Abstract
The study investigates the tensile, flexural and thermal properties of epoxy resin matrix reinforced with pristine as well as amino-functionalized multi-walled carbon nanotubes (MWCNTs, 0, 0.25 and 0.50 wt%). The combination of ultrasonication and magnetic stirring has been used for the fabrication of MWCNTs/epoxy composite samples. The epoxy composite reinforced with 0.50 wt% amino-functionalized MWCNTs exhibits superior mechanical and thermal properties. The tensile and flexural strengths of this composite are noticed to be higher by about 13.5 and 17%, respectively, as compared with the neat epoxy specimen. The improvement in properties offered by amino-functionalized MWCNTs/epoxy composites is attributed to uniform distribution of MWCNTs in epoxy matrix as well as better interfacial adhesion between MWCNTs reinforcement and epoxy matrix, when compared with those noticed for epoxy composite reinforced with pristine MWCNTs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Over the last few decades, the use of polymer matrix composites (PMCs) is increasing in various applications, viz. components of automobile industries, aerospace sector, defence, home furnishing, fabric, sports goods, etc. The application of PMCs has been recommended due to their low cost, light weight and simple processing techniques [1,2,3]. Polymer nanocomposites (PNCs) belong to the class of PMCs wherein the reinforcement has at least one of its dimensions on the order of nanometre (less than 100 nm) [4]. In comparison with PMCs, PNCs offer several advantages, not only in terms of their superior mechanical properties, but also in terms of excellent thermal, electrical, optical and other properties. Some of the most common nano-reinforcements employed in PNCs are nano-clay, carbon nano-fibres and carbon nano-tubes (CNTs) [5, 6].
Epoxy resins (a thermosetting polymer) are quite versatile and are extensively used in various applications, including adhesives, industrial paints and coatings, industrial tooling, encapsulation, potting, etc. [7,8,9]. In addition, the epoxy resins find extensive application as matrix material in PMCs. However, the use of neat epoxy resin is limited due to its extremely brittle nature, which results in its inferior mechanical properties. To overcome this issue, several attempts have been made by various researchers to enhance various mechanical properties of epoxy resin matrix by reinforcing it with different types of nanofillers, viz. \({\hbox {TiO}}_{2}\), ZnO, nano-clay, CNTs, \(\hbox {CaCO}_{3}\), etc. [6, 10, 11]. Amongst various nanofillers, CNTs are the most widely demanded due to their wide range of research and commercial applications, viz. structural composites, energy storage devices, electronic devices, bio-sensors and drug delivery systems [12,13,14]. The superior properties of CNTs have motivated the researchers to employ CNTs as reinforcement in epoxy resin, in order to achieve enhancement in various properties of neat epoxy resin [15,16,17]. However, exploration of full potential of reinforcing CNTs into epoxy matrix still remains a big challenge due to difficulty in achieving homogeneous dispersion of CNTs in epoxy and poor adhesion between CNTs and epoxy. The research carried out indicates that high aspect ratio and high van der Waals attraction forces in CNTs cause aggregation and agglomeration among themselves, which is responsible for poor load transfer between epoxy and CNTs, thus resulting in lower properties of epoxy/CNTs nanocomposites [18]. To enhance interfacial adhesion between CNTs and epoxy resin, various techniques, such as functionalization of CNTs, alignment of CNTs, ultrasonication and homogenization, have been employed [19,20,21,22]. Tariq et al [23] demonstrated that the use of ultrasonication results in better dispersion of multi-walled carbon nanotubes (MWCNTs) into epoxy matrix, especially at lower content of MWCNTs. The study noticed significant improvement in the tensile and flexural strengths of carbon fibre/epoxy composite reinforced with MWCNTs. Cividanes et al [24] investigated the effect of varying ultrasonication power and time on the curing behaviour of amino-functionalized CNTs/epoxy composite. The curing behaviour of CNT/epoxy composite was observed to depend on the sonication power. Liu et al [25] noticed that the peeling and tensile strengths of CNTs/epoxy bonding are remarkably enhanced when the surface of CNTs is functionalized by atmospheric-pressure helium/oxygen plasma. Cha et al [26] functionalized the surface of CNTs by polystyrene sulphonate and poly(4-aminostyrene), and used these functionalized CNTs to fabricate CNTs/epoxy nanocomposites. The functionalization of CNTs led to improved mechanical