Abstract
In this study, photodegradation of the non-biodegradable azo dye acid orange 7 (AO7) was selected as modal target in aqueous solution using graphene–TiO 2 (GR–TiO2) hybrid nanocomposite, was well investigated and elucidated. The crystal phase, special surface area, microscopic analysis of the GR–TiO2 and also, chemical state of the photocatalysts were studied by powdered X-ray diffraction (PXRD), Raman spectrum, field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), high resolution scanning electron microscopy (HRTEM), X-ray photoelectron spectroscopy (XPS), Brunauere Emmette Teller (BET) method and photoluminescence spectroscopy (PL). During photocatalysis, the GR–TiO2 nanocomposite improved photocatalytic performance compared with that of pure TiO 2 towards AO7 organic azo-dye degradation. The reusability test of photocatalytic activity was also examined. A conceivable reaction mechanism was suggested and nattered on the basis of tentative effects. Therefore, the GR–TiO2 nanomaterial can be widely used as a photocatalyst for treating the organic dye contaminant in the field of environmental protection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Graphene is considered to be a marvellous material and plays a vital role among all the members of the carbon group [1]. Since the invention of CNTs and C60, graphene is used in the applications of electronics and in the field of materials science [2]. Graphene has many super natural properties like high electrical conductivity, greater mechanical elasticity, huge surface area, high carrier transport mobility due to atomically dense and 2D sheet composed of sp 2-bonded carbon atoms ordered in a honeycomb structure [3]. When used to modify supplementary materials, graphene can retard the recombination of electron–hole pairs that are photochemical or electrochemical [5–7]. For this reason, graphene–supported semiconductor materials are considered as photocatalysts for degradation of pollutants as well as photocatalytic water splitting [8,9]. TiO2 is a non-toxic and excellent photocatalyst material which is used for the degradation of environmental pollutants among all the semiconductors [10]. TiO2–carbon nanocomposite material shows high photocatalytic activity compared with the bare TiO2. However, TiO2 has a high band gap (3.2 eV) and absorb little amount of solar light, about 5% in the ultraviolet region can be used [11]. Therefore, to enhance the activity of the solar light utilization, the progress of photocatalyst with improved efficiency under extended range of visible light irradiation is necessary. TiO2 very well combined with carbon-based materials to enhance photocatalytic efficiency and to overcome these type of problems [12]. Graphene–TiO2 nanocomposite is reasonable to predict great potential and more interfacial contact, hence considered as a advantageous material to improve the photocatalytic activity of TiO2. Water-soluble organic dye, acid orange 7, consists of azo functional group (–N=N–), acts as carcinogenic and causes severe health problems to the lives of people [13]. Photocatalysis is very cheap cost and ecofriendly method to degrade organic dyes [14–16]. From recent investigations, graphene–TiO2 composites were successfully fabricated and applied for photocatalysis. Muthirulan et al [17,18]demonstrated the fabrication of titanium dioxide (TiO2)–graphene (GR) (TiO2–GR) nanocomposites for acid orange 7 (AO7) degradation under UV irradiation. Liang et al [19] communicated that a hybrid material of graphene–TiO2 for Rh-B degradation. Hsieh et al [20] proposed that degradation of AO7 dye under visible light using Pt-TiO2/graphene nanocatalysts. However, very little work has been conducted on the photocatalytic activity of GR–TiO2 photocatalyst to acid orange 7. The purpose of this paper is fabrication and exploration of the potential of a hybrid material specifically graphene–TiO2 for the degradation of the AO7 dye under solar light. The decomposition kinetics and mechanism of the photocatalysts were also studied.
2 Experimental
2.1 Synthesis of graphene oxide
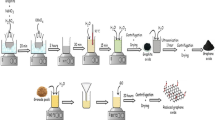
Graphene oxide was synthesized by the modified Hummers method [21]. In a typical procedure, about 5 g of graphite flakes were added to 115 ml of concentrated (98%) H2 SO 4 in an ice bath with constant stirring for 30 min. A 15 g of KMnO4 was added slowly to the above mixture with stirring and cooling for 30 min. Further, 2.5 g of NaNO3 was mixed with continuous stirring for 1 h, so that the temperature of the mixture was maintained below 288 K during the process. The temperature of mixture then raised to 313 K with water bath, and the mixture was stirred continuously for 30 min. Then, the mixture was diluted by 800–1000 ml of distilled water, then the temperature was raised to 371 K. Then H2 O 2 (30%) was added to the mixture until gas fruition was stopped, followed by filtering. The colour of the dispersion turned from black to yellow. The final reaction mixture was washed continuously with 1 M HCl (5%) and distilled water until the pH of the solution reached ∼7. Then, the washed compound was dried in vacuum oven at 333 K to obtain graphene oxide (GO). The chemical route synthesis is shown in figure 1.
