Abstract
Surface modification is necessary for titanium implants since it is unable to induce bone apposition. The beneficial effects of boron on bone formation, composition and physical properties make it suitable as a coating material. In the present study, surface properties of boron nitride (BN) coating on titanium implants were evaluated. Twenty-four implants and 12 abutments were coated with BN by RF-magnetron sputtering system. ATR–FTIR measurements were conducted to assess surface chemistry and morphology of BN-coated implants. Adhesion tests were performed by CSM nanoscratch test device to determine adhesion of BN to titanium surface. Surface profilometry and atomic force microscopy (AFM) was used to evaluate surface roughness. Mean roughness values were calculated. Contact angle measurements were done for evaluation of wettability. Surface characterization of coated implants was repeated after RF power of the system was increased and voltage values were changed to evaluate if these settings have an impact on coating quality. Three different voltage values were used for this purpose. Hexagonal-BN was determined in FTIR spectra. RF-coating technique provided adequate adherence of BN coatings to the titanium surface. A uniform BN coating layer was formed on the titanium implants with no deformation on the titanium surface. Similar roughness values were maintained after BN coating procedure. Before coating, the contact angles of the implants were in between 63∘ and 79∘, whereas BN coated implants’ contact angles ranged between 46∘ and 67∘. BN-coated implant surfaces still have hydrophilic characteristics. The change in voltage values seemed to affect the surface coating characteristics. Especially, the phase of the BN coating was different when different voltages were used. According to our results, BN coating can be sufficiently performed on pretreated implant surfaces and the characteristics of BN coated surfaces can be changed with the change in parameters of RF-magnetron sputtering system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Titanium is the most commonly used implant material due to its good mechanical properties and biocompatibility, good resistance to corrosion, no cell toxicity and very weak inflammatory response in peri-implant tissues [1]. Despite titanium biocompatibility, a positive modulation of biological process is limited because titanium is unable to induce bone apposition (osseoinduction) [2]. Furthermore, the biological process at the bone–implant interface are slower on smooth surfaces, so titanium surface treatment is carried out to minimize the healing time [3] and also to improve surface properties of titanium and increase bone–implant contact area.
Different titanium implant surface coatings have been evaluated in literature, such as plasma-sprayed hydroxyapatite (HA), titanium plasma spray (TPS) and fluoride. However, these coated surfaces have some disadvantages. HA coating has such problems as a thick coating layer, coating delamination, cohesive and adhesive failures and porosity of the coating layer [4]. In the absence of loading and during the insertion of TPS implants, the detachment of small titanium granules occurs. The loosening of particles during or after implantation endangers the safe application of rough coatings [5]. Although implants treated with fluorine has shown very successful clinical results, more research is still needed to enhance understanding of how fluoride promotes fast osseointegration [2].
Boron, a dark brown metalloid, and its derivatives have lots of biological effects on body such as stimulation of wound healing in vivo, release of growth factors and cytokines and increase in the extracellular matrix turn-over [6].
Benderdour et al[7] have used fibroblasts to investigate the role of boron in wound repair and found that it is not toxic on fibroblasts. Boron also stimulated some other cells such as endothelial cells involved in the formation of new blood vessels, angiogenesis occurs soon after boron treatment in vivo[7].
A topical boron-based compound (AN0128) has anti-inflammatory and anti-bacterial activities and can reduce alveolar bone loss in periodontitis by decreasing the inflammatory infiltrate. It has shown in vitro activity against several bacteria associated with periodontal diseases. Inhibition of lipopolysaccharide (LPS)-induced tumour necrosing factor (TNF)- α release from human monocytes, and thus, this compound exhibits both antibacterial and anti-inflammatory activities [8].
Boron nitride (BN), one of the boron derivatives, has several equilibrium polymorphs: such as cubic and hexagonal boron nitride. It has been utilized as a coating material for cutting tool applications due to its excellent mechanical and chemical properties. It is also used in cutting tools to shape and machine titanium implants [9]. When shaping and machining processes completed, BN particles remain on implants which cannot be removed after washing the implants with water. Thus, the implant inserted bone is exposed to BN and the residual BN particles on implant surfaces did not affect cell survival [9].