properties (Young’s modulus and tensile strength) of CNTs/epoxy composites. Cha et al [27], in their subsequent study, further noticed significant enhancement in tensile properties and fracture toughness of epoxy composite reinforced by melamine-functionalized CNTs (2 wt%). Kharitonov et al [28] also observed that the reinforcement of fluorinated CNTs into epoxy resin leads to remarkable enhancement in the tensile (35%) and flexural (58%) strengths as compared with that observed for pristine CNTs/epoxy composite. The study also indicated that the use of fluorinated CNTs as fillers does not worsen thermal stability of the CNTs/epoxy composite, though a slight increase in glassy temperature is noticed. Salam et al [29] employed amino-functionalized and carboxyl-functionalized MWCNTs to fabricate MWCNTs/epoxy composites. The study used combination of sonication and 3-roll mill techniques to disperse MWCNTs into epoxy. The study revealed that carboxyl-functionalized MWCNTs/epoxy composites have good dispersion and higher number of functional groups present, which result in increased cross-link density and superior flexural, thermal and thermo-mechanical properties. Mansoor et al [30] also observed that the incorporation of MWCNTs functionalized by hydrogen peroxide into epoxy matrix helps in improving the flexural and tensile properties of MWCNTs/epoxy composites. Srikanth et al [31] noticed that the reinforcement of amino-functionalized MWCNTs into epoxy resin matrix enhances the fracture toughness, attributed to increased cross-linked density of MWCNTs/epoxy composites. Cui et al [32] examined the effects of MWCNTs functionalization (carboxyl groups and amino-groups), MWCNTs loading, applied load and sliding speed on the friction and wear behaviour of the neat epoxy and MWCNTs/epoxy composites. The epoxy-reinforced amino-functionalized MWCNTs showed better performance as compared with neat epoxy. The epoxy composites having 0.5 wt% amino-functionalized MWCNTs exhibited the lowest wear rate, 41.3% lower than that noticed for neat epoxy.
Jiang et al [33] investigated the roles of inter-phase on the glass transition temperature (\(T_{\mathrm{g}})\) of epoxy matrix reinforced with either pristine or functionalized (amino and hydroxyl) CNTs. The study reveals that amino-functionalized CNTs/epoxy composites have the highest \(T_{\mathrm{g}}\), which is attributed to the formation of covalent bonds between amino-functionalized CNTs and cured epoxy. However, no such covalent bonding was observed between hydroxyl-functionalized CNTs and epoxy in hydroxyl-functionalized CNTs/epoxy composites.
The literature indicates that attempts have been made by the researchers to enhance the mechanical and thermal properties of epoxy resin using CNTs as reinforcement, which could be suitably employed in numerous high-performance applications, viz. aerospace, aviation, automotive, etc. [34, 35]. However, in most of the studies concerning pristine CNTs/epoxy composites, low enhancement in properties is reported due to agglomeration and entanglement of CNTs (owing to their inert nature and high aspect ratio), which results in weak interfacial adhesion between CNTs and epoxy [18, 36, 37]. To overcome these issues, the investigators have subjected CNTs to various treatments, viz. plasma, fluorination, carboxyl, hydroxyl, amino, melamine, hydrogen peroxide, silane, etc., prior to their reinforcement into epoxy matrix [24, 25, 27,28,29,30,31,32,33, 37]. The study conducted by Ma et al [37] reveals that amino-functionalized MWCNTs exhibit higher surface energy and much better wettability with epoxy resin than the pristine MWCNTs. Some of the reported works indicate enhancement in fracture toughness [31], wear resistance [32] and glass transition temperature [33]. In the light of this, it would be interesting to investigate the effect of reinforcing amino-functionalized MWCNTs into epoxy matrix on some other important mechanical properties, viz. tensile, flexural and thermal degradation behaviour. The present study is aimed to investigate the effects of reinforcing amino-functionalized MWCNTs, in different proportions (0.25 and 0.50 wt%), into epoxy matrix on the tensile, flexural and thermal degradation properties. For the purpose of comparison, results are also obtained for pristine MWCNTs/epoxy composites and neat epoxy. The combination of ultrasonication and magnetic stirring has been employed for the fabrication of MWCNTs/epoxy composite samples, so as to take care of agglomeration and dispersion issues of MWCNTs in epoxy matrix. In order to correlate the test results with the morphological features, the fracture surfaces of various samples have been examined under a scanning electron microscope (SEM).