2.2 Materials
Natural graphite flakes were commercially obtained from Sigma-Aldrich. In addition, titanium isopropoxide (TIP), sulphuric acid (H2SO4), potassium manganese oxide (KMnO4), sodium nitrate (NaNO3), sodium hydroxide (NaOH), hydrogen peroxide (H2 O 2), hydrochloric acid (HCl) and acid orange 7 dye, all these chemicals procured from the Merck and used without further refinement and ethanol was supplied by China Medicine Co. Ltd. The double-distilled water was used for the total process.
2.3 Synthesis of graphene–TiO 2 nanocomposite
To synthesize graphene–TiO2 nanocomposite, about 90 mg of as-prepared GO was spread in 60 ml of ethanol by ultrasonication for 1 h, then 1.5 ml of TIP was mixed into the suspension. The suspended mixture was stirred at room temperature for 30 min. Then, 15 ml of double-distilled water and 0.5 ml of HCl (1 M) was slowly added dropwise and the reaction mixture was stirred under N2 atmosphere at room temperature for 24 h. The product was then centrifuged and cleaned with double-distilled water to eliminate any remaining organic residue. Then, GR–TiO2 was dried at 353 K in a hot air oven.
2.4 Setting up of standard solution
Acid orange 7 was obtained from Merck and used without further refinement. 1000 mg l −1 solution was ready by dissolving suitable volume of dye in H2O and stored in dim-coated flask and diluted by adding required quantity of double-distilled H2O to the standard dye solution (according to necessity).
2.5 Characterization
The resulting materials were characterized by various techniques including powder X-ray diffractometry (PXRD) Bruker D8 using CuK α1 (1.5406 Å) and K α2 (1.54439 Å) radiations, Raman spectra were recorded using a WiTec alpha 200 SNOM system. Microanalysis of as-obtained materials were studied by a field emission scanning electron microscope (FESEM) imaging with energy dispersive spectroscopy (EDS) using a Carl Zeiss Supra 55 operating at 5 and 20 kV, structure analyses were conducted by a transmission electron microscope (TEM) and measurements were performed on a JEOL 3010 at 200 kV, ultraviolet–visible absorption spectra were recorded using Shimadzu-1800. X-ray photoelectron spectra (XPS) were noted down on a KRATOS AXIC 165 equipped with MgK α radiation. All binding energies were referenced to C 1s at 284.8 eV. N2 adsorption and desorption tests were conducted on Micrometrics ASAP 2020. The samples were outgassed at 150 ∘C for 12 h in a dynamic vacuum before physisorption measurements. The specific surface area was calculated using Brunauere Emmette Teller (BET) method. A photoluminescence spectrum (PL) was recorded by using JOBIN YVON Flurolog-3 spectrofluorometer with a YSOW Xenon lamp used as broad band source. The absorption of residual AO7 was resolute by visible spectrophotometer (Shimadzu-1800 UV–visible spectrometer) based on the absorbance at 483 nm wavelength. The pH of the solution was calculated by using digital Elico pH meter. Solar light intensity was checked by TES 1332A Lux meter.
2.6 Photocatalytic efficiency tests
Photocatalytic efficiency of the photocatalysts was esteemed by photodegradation of AO7 under solar light irradiation at 11.00 am and 1.00 pm in the summer season at Yogi Vemana University, Kadapa, Andhra Pradesh, India. The intensity of the solar light is found to be around one lakh flux. Organic dye solutions (30 ml, 20 mg l −1) containing 30 mg of catalysts were put in a closed round bottom flask. To ensure good absorption–desorption equilibrium, first sonicated for 5 min, and then stirred in dark condition for 30 min. For every regular time intervals, 3 ml of samples were taken in the presence of solar light irradiation. It is centrifuged and separated. This is recorded as base concentration C on. The absorption band maximum of supernatants in the AO7 dye was investigated by recording variations using a Shimadzu-1800 UV–visible spectrometer to calculate the optical absorption at 483 nm. This is denoted as C t. In the photocatalytic efficiency tests, the degree of degradation of the dye; in terms of the values of percentage degradation has been designed using the following correlation:
Here, C on is the initial concentration of the dye before solar light irradiation and C t is the final concentration of the dye at given time [17,18].