BN coating on cutting tools with RF-magnetron sputtering was successfully accomplished recently by our group [10]. In the present study, BN coating on titanium was aimed with the previous study protocol. In the light of the existing literature, this is the first report attempting to coat the titanium surface with BN by RF-magnetron sputtering. The coatings on titanium surfaces prepared from different commercially available implants were evaluated for adhesion, roughness and wettability. Moreover, different parameters were evaluated to increase the quality of the coating.
2 Materials and methods
Twelve sand blasted-acid etched (SLA) surface implants (StraumannⓇ, CH 4002, Basel, Switzerland), 12 abutments (StraumannⓇ, CH 4002, Basel, Switzerland) and 12 titanium implants produced for experimental purposes (XiveⓇ, Dentsply, Mölndal, Germany) were used in this study.
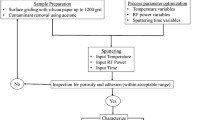
2.1 Preparation of implants for BN coating and BN-coating procedure
The implants were fixed to the aluminum moulds with melted bismuth. Then, the implants were ground and polished to obtain smooth surfaces (figure 1). They were also cleaned by ultrasonic method using nanocleans (detergent) and chemicals (acetone, isopropyl alcohol and ethyl alcohol). BN-coating procedure was performed in VaksisⓇ RF-magnetron sputtering system (PVD Hard-Coater, Vaksis R&D and Engineering, Ankara, Turkey) (figure 2). The RF-magnetron sputtering system consisted of a physical vapour deposition system and some auxiliary devices. RF-magnetron was utilized for reactive sputtering. To obtain boron nitride (BN) coatings, RF-magnetron was used with a hexagonal boron nitride target plate (hBN). A rectangular-shaped sintered hBN target plate had dimensions of 100 × 250 × 5 mm and was employed as a cathode. Before the coating process, holders were designed to hold the implants in the vacuum chamber. To ensure a homogeneous coating, designs of holders were carried out according to the shapes of moulds. The holders were also cleaned by ultrasonic method using nanocleans and chemicals. The system was then cleaned and general maintenance was carried out. After cleaning, moulds were attached to a substrate holder connected to a spindle mechanism which can be biased by a second RF power supply. Substrates can make either rotary or planetary motion. Also, the rotation speed and direction can be controlled. Turning speed is ∼25–30 rev min−1. The coating was carried out with sputtering technique under high vacuum. After substrates were attached to a substrate holder, the vacuum chamber was evacuated to a base pressure lower than 10 −2 Torr using a mechanical vacuum pump. At that time, hot water was circulated around the system for the evaporation of water molecules in the environment. When vacuum reached 3 × 10 −3 Torr, the turbo molecular pump was started. After the vacuum chamber is evacuated to 2 × 10−5 Torr, system was filled with Ar gas and plasma cleaning was performed with 250 W RF for ∼10 min. Then, the system was filled with N2 gas. The ratio of gases (Ar:N2) was 5:1. The flow rate of the working gases was adjusted by controllers installed on the system panel. During the deposition, magnetron power applied to the target plate was increased up to 700 W RF gradually and maintained at that value till the end of deposition. Heater temperature was measured by a thermocouple which was attached to heaters. Heater temperature was varied between 200∘ and 300∘C. Throughout the experiment, water was passed through the system. The growth of the films continued for 6 h for every experiment. Whole procedure was continued for ∼7 h.
2.2 Surface characterization of Ti implants
Attenuated total reflectance–Fourier transform infrared spectroscopy (ATR–FTIR) measurements, adhesion measurements and the analysis of the scratches by imaging were done on BN-coated Ti implants, whereas both roughness and wettability measurements were conducted on the uncoated and BN-coated implants to determine the effect of BN coating on roughness and wettability. ATR–FTIR spectra were performed in absorbance arranged from 1700 to 700 cm−1 at a spectral resolution of 8 cm−1 and 32 scans (Bruker IFS55, Germany). All spectra measured were baseline corrected, smoothed and analysed using spectroscopy software (Grams Suite 9.1, Thermo Fischer Scientific, PA).