2 Experimental
2.1 Materials used
High-quality LY556 epoxy resin (Bisphenol-A) and HY951 hardener (triethylenetetramine—TETA), with molecular structures shown in figure 1 [38], were purchased from Singhal Chemicals Corporation, Meerut, India. The pristine MWCNTs (see table 1) used in the study were procured from Reinste Nano Ventures Private Limited, New Delhi, India. The chemicals required for the purpose of amino-functionalization of MWCNTs (i.e. thionyl chloride (\(\hbox {SOCl}_{2})\), dimethyl formamide (DMF), TETA, ethanol and acetone) were purchased from Vivek Scientific Industries, Ambala Cant, India.
Molecular structure of (a) epoxy resin LY556 and (b) hardener HY951 [38].
2.2 Amino-functionalization of MWCNTs
Out of the total procured pristine MWCNTs, half the amount of MWCNTs was subjected to amino-functionalization, according to the procedure outlined in earlier study by Chandrasekaran et al [39]. For this purpose, 1.8 g of MWCNTs was refluxed with 72 ml mixture of sulphuric and nitric acids (mixed in 3:1 ratio) at \(40{^{\circ }}\hbox {C}\) for 7 h with the help of a magnetic stirrer in a fuming cupboard. The remaining MWCNTs, after refluxing, were washed with deionized water till a pH value of 7 was attained. The neutralized MWCNTs were then dried overnight at \(110{^{\circ }}\hbox {C}\) in a universal vacuum oven. The remaining 1 g of the oven-dried MWCNTs was suspended in 100 ml of \(\hbox {SOCl}_{2}\) and 40 ml of DMF in an inert atmosphere maintained at \(70{^{\circ }}\hbox {C}\) for 24 h. Excess amounts of \(\hbox {SOCl}_{2}\) and DMF were removed in the fuming cupboard. The resulting MWCNTs were then treated with 200 ml of TETA at \(120{^{\circ }}\hbox {C}\) for 96 h in the fuming cupboard. The treated MWCNTs were then washed with anhydrous ethanol and water to remove excess amount of TETA. The MWCNTs thus obtained were finally dried in the vacuum oven at \(120{^{\circ }}\hbox {C}\). Figure 2 schematically represents the chemical reactions occurring during the course of amino-functionalization of MWCNTs [40].
2.3 Fabrication of samples
The fabrication procedure of samples made of neat epoxy and MWCNTs/epoxy composites is described in the following sub-sections.
Schematic representation of amino-functionalization of MWCNTs [40].
2.3.1 Neat epoxy samples
The epoxy (LY556) and hardener (HY951) were mixed (in proportion 10:1 by weight) thoroughly in a glass beaker by continuously stirring with the help of a glass rod for around 2 min. The mixture was then poured slowly into a specially fabricated steel mould, consisting of slots conforming to the tensile and flexural specimens, as per ASTM D-638 and ASTM D-790, respectively. The mould was then allowed to cure at room temperature for 48 h. The cured specimens were ejected from the mould by applying mechanical pressure.
