Abstract
WS2 is a promising catalyst for the hydrogen evolution reaction. We have explored photocatalytic properties of ternary sulphoselenides of tungsten (WS x Se 2−x ) by the dye-sensitized hydrogen evolution. WS x Se 2−x solid solutions are found to exhibit high activity reaching 2339 μmol h−1 g−1 for WSSe, which is three times higher than that of WS2 alone (866 μmol h−1 g−1 ). The turnover frequency is also high (0.7 h−1 ). Such synergistic effect of selenium substitution in WS2 is noteworthy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transition metal dichalcogenides (TMDs) have emerged as an important class of materials with unique properties [1–4]. They have layered sandwiched X–M–X units (M = metal and X = chalcogenides) with strong in-plane bonding and weak out-of-plane van der Waals interaction between layers. Among the layered TMDs, MoS2 and MoSe2 have attracted the significant attention in the last few years due to their properties related to transistors [5,6], catalysis [8–10]and gas sensors [10]. They are also useful for hydrogen evolution catalysis. Theoretical and experimental studies indicate that the (0001) planes are catalytically inert, while (10–10) planes are catalytically active due to the low Gibbs free energy for hydrogen evolution reaction (HER) [11,12]. Various strategies have been employed to improve the HER either by creating much active edge sites [13–17]or by chemical modification with Fe, Co, Ni and Re [17,18]. The metastable 1T-phase exhibits improved catalytic activity for HER. Ternary composites of TMDs MoS2(1−x)Se2x [19,20] and MoS x Cl y [21] are reported to show improved performance compared with binary compounds in electrochemical HER. We have synthesized ternary WS x Se2−x (x = 0.0, 0.50, 1.0, 1.5, 2.0) and examined their photocatalytic HER activity. Interestingly, we found synergistic effect wherein the ternary sulphoselenides are superior catalysts compared with the binary compounds.
2 Experimental
Tungsten sulphoselenides, WS x Se2−x (x = 0, 0.5, 1, 1.5, 2.0), were synthesized by the solid-state reaction of a stoichiometric mixture of tungsten (W) (99.99 + % Sigma Aldrich) with sulphur (S) (99.99% Sigma Aldrich) and selenium (Se) (Sigma Aldrich) of 99.99% purity. The mixture was loaded in a high-quality quartz tube (12 mm OD, 10 mm ID, 22 cm length) and sealed under vacuum at 10−6 Torr. The sealed tube was introduced into a furnace and slowly heated to 850°C for over 15 h. The quartz tube was kept at the same temperature for 48 h and cooled slowly to room temperature. The products were also characterized by X-ray diffraction (XRD) using a Bruker D8 Advance X-ray diffractometer (Cu K α ; λ = 1.54 Å). Raman spectra were recorded with 514 nm Argon laser using a Jobin-Yvon Labram HR spectrometer. The scanning electron microscope (SEM) images were taken using a NOVA nano-FESEM. The BET surface area measurement was calculated by N2 adsorption using a Quantachrome Autosorb instrument at 77 K.
WS x Se2−x samples (x = 0.0, 0.50, 1.0, 1.5, 2.0) were dispersed in a solution of triethanolamine (TEAO, 15% v/v) in water. To this dispersion 0.014 mM of Eosin Y dye was added and the system purged with N2. Hydrogen evolution studies were carried out with a 100 W halogen lamp under constant stirring. The evolved gas was analysed over a period using a Perkin Elmer 580 C Clarus GC-TCD.
Turnover frequency (TOF) was calculated using the equation
3 Results and discussion
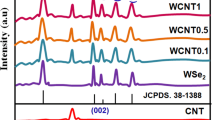
XRD patterns confirm the hexagonal 2H-structure for all the WS x Se2−x compositions, with a prominent peak due to the (002) reflection (figure 1a). There is a shift in the reflection to lower angles with increasing substitution of sulphur by selenium (figure 1b). Typical SEM images shown in figure 2a and b show the flake-like morphology of the samples. The lateral size of the flakes is ∼10 μm, while the thickness is < 1 μm. The composition of the samples was ascertained by energy-dispersive spectra (EDS).
Raman spectra, recorded using a 514 nm unpolarized Ar laser with backscattered geometry (figure 3a), show two prominent first-order modes E2g and A1g modes at 358 and 423 cm−1, respectively. The other first-order mode due to the longitudinal acoustic mode appears as a small hump at 177 cm−1. WSe2 shows a prominent band at 253 cm−1 due to the out-of-plane second-order mode of the longitudinal 2LA mode at 251 cm−1, which overlaps with the E\(^{1}_{2\mathrm {g}}\) mode [22–24]. With the increase in Se content of WS x Se2−x , a red-shift is observed in the A1g band but the E2g band shows no significant change (figure 3b). Disorder in the crystallites induces some broadening of the Raman peaks [25,26].