The quality of adhesion of BN films was evaluated by Micro Scratch TesterⓇ (CSM instruments, Switzerland). Loading was started from 0.5 N and progressively increased up to 30 N. Rockwell diamond indenter type with a radius of 100 μm was used. Two millimetres scratch was formed while indenter was moving at a speed of 4 mm min−1. Loading rate was 59.94 N min−1.
Scanning electron microscope (SEM) analyses (ZEISS EVO) were carried out for the analyses of scratched samples. The investigations were done both on scratches and the deformed regions near scratches. Coated surface analyses were done on etched metallographic specimens under 30 kV voltage and 2 nm working distance (WD) with LaB6 filament.
Roughness measurements were conducted using atomic force microscope (AFM, NanoSurf-Nanite-B Version 2.2 CSM) and profilometer (Taylor Hobson Precision Surtronic 25) before and after coating. AFM has a probe which does not damage the surface. A sensor tip mounted to the end of a small deflecting cantilever was brought into contact with the sample surface to be investigated. The sensor tip was moved across the surface in line scans. It produced a high resolution three-dimensional image of the surface. Also, the AFM software camera was used to check if the cantilever was at a correct distance to the surface. The measuring parameters used in the study were; the image size: 77 × 77 μm area, rotation: 0∘, time/line: 1 s, points/line: 512.
Topography-scan forward was taken cross section of scan which was in the centre of the scanned area. Profilometer instrument was used to determine the surface roughness and gives difference between the high and low points of a surface. The working principle of profilometer is that a diamond stylus is moved vertically in contact with a sample. Then, diamond stylus moved laterally across the sample for a specified distance and specified contact force. The results were evaluated using image plus software. The measuring parameters were; length of scrub: 4 mm; range: 100 μm.
Contact (wetting) angle measurements by sessile drop method were employed using a tensiometer (Attension Theta Lite Optical, Finland). All prepared uncoated and BN-coated Ti implant surfaces were tested with 0.25 μl droplet of distilled water at room temperature. At least four drops were placed on each surface and photographed during 3 s in which 36 images were captured. By an image program (OneAttension), drop images were analysed to know the average wetting angle.
After completion of the above mentioned coating and characterization procedures, RF power of the magnetron sputtering system was increased from 700 to 900 W and voltage values were changed to evaluate if these settings have an impact on coating quality. Then, three SLA surface implants (StraumannⓇ, CH 4002, Basel, Switzerland) were coated at these conditions. Three different voltage values (250, 100, 0 V) were used during coating process, then scratch test, FTIR, thickness and AFM measurements were performed on coated samples.
3 Results
3.1 Constant power (700 W) and voltage values
ATR–FTIR measurements in absorbance mode were conducted to assess surface chemistry and morphology of BN-coated Ti implants. Figure 3 demonstrates IR spectra of Straumann (S) Ti implant surface after BN coating. Almost same spectral pattern were also observed for BN coated Straumann abutment (SA) and Xive (X) Ti implant surfaces. They have absorption bands at around 1373 and 780 cm−1, can be attributed to characteristic peaks of hexagonal BN (h-BN). Another peak at around 920 cm−1 can be assigned to explosive BN (e-BN), which is a transition phase [11].
When adhesion measurements were performed, some critical load is determined; however, cracks were not observed during the analysis and good adhesions of the BN coatings were seen. These adhesions could be clearly seen in SEM images given for S implant in figure 4. In SEM images of the scratches, the traces occurring due to diamond indenter during scratch test were observed. However, these traces were not characterized as cracks. Similar SEM images were also obtained for all the samples.
The alteration in surface roughness of the samples was evaluated to assess the roughness changes after deposition of the coatings. Both two- (Ra and Rms) and three-dimensional (Sa and Sq) measurements were obtained. Ra (Sa for 3D) is the arithmetic mean deviation of profile (Ra) or a surface (Sa). Rms (or Rq) (Sq for 3D) is the root mean squared deviation of a profile (Rms) or a surface (Sq). Sq gives almost the same information as Sa but is slightly more sensitive to high peaks and low valleys [12].
The roughness values as measured by AFM and profilometer showed that these surfaces were smooth surfaces. The difference between the uncoated and coated Ti implants were found in the range of 0.11–3.44 μm, which indicated that BN coating did not alter the surface roughness. Each uncoated and coated implants show similar roughness values measured by profilometer as seen in table 1.