2.3.2 MWCNTs/epoxy composite samples
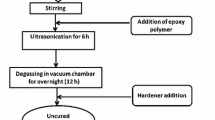
To fabricate MWCNTs/epoxy composite specimens, having either pristine MWCNTs (referred to as P-MWCNTs hereafter) or amino-functionalized MWCNTs (referred to as F-MWCNTs hereafter), the weighed amount of MWCNTs (0.25 and 0.50 wt%) was added to the measured amount of epoxy resin contained in a glass beaker. The beaker was then placed on a magnetic hot plate (Make-REMI, Model-5MLH) and stirring was carried out for 20 min at 2500 rpm. During the process, acetone was added intermittently to facilitate mixing by reducing the viscosity of MWCNTs/epoxy mixture. The mixture was thereafter sonicated in an ultrasonicator (Make-Wensar, Model-WUC Series, 2 l) at \(40{^{\circ }}\hbox {C}\) for 20 min. The sonicator was intermittently switched off for 10 s after every 2 min of sonication, so as to cool the mixture. In addition, the glass beaker, containing the mixture of epoxy and MWCNTs, was also surrounded by ice cubes, in order to maintain the temperature of the mixture below \(40{^{\circ }}\hbox {C}\) during the sonication process. Following sonication, the hardener was added to MWCNTs/epoxy mixture and manually mixed with the help of a glass rod for 2 min. The mixture was then poured into the steel moulds and allowed to cure at room temperature for 48 h, to obtain the tensile and flexural composite specimens.
2.4 Mechanical testing
The fabricated samples (neat epoxy, P-MWCNTs/epoxy and F-MWCNTs/epoxy composites) were subjected to the tensile and flexural tests. The tensile tests were performed at a cross-head speed of \(50~\hbox {mm}~\hbox {min}^{-1}\) on a universal testing machine (Make-Dark System Inc., Capacity 100 kN) using a load cell of 10 kN. During the tensile testing, the temperature and relative humidity (RH) were maintained at \(23{{^{\circ }}}\hbox {C}\) and 54%, respectively. The flexural tests were also conducted on the same machine, using again a load cell of 10 kN, at the temperature and RH level specified earlier for the tensile test. During the flexural testing, cross-head speed was maintained at \(1~\hbox {mm}~\hbox {min}^{-1}\) and span of the samples was kept at 60 mm. The tensile and flexural tests were performed on three similar samples, corresponding to each composition level (i.e. 0, 0.25 and 0.50 wt% of MWCNTs in epoxy matrix), and the average value of these results was used to represent the tensile and flexural strengths of the sample corresponding to a particular sample composition.
2.5 SEM
In order to reveal the distribution of MWCNTs in epoxy matrix and morphology of the fractured surfaces, the fractured surface of various tensile specimens was examined under the SEM (Make-JEOL, Model-JSM 6100). Prior to SEM examinations, the fractured surface of the specimens was made conductive by coating it with gold on a sputter coater.
2.6 Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy (FT-IR) analyses of P-MWCNTs and F-MWCNTs were conducted on an FT-IR spectrophotometer (Make-Perkin Elmer) by scanning the samples in the range of 4000–250 \(\hbox {cm}^{-1}\). FT-IR was carried out to ascertain the chemical changes occurring on the surface of MWCNTs during the course of amino-functionalization.
2.7 Thermogravimetric analysis
In order to investigate the effect of reinforcing P-MWCNTs and F-MWCNTs into epoxy matrix on the thermal degradation properties, thermogravimetric analysis (TGA) of various samples (neat epoxy and epoxy composite) was performed on a Mettler Toledo (Model: TGA/SDTA 851e). During TGA, the powdered form of various samples (weighing around 5 mg) was heated at a rate of \(10{{^{\circ }}}\hbox {C}~\hbox {min}^{-1}\) and weight loss of the samples was recorded at different temperatures.
