Abstract
Donor-doped lead-free Bi0.7Ba0.3(Fe0.7Ti0.3)1−x Nb0.66x O3 + 1 mol% MnO2 ceramics were prepared by a conventional oxide-mixed method and the effects of Nb-doping on microstructure, piezoelectric and ferroelectric properties of the ceramics were investigated. All the ceramics exhibit a pure perovskite structure with rhombohedral symmetry. The grain growth of the ceramics is inhibited after the addition of Nb doping. High electric insulation (R = 109–1010 Ω⋅cm) and the poor piezoelectric performance and weak ferroelectricity are observed after the addition of Nb2O5 in the ceramics. Different from the donor effect of Pb-based perovskite ceramics, the introduction of Nb into 0.7BiFeO3–0.3BaTiO3 degrades the piezoelectricity and ferroelectricity of the ceramics. The Bi0.7Ba0.3(Fe0.7Ti0.3)1−x Nb0.66x O3 + 1 mol% MnO2 ceramic with x = 0 exhibits the optimum piezoelectric properties with d 33 = 133 pN C−1 and k p = 0.29 and high Curie temperature (TC = 603∘C).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lead-based piezoelectric ceramics with a perovskite structure, represented by Pb(Zr,TiO3) (PZT) and PZT-based multi-system, have been widely used in transformers, sensors, actuators and other electromechanical devices due to their high piezoelectric response. However, the use of these lead-based ceramics has caused serious environmental problems because of the strong toxicity of lead oxide and its high vapour pressure during sintering process. Therefore, lead-free ceramics have attracted considerable attention in recent years.
As a classic single-phase multiferroic material, BiFeO3 (BFO) has attracted much attention due to its coexistence of ferroelectricity and ferromagnetism [1]. It has a rhombohedrally distorted pervoskite structure of R3c space group and possesses high ferroelectric Curie temperature (T C) of ∼830∘C and an antiferromagnet Néel temperature (T N) of ∼370∘C [2]. Therefore, BiFeO3 material may have potential applications in various advanced devices [1,3]. However, it is well known that pure BiFeO3 ceramic has a large electric leakage due to the reduction of Fe ions from Fe3+ to Fe 2+ during the sintering process [4]. In addition, low resistivity usually causes lower magnetoelectric effect [3,5]. The semiconducting property of BiFeO3 ceramic leads to high dielectric loss and poorly saturated ferroelectric hysteresis loops. Moreover, single-phase BFO with pure perovskite structure is difficult to synthesize due to the narrow temperature range of phase stabilization, and thus some impurity phases (e.g., Bi2Fe4O9, Bi25FeO40, etc.) are usually detected [1,4,6]. Hence, to enhance electric insulation and inhibit the formation of impurity phase of BFO, some effective methods such as A-site or B-site ions substituted by other ions (e.g., La3+, Mn4+, Ni2+, etc.) [7–9] and form solid solution of BiFeO3 with other ABO3 perovskite structure materials (e.g., BaTiO3 and Bi0.5K0.5TiO3, etc.) have been developed [4,10]. Among these BFO-based materials, BiFeO3–BaTiO3 (BFO–BT) ceramic is considered as one of the most promising multiferroic systems [11,12]. From previous studies, Mn-modified BFO–BT ceramics show good piezoelectricity and temperature stability [13–15]. On the other hand, it is well known that donor doping is an important approach to enhance the piezoelectric properties of perovskite piezoelectric ceramics. Among donor ions, Nb5+ is most frequently used to improve the piezoelectricity of piezoelectric materials as a donor aid. For instance, the piezoelectric property of pure PZT (d 33 ∼ 240 pC N−1) is increased significantly after the doping of Nb (d 33 ∼ 385–640 pC N −1) [16–19]. However, to our knowledge, there is no report on the effect of Nb doping on the piezoelectric and ferroelectric properties of BFO–BT ceramics. Therefore, in this work, Nb-doped BiFeO3–BaTiO3 ceramics, Bi0.7Ba0.3(Fe0.7Ti0.3)1−x Nb0.66x O3+1 mol% MnO 2 (x = 0, 0.0025, 0.05, 0.075, 0.01, 0.0125, 0.015, 0.0175 and 0.02), were prepared by the solid-state reaction method and the phase structure, piezoelectric and ferroelectric properties of the ceramics were investigated.