Similar results were obtained for AFM values (table 2). In this study, the surface roughness values attained by AFM showed that the roughness values are in the range of 2.09–2.80 nm after BN-coating even though the implants have either higher (9.47 nm) or lower (1.03 or 1.65 nm) roughness values or different grades of titanium (low grade for X, high grade for S) beforehand. Large differences were not seen between uncoated and coated samples in terms of implant type. AFM images showing the surface roughness are provided for uncoated S implant in figure 5 and BN-coated S implant in figure 6a and b.
Contact angle measurements with distilled water were carried out to investigate surface hydrophobicity or hydrophilicity of implants. Table 3 listed contact angle of each Ti implant group before and after BN coating. Before BN coating, the contact angles of the implants were between 63∘ and 79∘, whereas with BN coating, all the implants contact angles ranged between 46∘ and 67∘. The photographs of contact angle measurements before and after BN coating were shown in figures 7 and 8. This showed that although BN coating is thin, it helped to obtain a hydrophilic surface with a comparable contact angle.
3.2 Constant power (900 W) and different voltage values
3.2.1 Scratch test
Tests were performed with 0.5–30 N load. Critical load for start of the scratch was 1.56 N when the RF system was 900 W, 100 Vb + 0 Vb and 3 N for 900 W, 0 Vb.
3.2.2 ATR–FTIR
In the first implant surface (900 W, 250 Vb), the thickness of the coating is below 25 nm, sufficient intensity could not be obtained for deconvolution of the curve and the respective analysis could not be made.
There were multi-phase structures in the surface of the second implant (900 W, 100 Vb + 0 Vb). High amounts of amorphous-BN (a-BN) and cubic-BN (c-BN) together with BN and e-t-BN phase exist in the coating (figure 9). In the third implant surface (900 W, 0 Vb), large amount of turbostratic-BN (t-BN) and explosive-BN (e-BN) phases are observed (figure 10).
The dependence of allotropes on substrate RF voltage is shown in figure 11.
3.2.3 Thickness measurement
Optical thickness measurement results performed by F20 device is as follows.
Coating thickness measurement could not be detected in the first implant (900 W, 250 Vb) because it was under 25 nm.
Thickness of second implant was measured as 3345 nm (900 W, 100 Vb + 0 Vb), while coating thickness of third implant was 3292 nm (900 W, 0 Vb).
3.2.4 AFM
The surface measurements conducted for the first implant (900 W, 250 Vb) were Ra: 0.03 μm, Rq: 0.04 μm; Ra: 0.019 μm and Rq: 0.032 μm for the second implant (900 W, 100 Vb + 0 Vb) and Ra: 11.5 μm, Rq: 16.6 μm for the third implant (900 W, 0 Vb).
4 Discussion
The implant surface is very important to obtain osseointegration and to increase the survival time of the implants. The quality of the surfaces can be significantly improved by modifying the surfaces through the application of a coating. The present study was the first that demonstrated the boron nitride coating on titanium implants by RF-magnetron sputtering method and evaluated the surface properties of that coating.
Boron is an essential micronutrient of organisms and plays an important role in osteogenesis and in the maintenance of bone [13]. Gorustovich et al[14] evaluated in vivo biocompatibility of bioactive glass modified by boron and found that the implantation of boron-modified particles induced a rise in bone formation in the marrow of rat tibia. Boron was reported to regulate mRNA expression in mineralized-tissue associated proteins and was a dose-dependent regulator on osteoblastic cells [15]. The use of boron to induce bone formation in tissue engineering makes it reasonable as a coating material. Recently, an environmentally friendly method of BN coating on cutting and forming materials has been introduced by Kaftanoğlu et al[10]. The similar method was used in the present study to coat BN on titanium surfaces.
The surface quality of the titanium implants will depend on its chemical, physical, mechanical and morphological properties. These properties are also very important to obtain good osseointegration and increase in long-term success.
In this study, FTIR spectra were obtained to observe the presence of BN on Ti implants. In the spectra, peaks around 1373 and 780 cm−1 assigned to h-BN were determined. Ansaloni et al[16] presented similar results in which the asymmetric bonds were at 1380 and 775 cm−1. Vilcarromero et al[17] determined the absorption band around 1380 and 780 cm−1, which are related to hexagonal phase of the BN samples. BN phases seem to depend on the power and voltage of RF system. When different voltage values tested, different phases of BN occurred (figure 11).