3 Results and discussion
3.1 FT-IR analysis of P-MWCNTs and F-MWCTs
FT-IR spectral analysis of both P-MWCNTs and F-MWCNTs, as shown in figure 3, investigates the effect of modification at the amino-group present in F-MWCNTs. FT-IR spectra of F-MWCNTs show characteristic bands due to the N–H bond of amide linkage at \(3777.68~\hbox {cm}^{-1}\), absorption band at \(1562.61~\hbox {cm}^{-1}\) representing N–H scissoring vibrational mode and absorption band in the range of \(1228.61{-}1297.61~\hbox {cm}^{-1}\) corresponding to C–N stretching vibrations, which are absent in the case of P-MWCNTs. All other absorption peaks such as the broad absorption band at \(3717.63~\hbox {cm}^{-1}\) due to free O–H stretching, the vibrational peak at \(3001.61~\hbox {cm}^{-1}\) corresponds to \(=\)C–H stretching, the strong absorption at \(1828.60~\hbox {cm}^{-1}\) due to C\(=\)O asymmetrical vibration, the vibrational stretching at \(1494.60~\hbox {cm}^{-1}\) attributed to the presence of C\(=\)C linkage and the bending vibration at 920.61 \(\hbox {cm}^{-1}\) attributed to C–H bending vibrational mode are present in both P-MWCNTs and F-MWCNTs [41, 42]. Thus, it is revealed from the FT-IR spectra that the structure of P-MWCNTs remains intact with structural modification occurring primarily to form the amide linkage to obtain the desired F-MWCNTs.
3.2 Mechanical properties
The variation of tensile and flexural properties (strength and modulus) of various samples, as per composition mentioned in table 2, is shown in figures 4 and 5. The tensile as well as flexural strengths of F-MWCNTs/epoxy composites are observed to be superior in comparison with neat epoxy as well as P-MWCNTs/epoxy composites (figure 3). Both the strengths (tensile and flexural) are noticed to increase with the increase in content of MWCNTs from 0.25 to 0.50 wt% in both P-MWCNT- and F-MWCNT-reinforced epoxy composites. It appears that the incorporation of MWCNTs into epoxy matrix enhances the cross-link ratio and blocks the molecular motion of the epoxy matrix, thereby resulting in enhanced strengths of the MWCNTs/epoxy composites as compared with neat epoxy [13]. Composite specimen-3 (i.e. 0.50 wt% F-MWCNTs/epoxy) exhibits the highest tensile and flexural strengths amongst the various specimens. The tensile and flexural strengths noticed for composite specimen-3 are, respectively, higher by about 13.5 and 17% than that observed for neat epoxy specimen-1. Composite specimen-4 with 0.25 wt% P-MWCNTs in epoxy matrix has the lowest tensile and flexural strengths. Similar to variation of strengths shown in figure 3, composite specimen-3 also exhibits the highest Young’s as well as flexural modulus (figure 4) while the lowest values of both the modulii are observed for neat epoxy specimen-1. The increase observed in Young’s modulus and flexural modulus on reinforcing MWCNTs into epoxy matrix is attributed to high aspect ratio, high modulus and high strength of MWCNTs [13]. The values of Young’s and flexural modulii noticed for composite specimen-3 are, respectively, higher by about 14.5 and 30% than that observed for neat epoxy specimen-1.
SEM examination of the tensile fractured surfaces of various specimens was conducted to explain the observed variation in tensile and flexural behaviours of different samples (figure 6a–d). It is revealed from the SEM images that there is agglomeration of MWCNTs in P-MWCNTs/epoxy composites (figure 6a and c). However, the distribution of MWCNTs is relatively finer and uniform in F-MWCNTs/epoxy composites (figure 6b and d). It appears that amino-functionalization of MWCNTs helps in reducing the agglomeration and facilitates uniform dispersion of F-MWCNTs in epoxy matrix. The reported facts are in agreement with the TEM observations of P-MWCNTs and F-MWCNTs under similar treatment conditions [39] and the work reported by Ma et al [37]. The use of amino-functionalized MWCNTs also appears to increase the bridges between MWCNTs and epoxy, thereby improving their interfacial adhesion [37, 43]. During the course of composite fabrication, chemical interaction occurs between the amine group attached to F-MWCNTs and epoxy molecule, causing ring-opening reactions followed by cross-linking reactions, as depicted in figure 7 [39]. As a result of this, formation of covalent bond takes place between F-MWCNT and epoxy matrix, which results in better load transfer between F-MWCNTs and epoxy matrix and leads to superior mechanical performance of F-MWCNTs/epoxy composites than noticed for P-MWCNTs/epoxy.
