of \(42.6\hbox { g eq}^{{-1}}\). O-MMT clay (NANOMER I.31 PS) was purchased from Sigma-Aldrich.
2.2 Characterization techniques
2.2.1 Infrared spectroscopy
Fourier transform infrared (FTIR) spectra were recorded on a Testcan Shimadzu Infra-Red-spectrophotometer (model 8000) within the wavenumber range of \(4000{-}600 ~\hbox {cm}^{{-1}}\) at \(25^{\circ } \hbox {C}\).
2.2.2 Thermogravimetric analysis
Thermogravimetric analysis was performed on a TGA-50H Shimadzu Thermogravimetric Analyzer. Samples were heated from 0 to \(600^{\circ } \hbox {C}\) in a platinum pan at a heating rate of \(10^{\circ } \hbox {C}\,\hbox {min}^{{-1}}\) under an \(\hbox {N}_{{2}}\) atmosphere with a flow rate of \(25\hbox { ml min}^{{-1}}\).
2.2.3 X-ray diffraction (XRD)
The spacing of MMT was measured by using a PAN analytical XRD equipment model X’Pert PRO with a secondary monochromator, Cu-radiation (\(\lambda = 1.542\) Å) at 50 kV, 40 mA and scanning speed \(0.02^{\circ }\,\hbox {s}^{{-1}}\). The diffraction peaks between \(2\lambda = 2\) and \(10^{\circ }\) (or \(2{-}60^{\circ }\)), corresponding spacing (d, Å) and relative intensities \(({I/I}_{\mathrm {0}})\) were obtained.
2.2.4 Scanning electron microscopy (SEM)
SEM was used to characterize the pristine epoxy and epoxy/nanoclay composites with a model Quanta 250 FEG (Field Emission Gun) attached with an EDX unit (Energy Dispersive X-ray Analyses), with accelerating voltage 30 kV (magnification \(14\times \) up to 1,000,000 and resolution for Gun 1n).
2.2.5 Viscosity measurement
A Brookfield viscometer was used for measurement of the viscosity of DGEBA resin loaded with 0, 3, 5 and 7 wt% of O-MMT. The measurements were performed according to ASTM D 4878-93.
2.2.6 Gel time measurement
The gel time was measured by a manual method, which depends on the estimation of the rheological nature of the resin through observation during stirring and direct the resin with the probe [21].
2.2.7 Compressive strength
Compressive strength was measured by using equipment from Matest Srl Brembate Sopra 24030, Italy, with a capacity of 1500 kN, model no. C038, grade A, according to ASTM 579.01 method B.
2.2.8 Chemical resistance
The chemical resistance of specimens of the pristine epoxy and epoxy/O-MMT composites was studied using the ASTM D 543.06 method. Different chemical reagents were used such as 10% HCl (v/v) and 10% \(\hbox {CH}_{\mathrm {3}}\hbox {COOH}\) (v/v) to investigate the effect of acidic environments on the composites.
2.3 Experimental techniques
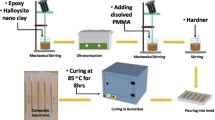
DGEBA resin was heated up to \(80^{\circ } \hbox {C}\). Different amounts of O-MMT with 3, 5 and 7 wt% based on the resin were dispersed at \(80^{\circ } \hbox {C}\) for 2 h using a high shear force mechanical stirrer. Resins were degassed under vacuum until no bubbles were observed and then mixed with stoichiometric ratios of IPD at room temperature. Uncured epoxy and nanoclay/epoxy composites were degassed again and then cast into steel moulds previously treated with a mould-releasing agent. All samples were subsequently stored at room temperature for 7 days for curing, then removed from the moulds and post cured for 2 h at \(100^{\circ } \hbox {C}\) and then for 2 h at \(160^{\circ } \hbox {C}\).
3 Results and discussion
3.1 XRD
XRD pattern of O-MMT clay showed a single peak at \(2\theta = 3.94\) indicating the (001) basal plane identifying the interlayer structure of the clay [22]. To identify the expansion of O-MMT in epoxy composites, the position of the (001) peak of O-MMT was compared with different XRD patterns. In the case of epoxy composite with 3 wt% of O-MMT, the peak at \(2\theta = 3.97\) had completely disappeared which indicates a good expansion between epoxy and O-MMT. While for 5 and 7% O-MMT, the XRD patterns showed peaks at \(2\theta = 3.4\) and 3.35, respectively, which indicate an intercalated structure or agglomeration of O-MMT onto the epoxy (figure 1).