3 Results and discussion
3.1 XRD patterns
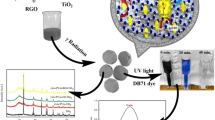
The crystallographic structure of the GR–TiO2 nanocomposite, TiO2, graphite oxide and graphite was evidenced by XRD measurements. Figure 2a exhibits intense crystalline peak at 26.46 ∘. This i denoted as graphite. After oxidation, the peak shifts to 9.92 ∘, which corresponds to graphite oxides. It is found from figure 2d that the peaks at 2 𝜃 values of 25.47, 29.46, 38.13, 44.67, 45.18, 63.19 and 69.96 ∘ which can be indexed to (101), (004), (200), (105), (211), (204) and (116) from the graphene–TiO2 which was relatively the same as that from anatase TiO2 crystal planes (figure 2c) (JCPDS file no: 89-4921) [22]. Due to the low amount of graphene, there is no characteristic diffraction peak observed at XRD pattern.
3.2 Raman spectroscopy analysis
The presence of both carbon and TiO2 can be confirmed from the Raman spectra. Figure 3 shows the Raman spectra of graphite, graphite oxide, TiO2 and GR–TiO2 nanocomposites. From figure 3d, Raman spectra clearly seen as vibration peaks at 397 (B1g), 518 (A1g) and 637 cm−1 (E1g) which are characteristics of anatase TiO2[23]. The D band represents the sp3 defects in carbon, and the G band is plane vibrations of sp 2 bonded carbons. The D and G bands intensity ratio typically reveals the order of defects in graphene [24]. Raman spectrum of GR–TiO2 showed the presence of D and G bands at 1364 and 1589 cm−1, respectively (figure 3d). In comparison to GO (I D/ I G = 0.99) and GR–TiO2 (I D/ I G = 1.02), the increased I D/ I G peak intensity ratio is in the GR–TiO2. This demonstrates the strong interaction between graphene and TiO2.
3.3 Morphology and EDS study
Figure 4 shows the FESEM images of as-prepared GR–TiO2 which possesses sphere morphology. Energy dispersive spectroscopy (EDS) proved the presence of carbon and TiO2 in those materials and is shown in figure 4c. These spectra indicate TiO2 with high purity which have been successfully synthesized in this study. The C element should mainly originate from graphene. The oxygen and Ti element mainly come from the TiO2. Figure 4d shows Ti element of strong K α at 4.51 KeV and K β peak at 4.92 keV, while a moderate K α peak of the O element at 0.52 keV and also listed about the samples elemental microanalyses (wt%). Figure 5 shows the GR–TiO2 catalyst TEM images and it can be observed that spherically polycrystalline TiO2 nanoparticles with average facet of 3–6 nm are supported on the translucent graphene (figure 5a and b). Hence, in this structure, the graphene acts as a platform for TiO2 components. Figure 5b shows the SAED pattern of the graphene–TiO2 material, in this, lattice spacing of TiO2 is 3.5 Å. As shown in figure 5c and d, HRTEM images evidently illustrate the crystalline nature of the GR–TiO2 nanocomposite.
3.4 XPS exploration
XPS was analysed to the surface chemical formation of the hybrid materials. The XPS survey spectrum of graphene–TiO2 clearly presents the photoelectron peaks of Ti, O and C, confirming the existence of GR in the sample (figure 6a) in graphene–TiO2. From the panel b, it can be observed that 285 eV, which may be assigned to the elemental carbon, carbonates and Ti–O–C bond. The corresponding XPS spectrum of the two well-determined peaks at 461 and 467 eV were observed from the Ti 2p core-level spectrum (figure 6b), which can be assigned to Ti 2p 1/2 and Ti 2p 3/2 spin orbital components in TiO2, respectively. For the O 1s electrons (as shown in figure 6d), sample exhibits a strong peak at around 533.5 eV corresponding to the Ti–O bond [24].