The poor mechanical performance exhibited by P-MWCNTs/epoxy composites may be attributed to the presence of voids (figure 4a) and agglomeration of P-MWCNTs (figure 4c), which is more at higher MWCNTs content (0.50 wt%). The presence of voids observed in figure 4a might be due to improper mixing of epoxy and CNTs. The agglomeration of P-MWCNTs occurs due to their high surface area, \(\uppi \)–\(\uppi \) bonding and van der Waals forces of attraction [18].
3.3 Thermal properties
The temperatures corresponding to different extents of weights loss (i.e. 30, 50 and 70 wt%) of various samples have been extracted from the results of TGA and are reported in table 3. Owing to high thermal conductivity of MWCNTs (on the order of 3000 W (m K)\(^{-1})\) [13], the reinforcement of MWCNTs into epoxy matrix helps in dissipating more heat, thereby increasing thermal stability of neat epoxy resin. Further, relatively finer and uniform distribution of F-MWCNTs into epoxy matrix, as pointed out in previous section, results in higher thermal stability than that noticed for P-MWCNTs/epoxy composites. Amongst various samples, the epoxy composite having 0.50 wt% F-MWCNTs shows the highest thermal stability, which may be due to better interfacial adhesion between F-MWCNTs and epoxy matrix, as revealed from the SEM examinations.
4 Conclusions
The study investigated the mechanical and thermal properties of epoxy matrix composites reinforced with MWCNTs (pristine as well as amino-functionalized) at 0.25 and 0.50 wt% of MWCNTs. The study reveals that epoxy nanocomposite reinforced with 0.50 wt% amino-functionalized MWCNTs exhibits superior tensile and flexural properties. The tensile and flexural strengths noticed for 0.50 wt% F-MWCNTs/epoxy composite are observed to be higher, respectively, by about 13.5 and 17% than that noticed for the neat epoxy sample. Similarly, Young’s and flexural modulii noticed for 0.50 wt% F-MWCNTs/epoxy composite are, respectively, higher by about 14.5 and 30% than that observed for the neat epoxy sample. The tensile and flexural properties of MWCNTs (both pristine and functionalized) improve with the increase in content of reinforcement from 0.25 to 0.50 wt%. Similar to mechanical properties, the reinforcement of either P-MWCNTs or F-MWCNTs into epoxy enhances its thermal stability. The epoxy composite reinforced with 0.50 wt% F-MWCNTs shows the highest thermal stability. The improvement in mechanical and thermal properties of F-MWCNTs/epoxy composites is attributed to better interfacial adhesion between MWCNTs reinforcement and epoxy matrix and uniform dispersion of MWCNTs, when compared with those noticed for P-MWCNTs/epoxy composites.
References
Lorandi N P, Cioffi M O H and Shigue C Jr H L O 2018 Mater. Res. 21 1
Luz F S D, Monteiro S N, Lima E S and Júnior E P L 2017 Mater. Res. 20 23
Amorim F D C, Souza J F B D and Reis J M L D 2018 Mater. Res. 21 1
Hollaway L C 2014 in High performance textiles and their applications J Bai (ed) (Cambridge: Woodhead Publishing Limited) p 366
Gupta R K, Kennel E B and Kwang-Jea K (eds) 2010 Polymer nanocomposites handbook (Florida: Taylor and Francis Group)
Pinto D, Bernardo L, Amaro A and Lopes S 2015 Constr. Build. Mater. 95 506
Saba N, Jawaid M, Alothman O Y, Paridah M T and Hassan A 2015 J. Reinf. Plast. Compos. 35 447