3.2 FTIR
FTIR spectrum of cured epoxy at room temperature shows the characteristic peaks of the epoxide ring at 763, 829 and \(913\, \hbox {cm}^{{-1}}\). These peaks indicate the presence of epoxide ring residue. In the spectrum of the cured epoxy at elevated temperatures, these peaks disappeared, which indicates the complete curing of the epoxy resin (figure 2). Therefore, curing of epoxy at elevated temperatures was chosen to obtain fully cured epoxy resin.
3.2.1 Intercalation of O-MMT nanoclay with the epoxy resin
As shown in scheme 1, the amino group of modified MMT attacks the epoxide ring of DGEBA covalently. In figure 3a, the IR spectrum of DGEBA resin showed the characteristic peaks of the epoxide ring and hydroxyl group at 914 and \(3499\, \hbox {cm}^{{-1}}\), respectively. However, the IR spectrum of DGEBA resin with O-MMT (figure 3b) showed the same peaks in addition to a peak at \(3624 \,\hbox {cm}^{{-1}}\), which is characteristic of the hydroxyl groups of MMT. A comparison of the two spectra shows that the intensity of the epoxide group decreases relative to that of the hydroxyl group. These results are in agreement with the result of cured epoxy using polyetheramine and reinforced with organo-modified Cloisite [23]. Therefore, the use of modified clay gave better results than unmodified clay due to the formation of covalent bond between clay and epoxy which improves the expansion of clay through epoxy.
3.3 Thermogravimetric analyses of epoxy and its different nanocomposites
Different epoxy composites with different weight percents of clay such as 3, 5 and 7 wt% were prepared. As shown in figure 4, there is no significant change in the thermal stability of the epoxy resin after loading with clay. There is no change in the initial decomposition temperature at \(370^{\circ } \hbox {C}\) for all investigated samples. Therefore, the presence of the clay has no effect on the thermal decomposition of epoxy. On the contrary, at a high temperature of \(500^{\circ } \hbox {C}\), the mass loss% of epoxy/O-MMT with 3, 5 and 7 wt% showed an increase in weight and an increase in the O-MMT content of 12, 15 and 20%, respectively (figure 4). The increase in the residual mass% as a function of the clay content is probably due to the non-decomposable inorganic oxides originating from MMT.
3.4 Gel time measurement
As illustrated in figure 5, the time of gelation process of epoxy and its composites was measured as a function of weight% of O-MMT clay added. The investigated gel time was 135 to 95, 80 and 55 min for the epoxy and composites with different wt% of O-MMT were 0, 3, 5 and 7%, respectively. The data revealed the decrease of gel time with increasing wt% of O-MMT owing to the presence of amine groups on modified clay, which acts as an accelerator for the curing process, this is in good agreement with the FTIR result. Based on literature, tertiary amine is usually added to accelerate the curing process of the epoxy [4].
3.5 Viscosity measurements
The viscosity values of epoxy in the presence and absence of clay with different weight percents are measured in the absence of cross-linking agents. As shown in table 1, the viscosity values of epoxy in the presence of 3, 5 and 7 wt% of O-MMT clay are higher than the viscosity value of pure epoxy by 25, 56 and 81%, respectively. The increase in the viscosity originates from the interaction of clay with the resin epoxy [4]. These results are supported by the results obtained from FTIR analysis and gel time measurement.
3.6 Morphology of the composites
As shown in figure 6, the image of unmodified epoxy (figure 6a) shows its fracture nature of surface. The images of its composites with 5 and 7 wt% show the agglomeration of clay on the surface and this agglomeration is not present in the case of the composite with 3 wt% O-MMT clay.
3.7 Chemical resistance
Specimens of epoxy and its composites with different weight percents of clay as 3, 5 and 7% were exposed to acidic environments such as hydrochloric acid (HCl) and acetic acid with the same concentration. In the case of HCl after 28 days, neat epoxy and its composites with 3, 5 and 7% showed the uptake percent as 1.62, 1.64, 1.55 and 1.33, respectively. This could be explained by a reaction of high clay content as intercalated and agglomerated, which increase the ability of absorption of the acid solution. In addition, the presence of tertiary amine increases the absorbance of acid to form a quaternary salt [24]. In contrast, the composite with 3% showed a slight increase in weight compared to the epoxy due to its good expansion of clay into epoxy which made a barrier for the absorption of acid solution [25]. Therefore, the composite with the highest clay content (\(\hbox {wt}{\%} = 7{\%}\)) has the highest rate of absorption of acid solution that leads to an increase in the corrosive rate of epoxy. As shown in figure 7, epoxy and its composites with 5 and 7 wt% show lower acid uptake compared to neat epoxy and its composite with 3 wt%. The rate of absorption of HCl is higher than that of acetic acid and that could be explained by the inorganic acid forming a quaternary salt easier than an organic acid and in turn leads to the rate of corrosive increase in the case of HCl.