BET surface areas of WS x Se2−x composition are in the 4–6 m2 g−1 range with type-III isotherm (figure 4). Catalytic activities of these compounds would, therefore, be independent of the surface area. Dye-sensitized photochemical reaction was performed using TEAO as a sacrificial agent with Eosin Y as a photosensitizer. Eosin Y, on the absorption of photon, undergoes a transition from a singlet to a lowest lying triplet state (EY3∗) through intersystem crossing. EY3∗ takes up an electron from TEAO and gets converted to EY−. EY− is highly reactive and transfers the electron to the hydrogen evolution site [8,9]. The activities in the first 3 h of WS2 and WSe2 were 691 and 1732 μmol h−1 g−1 of hydrogen with TOF of 0.17 and 0.59 h−1, respectively. The activities of the solid solutions of WS1.5Se0.5, WS1.0Se1.0 and WS0.5Se1.5 compounds were 880, 1567 and 1202 μmol h−1 g−1 with TOF of 0.24, 0.36 and 0.38 h−1, respectively (figure 5a). The activity of the catalyst increases with further addition of the dye, where activities of WS2, WS1.5Se0.5, WS1.0Se1.0, WSe0.5 S 1.5 and WSe2 are 866, 955, 2339, 1491 and 1480 μmol h−1 g−1 with TOF of 0.21, 0.26, 0.70, 0.47 and 0.51, respectively. The activity of bulk WSe2 (1732 μmol h−1 g−1) is slightly higher than that of WS1.0Se1.0 (1567 μmol h−1 g−1) in the first 3 h but decreases after 3 h. The activity of WSSe is 2339 μmol h−1 g−1, whereas it is 1480 μmol h−1 g−1 for WSe2 (figure 5a and b). However, we observe a
decrease in the activity of WSe2 on further addition of dye after 3 h, suggesting that it will not be an optimal choice as a long-term catalyst. Figure 5b and c and table 1 show comparative activities and TOF of these compounds, where we can clearly see the enhanced hydrogen evolution (2–3 times higher than that from WS2) of the solid solution with a maximum at WS1.0Se1.0, which indicates synergistic effect. The introduction of selenium into WS2 induces a slight distortion in the structure because of the larger radius of selenium as compared with S. This could facilitate bond breaking of the molecules adsorbed on the basal plane. The basal plane with unsaturated bonds provides favourable hydrogen binding energy from the tensile regions on the surface, with active sites mainly located in the basal plane [27,28]. Previous studies of alloys of Se, S and W suggest that distortions can make the surface active and thus provide more sites for hydrogen samples compared with the binary compounds [29–31]. Hydrogen evolution was stable for 5 cycles (over a period of 15 h) as shown in figure 6a. There was no change in the sample before and after the HER (figure 6b and c).
4 Conclusions
In summary, we have synthesized layered binary solid solution of WS2 and WSe2 by a sealed tube reaction and characterized it appropriately. Visible-light dye-induced photocatalytic study indicates higher hydrogen evolution studied in WS x Se2−x compared with the parent binary counterparts, showing maximum yield at WSSe. The result suggests a synergistic effect of substitution of Se in the HER activity of the solid solutions.
Highlights
-
1.
Bulk synthesis of ternary transition metal dichalcogenides, tungsten sulphoselenide
-
2.
Composition-dependent X-ray diffraction patterns of sulphoselenide of tungsten
-
3.
Composition-dependent Raman
-
4.
Photochemical hydrogen evolution
-
5.
Role of anion substitution in hydrogen evolution of tungsten sulphoselenides.