There are no in vitro or in vivo experiments are present in the literature concerning the BN films as a coating on dental implants. The present study demonstrated that implant surfaces can be coated successfully with boron nitride by magnetron sputtering technique. The differences in implant trademark and titanium grade were not seemed to affect the success of BN coating on surfaces.
The coating method used in this study – RF-magnetron sputtering – has advantages like lower deposition temperature, possibility to deposit thinner coatings and to deposit sharp edges and complex forms [18]. The possibility of delamination of coating from the surfaces was thought to be eliminated with this method. The increased thickness of a coating leads to an increased chance of coating accumulating residual stresses. The accumulation of residual stresses in any coating has been shown to weaken its structure, making it more vulnerable when forces are applied [19]. Furthermore, the fracture of the coatings that occurs in and along the interface of the coating during implant placement and during load-bearing process are major problems [19]. The good adherence of the coating is critical for the success of dental implant therapy. SEM images showed good adhesion of BN coating of the samples. When scratches were evaluated, BN coating seems to bond tighter to the titanium implants. In addition, when magnetron power increased and different voltage values tested, it was observed that the applied power and voltage could affect the coating adherence.
When the surface area of implants increase by roughening process, greater bone-to-implant contact can be provided than smooth surfaces. In the present study, as grinded, polished and then coated implants were evaluated by profilometer, they exhibited low roughness (Ra) values before (0.92–11.95 μm) and after (1.64–15.39 μm) coating. Al-Radha et al[20] reported Ra values between 0.043 to 0.15 μm in different surfaces which were ground and then polished. On the other hand, Jung et al[21] reported higher roughness values for the HA-coated implant that was 1.83 ± 0.06 μm. In a consensus report, it was suggested that the moderately rough and rough surfaces provided enhanced bone integration compared with smooth and minimally rough surfaces [22].
The type of titanium (its hardness) affects the roughness obtained in the treatment [23]. The same treatment can produce different roughness patterns in different grades of titanium [24]. BN coating on Si substrates by RF-magnetron sputtering was studied by Lousinian et al[25]. This study showed that BN films are ultra-smooth with Rms values around 0.3 nm obtained by AFM. The difference between the AFM and profilometer results is that AFM measures the surface roughness area and profilometer measures the line roughness. To sum up the roughness results of profilometer and AFM, it can be concluded that BN coating on titanium surfaces did not change the roughness of surface considerably. However, the change in the power and the voltage of the system seemed to have an effect on roughness parameters. It can be suggested that the coating was thin and uniform so that the original surface roughness was maintained [21].
Surface wettability can directly influence biological process between bone and implant. Hydrophilicity have a pivotal role in the primary action of an implant with a physiological environment [26]. The angle of an intersection of a line tangent to the liquid and the surface of the solid where it contacts, is the contact angle that affects wettability of the surfaces. A low contact angle indicates good wettability, whereas a high contact angle results in poor wettability [27]. The contact angles of different implants can range from 0∘ to 180∘. Contact angle values lower than 90∘ are considered as hydrophilic and above 180∘ are considered as hydrophobic. Mekayarajjananonth et al[27] indicated that the surface preparation of implants affected wettability. Commercially pure Ti implants exhibit contact angles vary between 24∘ to 97∘[28]. On the other hand, Elias et al[3] reported that the contact angles of machined, acid-etched, sandblasted and anodized surfaces were 85.20, 94.24, 79.86 and 47.25, respectively. In a study done by Lousinian et al[25], the contact angle measurements of BN films ranged between 55∘ and 71∘. In this study, contact angle measurements ranged between 63∘ and 79∘ for uncoated samples and 46–67∘ for coated samples. Thus, all coated implants demonstrated hydrophilic surface properties. As seen from table 3, the S implants had similar contact angles when coated with BN, but X implant had lower contact angle. This can be due to the chemical composition of the implant (low-grade titanium).