2 Experimental
Ceramics of Bi0.7Ba0.3(Fe0.7Ti0.3)1−x Nb0.66x O3+1 mol% MnO 2 with x = 0, 0.0025, 0.05, 0.075, 0.01, 0.0125, 0.015, 0.0175 and 0.02 were fabricated in air by the conventional solid-state reaction. Stoichiometric amounts of Bi2O3 (99%), Fe2O3 (99%), BaCO3 (99%), TiO 2 (98.5%) were weighed and calcined at 800∘C for 4 h in a crucible. Subsequently, the target amount of Nb2O5 (99.99%) and MnO 2 (99.9%) additives were added after the calcination. The powders were pressed into pellets and sintered at 960∘C for 2 h. Both surfaces of the sintered ceramics fired silver electrodes at 650∘C for 30 min. The samples were pooled at 100–120∘C for 30 min and then cooled to room temperature in a silicone oil bath under a d.c. field of 5 kV mm −1.
The crystal structure was determined by use of an X-ray diffractometer with CuK α (λ = 1.540598 Å) radiation (Smart Lab; Rigaku, Tokyo, Japan), 0.01 ∘ scan step and continuous scanning type in the 2 𝜃 range of 20–70 ∘. The lattice parameters of the ceramics were refined by the Rietveld refinement using a general diffraction/reflectivity analysis programme MAUD [20]. The microstructure of the samples was examined by scanning electron microscopy (SEM, FEI-Quanta 250). The relative dielectric constant ε r and loss tangent tan δ at 1 MHz were measured as a function of temperature using an LCR meter (Agilent E4980A) and temperature controlled probe stage (Linkam TS1500E). The polarization–electric field (P–E) loops at room temperature were determined using a ferroelectric tester (Premier II, Radiant Technologies Inc.). The piezoelectric constant d 33 was measured using a piezo- d 33 meter (ZJ-6A, China). The planar mode electromechanical coupling coefficient k P was measured by the resonance method according to the IEEE Standards 176 using an impedance analyzer (Agilent 4294A).
3 Results and discussion
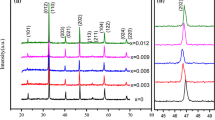
Figure 1 shows the room temperature XRD patterns of Bi0.7Ba0.3(Fe0.7Ti0.3)1−x Nb0.66x O3 ceramics. All ceramics exhibit pure perovskite phase and no second phases could be detected. As well known, the radius of Nb5+ (0.69 Å) is close to that of Ti4+ (0.60 Å) and Fe3+ (0.64 Å). Therefore, Nb5+ ions most likely enter the B sites and substitutes for Ti4+ or Fe3+, serving as donor-type dopant. The ceramic with x = 0 exhibits rhombohedrally distorted structure, which is in accordance with previous reports of the BFO–BT-x system with x < 33 mol% [21]. A general diffraction/reflectivity analysis programme MAUD was used to perform a full-pattern matching using the Rietveld method and refine the cell parameters. According to the rhombohedral structure (R3c), the compositional dependences of the lattice parameters (a, c and V) and fitting parameters (R wp and S) of the BFO–BT–Nb-x ceramics are shown in table 1. From table 1, for all the refinements, the values of the reliability R wp of 9.81–13.88% (<15%) and the goodness-of-fit indicator S of 1.27–1.83 (<2) are obtained, suggesting a good matching between the observed and calculated patterns. Diffraction peaks of all the ceramics can be indexed and refined to the rhombohedral structure R3c by the Rietveld method. The observed lattice parameters a, c and V remain the slight fluctuations of 5.6478–5.6511 Å 3/13.8207–13.8399 Å 3/382.0930–382.5182 Å 3. From figure 1b, diffraction angle has a slight shift to lower diffraction angel, indicating that the lattice distortion of the ceramics was dependent on the doping amount of Nb2O5.
Figure 2 shows the SEM graphs of the surface microstructure of the Bi0.7Ba0.3(Fe0.7Ti0.3)1−x Nb0.66x O3 ceramics with x = 0–0.02. From figure 2a, the ceramic without doping (i.e., x = 0) is well-crystallized with an average grain size D A of 4.22 μm and have clear grain boundaries. However, the grain sizes decrease significantly to 0.91 μm with x increasing to 0.02, indicating that the doping of Nb inhibits the grain growth of the ceramics, which may be ascribed to the concentration of doping ions near the grain boundaries and reduction of the grain-boundary mobility. This mechanism for reducing grain-boundary mobility has been studied theoretically as it applies to the recrystallization of metals [22,23]. Therefore, this inhibition of grain growth of the ceramics may be mainly attributed to the grain boundary aliquation [24,25]. Similar phenomenon of the suppression of grain growth induced by donor doping has been frequently observed in the ceramics such as La-modified K0.5Na0.5NbO3[26] and PZT [27], Nb-modified Pb(Ti,Zr)O 3[22] and BaTiO3–Bi(Mg 1/2Ti1/2)O3–BiFeO3 ceramics [24]. For the present ceramics, uniform grains and clearly demarcated grain boundaries are observed for bulk components as shown in figure 2a–g. However, the ceramics with x = 0.0175 and 0.02 sintered at 960∘C melt down with ambiguous grain boundaries due to the over high sintering temperature for the two components.