References
Chhowalla M, Shin H S, Eda G, Li L, Loh K P and Zhang H 2013 Nat. Chem. 5 63
Rao C N R, Maitra U and Waghmare U V 2014 Chem. Phys. Lett. 609 172
Rao C N R, Matte H S S R and Maitra U 2013 Angew. Chem. Int. Ed. 52 13162
Rao C N R and Maitra U 2015 Annu. Rev. Mater. Res. 45 29
Radisavljevic B, Radenovic A, Brivio J, Giacometti V and Kis A 2011 Nat. Nanotechnol. 6 147
Late D J, Liu B, Matte H S S R, Dravid V P and Rao C N R 2012 ACS Nano 6 5635
Chen J, Wu X-J, Yin L, Li B, Hong X, Fan Z, Chen B, Xue C and Zhang H 2015 Angew. Chem. Int. Ed. 127 1226
Maitra U, Gupta U, De M, Datta R, Govindaraj A and Rao C N R 2013 Angew. Chem. Int. Ed. 52 13057
Gupta U, Naidu B S, Maitra U, Singh A, Shirodkar S N, Waghmare U V and Rao C N R 2014 APL Mater. 2 092802
Late D J, Huang Y K, Liu B, Acharya J, Shirodkar S N, Luo J, Yan A, Charles D, Waghmare U V, Dravid V P and Rao C N R 2013 ACS Nano 7 4879
Lauritsen J V, Kibsgaard J, Helveg S, Topsoe H, Clausen B S, Laegsgaard E and Besenbacher F 2007 Nat. Nanotechnol. 2 53
Jaramillo T F, Jørgensen K P, Bonde J, Nielsen J H, Horch S and Chorkendorff I 2007 Science 317 100
Eng A Y S, Ambrosi A, Sofer Z, Simek P and Pumera M 2014 ACS Nano 8 12185
Xie J, Zhang J, Li S, Grote F, Zhang X, Zhang H, Wang R, Lei Y, Pan B and Xie Y 2013 J. Am. Chem. Soc. 135 17881
Liu N, Kim P, Kim J H, Ye J H, Kim S and Lee C J 2014 ACS Nano 8 6902
Faber M S and Jin S 2014 Energy Environ. Sci. 7 3519
Chettri M, Gupta U, Yadgarov L, Rosentveig R, Tenne R and Rao C N R 2015 Dalton Trans. 44 16399
Merki D, Vrubel H, Rovelli L, Fierro S and Hu X 2012 Chem. Sci. 3 2515
Kiran V, Mukherjee D, Jenjeti R N and Sampath S 2014 Nanoscale 6 12856
Xu C, Peng S, Tan C, Ang H, Tan H, Zhang H and Yan Q 2014 J. Mater. Chem. A 2 5597
Zhang X, Meng F, Mao S, Ding Q, Shearer M J, Faber M S, Chen J, Hamers R J and Jin S 2015 Energy Environ. Sci. 8 862
Cong C, Shang J, Wu X, Cao B, Peimyoo N, Qiu C, Sun L and Yu T 2014 Adv. Opt. Mater. 2 131
Berkdemir A, Gutierrez H R, Botello-Mendez A R, Perea-Lopez N, Elias A L, Chia C-I, Wang B, Crespi V H, Lopez-Urias F, Charlier J-C, Terrones H and Terrones M 2013 Sci. Rep. 3 1755
Elias A L, Perea-Lopez N, Castro-Beltran A, Berkdemir A, Lv R, Feng S, Long A D, Hayashi T, Kim Y A, Endo M, Gutierrez H R, Pradhan N R, Balicas L, Mallouk T E, Lopez-Urias F, Terrones H and Terrones M 2013 ACS Nano 7 5235
Nakamura K, Fujitsuka M and Kitajima M 1990 Phys. Rev. B 41 12260
Gouadec G and Colomban P 2007 Prog. Cryst. Growth Charact., Mater. 53 1
Voiry D, Salehi M, Silva R, Fujita T, Chen M, Asefa T, Shenoy V B, Eda G and Chhowalla M 2013 Nano Lett. 13 222
Somorjai G A 2002 Topics Catal. 18 158
Wang F, Li J, Wang F, Shifa T K A, Cheng Z, Wang Z, Xu K, Zhan X, Wang Q, Huang Y, Jiang C and He J 2015 Adv. Funct. Mater. 25 6077
Xu K, Wang F, Wang Z, Zhan X, Wang Q, Cheng Z, Safdar M and He J 2014 ACS Nano 8 8468
Fu Q, Yang L, Wang W, Han A, Huang J, Du P, Fan Z, Zhang J and Xiang B 2015 Adv. Mater. 27 4732
Acknowledgements
We would like to sincerely thank Prof C N R Rao, FRS, for his constant guidance, support and encouragement. We would also like to thank Sonu K P for BET measurements. DSN is thankful to DST, Government of India, for the award of the post-doctoral fellowship for nanoscience and nanotechnology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
GUPTA, U., NARANG, D.S. Visible-light-induced hydrogen evolution reaction with WS x Se2−x . Bull Mater Sci 40, 329–333 (2017). https://doi.org/10.1007/s12034-017-1377-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-017-1377-1