3.5 BET and PL spectra study
The specific surface area of the prepared graphene–TiO2 nanocomposites calculated by the multipoint BET method. Surface area of GR–TiO2 (162.3 m 2 g −1) was lower than that of GO (186 m 2 g −1) due to the high density and low surface area of TiO2 polycrystalline nanoparticles (figure 7a). PL emission spectrum is useful to disclose the efficiency of charge carrier catching, immigration and distribution. The excitation wavelength of catalyst is 320 nm. In the literature, it was reported that PL of TiO2 and 0.3% GR–TiO2 composite were in the range of 350–550 nm and the electron–hole recombination rate was lower for GR–TiO2-based composites (figure 7b).
3.6 Photocatalytic activities and photodegradation mechanism
Figure 8a shows photocatalytic skills of altered samples irradiated with solar light. The adsorption of these solutions decreased gradually with irradiation time. The characteristic adsorption of AO7 almost disappeared after 60 min, and the dye of AO7 solution changed gradually from golden yellow to colourless. The GR–TiO2 shows the degradation of AO7 removal is 99%, TiO2 removal is 47%, GR removal is 20% and mechanical mixing of GR + TiO2 is 66%, suggesting that GR–TiO2 is active catalyst. Figure 8b shows the absorption curves progressively with rising exposure time from 0 to 60 min. Figure 8c shows the optimum value of GR–TiO2 catalyst (0.3% GR) exhibited even better photocatalytic performance than that of P25 under solar light. Pure TiO2 rate constant, k = 0.065 min −1 and 0.3% GR–TiO2 nanocomposite rate constant, k = 0.112 min −1. Therefore, the degradation efficiency of GR–TiO2 composites is slightly two times higher than that of bare TiO2. The photocatalyst reusability was important property for effective practical applications. Three successive batch runs of the AO7 degradation reaction were conducted by using the recycled catalysts under the tested reaction conditions. The next run was carried out by recovering the catalyst from the above reaction mixture and was washed with double-distilled water and dried. The recycling test results of the most active GR–TiO2 catalyst, represented by AO7 decolorization, are shown in figure 8d. After the third run, the AO7 removal was still about 92% at 60 min, demonstrating the satisfactory reusability of the catalyst. Figure 9 shows the degradation mechanism of photocatalyst, where the photo-generated electrons transferred to excited state of conduction band (CB) from valance band (VB). Therefore, photo-generated holes are created on VB of TiO2 spheres [25]. Subsequently, the photo-generated electrons will get transferred to graphene, which thoroughly contact on the surface of TiO2 polycrystal spheres. Effective charge separation of photo-generated electrons and holes were maintained by graphene. Reactive oxygen species were formed by reaction of trapped electrons in graphene with the dissolved oxygen. Owing to this, decreased electron–hole recombination occurred. TiO2 surface contains photo-generated electrons stucked directly by the dissolved oxygen to give reactive oxygen species, which react with water to give hydroxyl radicals, which is a strong oxidant towards degradation of AO7 [26–28]. A schematic illustration of the mechanism of improved photocatalytic efficiency of the GR–TiO2 nanocomposites is shown in figure 9. The degradation mechanism was exposed as follows:
(a) The evaluation of degradation of AO7 at different catalysts such as GR, TiO2, mechanical GR + TiO2 under solar light irradiation. (b) The display of UV–Vis spectra for AO7 solution during the photocatalysis process with GR–TiO2. (c) Photocatalytic degradation rate constant (k) of AO7 for the photocatalysts. (d) Re-stability of GR–TiO2 over AO7 dye.

4 Conclusions
Fabrication of graphene–TiO2 nanocomposite has been facile synthesized by a chemical process. The FESEM, TEM and HR-TEM show that the GR–TiO2 nanoparticles were randomly anchored onto the graphene sheets. Photodegradation activity tests exposed that GR–TiO2 material shows superior catalytic efficiency for AO7 dye compared to bare TiO2, GR and mechanical mixture GR + TiO2. This signifies that the electron transfer between GR and TiO2 will greatly hinder the recombination of photo-induced charge carriers and extends the lifetime of electrons, which keeps in the improvement the lifetime of electrons of photocatalytic performance. GR–TiO2 composites also show a superior stability due to the cycling tests. Therefore, solar light-sensitive catalyst facilities are used for environmental applications.