Gibson G 2017 in Brydson’s plastics materials M Gilbert (ed) 8th ed. (Cambridge: Elsevier) p 773
Mittal V, Saini R and Sinha S 2016 Compos. Part B: Eng. 99 425
Shukla M K and Srivastava D 2015 Int. J. Sci. Res. 5 1692
Islam M E, Mahdi T H, Hosur M V and Jeelani S 2015 Procedia Eng. 105 821
Kepple K L, Sanborn G P, Lacasse P A, Gruenberg K M and Ready W J 2008 Carbon 46 2026
Zakaria M R, Kudus M H A, Akil H M and Thirmizir M Z M 2017 Compos. Part B: Eng. 119 57
Irshidat M R, Al-Saleh M H and Almashagbeh H 2016 Mater. Des. 89 225
Gojny F H, Wichmann M H G, Fiedler B and Schulte K 2005 Compos. Sci. Technol. 65 2300
Laurenzi S, Pastore R, Giannini G and Marchetti M 2013 Compos. Struct. 99 62
Le H R, Howson A, Ramanauskas M and Williams J A 2012 Tribol. Lett. 45 301
Ervina J, Mariatti M and Hamdan S 2016 Procedia Chem. 19 897
Gojny F H, Nastalczyk J, Roslaniec Z and Schulte K 2003 Chem. Phys. Lett. 370 820
Dutta A K, Penumadu D and Files B 2004 J. Mater. Res. 19 158
Ahn S N, Lee H J, Kim B J, Tan L S and Baek J B 2008 J. Polym. Sci. A: Polym. Chem. 46 7473
Moaseri E, Karimi M, Baniadam M and Maghrebi M 2014 Compos. Part A 64 228
Tariq F, Shifa M and Baloch R A 2018 Arab. J. Sci. Eng. 43 5937
Cividanes L D S, Simonetti E A N, Oliveira J I S D, Serra A A, Barboza J C D S and Thim G P 2015 Polym. Compos. 38 1964
Liu X, Xu F, Zhang K, Wei B, Gao Z and Qiu Y 2017 Compos. Sci. Technol. 145 114
Cha J, Jin S, Shim J H, Park C S, Ryu H J and Hong S H 2016 Mater. Des. 95 1
Cha J, Jun G H, Park J K, Kim J C, Ryu H J and Hong H S 2017 Compos. Part B: Eng. 129 169
Kharitonov A P, Simbirtseva G V, Tkachev A G, Blohin A N, Dyachkova T P, Maksimkin A et al 2015 Compos. Sci. Technol. 107 162
Salam M B A, Hosur M V, Zainuddin S and Jeelani S 2013 Open J. Compos. Mater. 3 1
Mansoor M, Shahid M and Habib A 2014 Arab. J. Sci. Eng. 39 6411
Srikanth I, Kumar S, Kumar A, Ghosal P and Subrahmanyam C 2012 Compos. Part A 43 2083
Cui L J, Geng H Z, Wang W Y, Chen L T and Gao J 2013 Carbon 54 277
Jiang C, Jiang D, Zhang J, Lin S, Shang X and Ju S 2017 Polym. Compos. 39 1129
Silva V A D and Rezende M C 2018 Mater. Res. 21 1
Kausar A, Rafique I and Muhammad B 2016 Polym. Plast. Technol.Eng. 55 1167
Yeh M K, Hsieh T H and Tai N H 2008 Mater. Sci. Eng. A 483–484 289
Ma P C, Mo S Y, Tang B Z and Kim J K 2010 Carbon 48 1824
Rao S and Rao R M V G K 2008 Polym. Test. 27 645
Chandrasekaran V, Santare M, Krishnan P and Advani S 2011 Compos. Interface 18 339
Wang J, Fang Z, Gu A, Xu L and Liu F 2006 J. Appl. Polym. Sci. 100 97
Monthe G C and de Miranda I C 2009 J. Therm. Anal. Calorim. 97 661
Silverstein R M, Bassler G C and Morill T C 1991 Spectrometric identification of organic compounds (New York: John Wiley and Sons Ltd.)
Moaseri E, Hasanabadi S, Maghrebi M and Baniadam M 2015 J. Compos. Mater. 49 1961
Acknowledgements
The authors are thankful to the staff members of CIPET, Amritsar (India), and CIL, Punjab University, Chandigarh (India), for providing the testing facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kashyap, A., Singh, N.P., Arora, S. et al. Effect of amino-functionalization of MWCNTs on the mechanical and thermal properties of MWCNTs/epoxy composites. Bull Mater Sci 43, 43 (2020). https://doi.org/10.1007/s12034-019-2012-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-019-2012-0