BN coating on titanium implant surfaces was successfully done in this study for the purpose of osteoblastic activity induction properties of boron. The limitations for the study were attributed to the difficulties in characterization of surfaces with the form of implant threads. BN coating is thought to have promising clinical application in dental implants. A thin and homogeneous coating on samples was achieved. We did not expect to change the surface characteristics of implants with BN coating, thus maintaining the previous surface characteristics after coating procedure was thought to be a success.
5 Conclusion
In vitro analysis of BN coating on titanium surfaces encourage us to test those coatings in in vivo conditions. Further, the implants will be aimed to be coated with BN, while they will be in a rotating position in the RF-magnetron sputtering system. Besides, surface characteristics of BN coating can be improved by the change of power and energy of the magnetron sputtering system. Investigations on the implants in terms of application and clinical implications are required.
References
Franchi M, Bacchelli B, Giavarasi G et al 2007, J. Periodontol. 78 879
Avila G, Misch K, Galindo-Moreno P et al 2009, Implant Dent. 18 17
Elias C N, Oshida Y, Lima J H et al 2008, J. Mech. Behav. Biomed. Mater. 1 234
Yeo I S, Han J S and Yang J H 2008 J. Biomed. Mater. Res. B Appl. Biomater. 87 303
Franchi M, Bacchelli B, Martini D et al 2004, Biomaterials 25 2239
Dzondo-Gadet M, Mayap-Nzietchueng R, Hess K et al 2002, Biol. Trace Elem. Res. 85 23
Benderdour M, Hess K, Dzondo-Gadet M et al 1998, Biochem. Biophys. Res. Commun. 246 746
Luan Q, Desta T, Chehab L et al 2008, J. Dent. Res. 87 148
Koga K, Kaji A, Hirosaki K et al 2006, Toxicol. In Vitro 20 1370
Kaftanoğlu B and Dökmetaş N 2014 Int. J. Sustain. Manufac. 3 143
Yu L, Gao B, Chen Z et al 2005, Chinese Sci. Bull. 50 2827
Wennerberg A and Albrektsson T 2009 Clin. Oral Impl. Res. 20 172
Nielsen F H 2004 Biofactors 20 161
Gorustovich A A, López J M, Guglielmotti M B et al 2006, Biomed. Mater. 1 100
Hakki S S, Bozkurt B S and Hakki E E 2010 J. Trace Elem. Med. Biol. 24 243
Ansaloni L M S and de Sousa E M B 2013 Mater. Sci. Appl. 4 22
Vilcarromero J, Carreno M N P and Pereyra I 2000 Thin Solid Films 373 273
Cesur H and Kaftanoglu B 2009 5th International Conference and Exhibition on Design and Production of Machines and Dies/Molds. 18–21 June, Aydin, Turkey
Vasanthan A, Kim H, Drukteinis S et al 2008, J. Prosthodont. 17 357
Al-Radha A S, Dymock D, Younes C et al 2012, J. Dent. 40 146
Jung U W, Hwang J W, Choi D Y et al 2012, J. Periodontal. Implant. Sci. 42 59
Lang N P and Jepsen S 2009 Clin. Oral Implants Res. 20 228
Rosa M B, Albrektsson T, Francischone C et al 2012, Appl. Oral Sci. 20 550
Elias C N and Meirelles L 2010 Expert Rev. Med. Devices 7 241
Lousinian S, Kalfagiannis N and Logothetidis S 2009 Solid State Sci. 11 1801
Stadlinger B, Ferguson S J, Eckelt U et al 2012, Br. J. Oral Maxillofac. Surg. 50 74
Mekayarajjananonth T and Winkler S 1999 J. Oral Implantol. 25 230
Gittens R A, Scheideler L, Rupp F et al 2014, Acta Biomater. 10 2907
Acknowledgements
We thank BOREN Competence Centre for Boron Coatings and Metal Forming Center of Excellence at ATILIM University, for the support of experimental investigations.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
TThis research was supported by The Scientific and Technical Research Council of Turkey (TUBITAK) Research Grant, with the project number of 111S111.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
GÖKMENoĞLU, C., ÖZMERIÇ, N., ÇAKAL, G. et al. Coating of titanium implants with boron nitride by RF-magnetron sputtering. Bull Mater Sci 39, 1363–1370 (2016). https://doi.org/10.1007/s12034-016-1273-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-016-1273-0