3.8 Application
There are many research studies on epoxy mortars to improve its mechanical properties [26, 27]. One of the important characteristics of epoxy mortars is its compressive strength. The different mortars of epoxy and its composites are prepared on cubic shape as shown in figure 8. The dimensions of the shape were according to ASTM 579.01 method B. The compressive strength of pure epoxy was measured and found to be equal to 52 MPa. The epoxy/O-MMT composite with 3 wt% has a compressive strength equal to 72 MPa, that means that the compressive strength of cured epoxy was improved by \(\sim 40{\%}\). In the case of higher contents of O-MMT, there is no improvement in the compressive strength of epoxy (figure 8). This could be explained by the good expansion of O-MMT through epoxy resin which leads to a good compressive strength of epoxy.
4 Conclusions
DGEBA epoxy/O-MMT was prepared by in-situ polymerization. The prepared composites were characterized by using FTIR, XRD and SEM. The effect of organically modified clay enhancing the gelation process was observed by decreasing the time of gel and increasing its viscosity. The presence of O-MMT with different weight percents had no effect on the thermal behaviour of epoxy. The good expansion of 3 wt% of O-MMT was proved by XRD and SEM analyses. DGEBA/O-MMT with 3 wt% improved the compressive strength of epoxy by \(\sim 40{\%}\). However, the increase in the weight% of O-MMT did not show an improvement in the compressive strength.
References
Reis J, Amorim F, Silva A and Costa Mattos H 2015 Int. J. Adhesion Adhes. 58 88
Souza J P B and Reis J M L 2013 Appl. Adhes. Sci. 6 1
Zabihi O, Ahmadi M, Nikafshar S, Preyeswary K C and Naebe M 2018 Compos. B Eng. 135 1
Mohan T and Kumar M 2005 Polym. Int. 54 1653
Nigam V, Setua D, Mathur G and Kar K 2004 J. Appl. Polym. Sci. 93 2201
Su W F, Huang H W and Pan W P 2002 Thermochim. Acta 392–393 391
Tack J L and Ford D M 2008 J. Mol. Graph. Model. 26 1269
Pavlidou S and Papaspyrides C D 2008 Prog. Polym. Sci. 33 1119
Ray S S and Okamoto M 2003 Prog. Polym. Sci. 28 1539
Kaya E, Tanoglu M and Okur S 2008 J. Appl. Polym. Sci. 109 834
Parimalam M, Islam M R, Yunus R and Rashidi N M 2019 Prog. Color Colorants Coat. 12 71
Liu W, Hoa S and Pugh M 2005 Compos. Sci. Technol. 65 307
Wang L, Wang K, Chen L, Zhang Y and He C 2006 Compos. A, Appl. Sci. Manuf. 37 1890
Liu T, Tjiu W, Tong Y, He C, Goh S and Chung T 2004 J. Appl. Polym. Sci. 94 1236
Zeng Q H, Yu A B, Lu G Q and Paul D R 2005 J. Nanosci. Nanotechnol. 5 1574
Wang M, Fan X, Thitsartarn W and He C 2015 Polymer 58 43
Khanbabaei G, Aalaie J, Rahmatpour A, Khoshniyat A and Gharabadian M A 2007 J. Macromol. Sci. Part B 46 975
Jumahat A, Soutis C, Mahmud J and Ahmad N 2012 Procedia Eng. 41 1607
Fraga F, Vazquez E, Rodrıguez-Nunez E and Martınez-Ageitos J M 2008 Polym. Adv. Technol.19 1623
Pinnavaia T J and Beall G W 2001 Polymer clay nanocomposites (New York: Wiley - Wiley series in Polymer Science)
Shimkin A A 2016 Russ. J. Gen. Chem. 86 1488
Tbrrbonsrt R 1973 Am. Mineral. 58 73
Rỳznarová B, Zelenka J, Lednický F and Baldrian J 2008 J. Appl. Polym. Sci. 109 1492
Yang T, Zhang C, Zhang J and Cheng J 2014 Thermochim. Acta 577 11
Ribeiro M C S, Rodrigues J A, Ferreira A J M and Marques A T 2008 Mater. Sci. Forum. 587–588 903
Haurie L, Lacasta A M, Ciudad A, Realinho V and Velasco J I 2013 Constr. Build. Mater. 42 266
Yemam D M, Kim B J, Moon J Y and Yi C 2017 Materials (Basel) 10 246
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sabaa, M.W., Abdelhakim, M. & Soliman, S.M.A. Characterization and application of cured epoxy resin reinforced with montmorillonite. Bull Mater Sci 43, 126 (2020). https://doi.org/10.1007/s12034-020-02100-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-020-02100-y