References
Stankovich S, Dikin D A, Dommett G H B, Kohlhaas K M, Zimney E J, Stach E A et al 2006 Nature 442 282
Geim A K and Novoselov K S 2007 Nat. Mater. 6 183
Khalid N R, Ahmed E, Hong Z, Sana L and Ahmed M 2013 Curr. Appl. Phys. 13 659
Lee J S, You K H and Park C B 2012 Adv. Mater. 24 1084
Xiang Q, Yu J and Jaroniec M 2012 Chem. Soc. Rev. 41 782
Xiang Q and Yu J 2013 J. Phys. Chem. Lett. 4 753
Zhang N, Zhang Y H and Xu Y J 2012 Nanoscale 4 5792
Yang M Q and Xu Y J 2013 Phys. Chem. Chem. Phys. 15 19102
Yang M Q, Zhang N, Pagliaro M and Xu Y J 2014 Chem. Soc. Rev. 43 8240
Habisreutinger S N, Schmidt-Mende L and Stolarczyk J K 2013 Angew. Chem. Int. Ed. 52 2
Khan M M, Ansari S A, Pradhan D, Ansari M O, Han D H, Lee J et al 2014 J. Mater. Chem. A 2 637
Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V and Firsov A 2004 Science 306 666
Kryukova G N, Zenkovetsa G A, Shutilova A A, Wilde M, Günther K, Fassler D et al 2007 Appl. Catal. B Environ. 71 169
Zhang J, Wang P, Sun J and Jin Y 2014 ACS Appl. Mater. Interf. 6 19905
Perreault F, Fonseca de Fariaa A and Elimelech M 2015 Chem. Soc. Rev. 44 5861
Yuan L, Yu Q Q, Zhang Y H and Xu Y J 2014 RSC Adv. 4 15264
Muthirulan P, Nirmala Devi C and Meenakshi Sundaram M 2014 Ceram. Inter. 40 5945
Muthirulan P, Nirmala Devi C and Meenakshi Sundaram M 2014 Adv. Mater. Lett. 5 163
Liang Y Y, Wang H L, Casalongue H N S C, Chen Z and Dai H J 2010 Nano Res. 3 701
Hsieh S H, Chen W J and Wu C T 2015 Appl. Surf. Sci. 340 9
Hummers W S and Offeman R E 1958 J. Am. Chem. Soc. 80 1339
Lui G, Liao J Y, Duan A, Zhang Z, Fowler M and Yu A 2013 J. Mater. Chem. A 1 12255
Akhavan O, Ghaderia E and Rahimi K 2012 J. Mater. Chem. 22 23260
Kim J, Khoh W H, Wee B H and Hong J D 2015 RSC Adv. 5 9904
Sun L, Zhao Z, Zhou Y and Liu L 2012 Nanoscale 4 613
Gu L, Wang J, Cheng H, Zhao Y, Liu L and Han X 2013 ACS Appl. Mater. Interf. 5 3085
Zhang H, Guo L H, Wang D, Zhao L and Wan B 2015 ACS Appl. Mater. Interf. 7 1816
Venkata R P, Viswadevarayalu A, Janardhan R K, Varada R A and Adinarayana R S 2016 Korean J. Chem. Eng. 33 456
Park C Y, Kefayat U, Vikram N, Ghosh T, Oh W C and Cho K Y 2013 Bull. Mater. Sci. 36 869
Acknowledgements
The present work was economically supported by University Grant Commission, New Delhi (Grant no. 2-362/2013 (SR), and instrumental analysis supported by the University of Hyderabad, IIT Madras, Sri Venkateswara University, Tirupati and IICT Hyderabad is gratefully acknowledged. We are also thankful to Dr R V Jayanth Kasyap, Asst. Professor in English, Yogi Vemana University, Kadapa, India, for verifying the linguistic correctness.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
POSA, V.R., ANNAVARAM, V. & SOMALA, A.R. Fabrication of graphene–TiO2 nanocomposite with improved photocatalytic degradation for acid orange 7 dye under solar light irradiation. Bull Mater Sci 39, 759–767 (2016). https://doi.org/10.1007/s12034-016-1215-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-016-1215-x