Figure 3a shows the leakage current density J of the Bi0.7Ba0.3(Fe0.7Ti0.3)1−x Nb0.66x O3 ceramics at room temperature as a function of the electric field E; while figure 3b gives the dependences of the resistivity R of the Bi0.7Ba0.3 (Fe0.7Ti0.3)1−x Nb0.66x O3 ceramics on the electric field E. As a comparison, the J and R of pure BiFeO3 prepared by an ordinary oxide sintering technique (sintered at 775∘C for 2 h) are also shown in figure 3. From figure 3a and b, all the ceramics with x = 0–0.02 exhibit the low J values of 10 −5–10 −4 A cm −2 and high R values of 10 9–10 10 Ω⋅cm, suggesting that the Bi0.7Ba0.3(Fe0.7Ti0.3)1−x Nb0.66x O3 ceramics with x = 0–0.02 possess much better electric insulation than pure BiFeO3 ceramics (J = 8.18×10−4 A cm−2, R = 2.62 × 107 Ω⋅cm). As well known, the poor electric insulation of pure BiFeO3 is due to the valence transformation of Fe ions from Fe3+ to Fe 2+ and the creation of oxygen vacancies for charge compensation during sintering [28]. The improvement in the electric insulation of the present ceramics may be ascribed to partial substitutions of Mn4+ and/or Ti4+ for Fe3+ in the ceramics which could suppress the formation of \(\text {Fe}_{\text {Fe}^{3+}}^{2+} \) and V Ö[29,30]. As a result, the high resistivity of the ceramics sintered at 960∘C for 2 h is obtained.
Figure 4a shows the polarization–electric field (P−E) hysteresis loops of the Bi0.7Ba0.3(Fe0.7Ti0.3)1−x Nb0.66x O3 ceramics measured at 6.0 kV mm −1, while figure 4b shows the variations of the remanent polarization P r and coercive field E c with x. The saturated ferroelectric hysteresis loops were observed at x = 0. However, as the Nb content increases, the loops become narrower, indicating the degradation of ferroelectric activity. From figure 4b, the values of the remanent polarization P r and coercive field E c are 19.68 μC cm −2 and 3.73 kV mm −1, respectively. However, P r decreases from 6.38 to 3.11 μC cm −2 and E c decreases from 4.53 to 2.41 kV mm −1 as x increases from 0.0025 to 0.02. Similar results about Nb-doping (Bi, Na)TiO3–BaTiO3 ceramics have been reported [31]. This is clearly different from the effect of donor ions on the ferroelectricity of Pb-based perovskite ceramics.
Figure 5 shows the variation of piezoelectric constant d 33, planar electromechanical coupling factor k p, relative dielectric constant ε r and loss tangent tan δ with x for the Bi0.7Ba0.3(Fe0.7Ti0.3)1−x Nb0.66x O3 ceramics. Similar piezoelectric properties were observed in Nb-doped PZT systems [32,33]. From figure 5a, it can be noted that the observed d 33 decreases significantly from 133 to 19 pC N −1 and k p decreases from 0.29 to 0.14 as x increases from 0 to 0.02, respectively. In general, Nb5+ plays a role as a donor to reduce the concentration of oxygen vacancies which was considered to be the origin of domain wall clamping, leading to an improvement in piezoelectricity and ferroelectricity of perovskite piezoelectric ceramics. However, after the addition of Nb, the present ceramics exhibit degradation in piezoelectricity and ferroelectricity. Further studies are needed to clarify the reason for the decrease in the d 33 of Bi0.7Ba0.3(Fe0.7Ti0.3)1−x Nb0.66x O3 ceramics, especially, the effect of oxygen vacancies and crystal structure on electrical properties of the ceramics. While the observed ε r and tan δ exhibit no obvious dependence on x as shown in figure 5b. From figure 5b, the observed dielectric constant increases significantly from 556 to 615 as x increases from 0 to 0.0025 and then increases slightly from 615 to 647 with x = 0.0025–0.0175. Finally, the observed dielectric constant decreases significantly to 569 as x further increases to 0.02.
It is known that the donor doping is the most important method for improving the piezoelectricity of the lead-based piezoelectric ceramics. Table 2 shows the dielectric, piezoelectric and ferroelectric properties of some donor-doped ceramics. Obviously, after donor doping, the d 33 of the lead-based ceramics (PZT, PSZT, PZN–PZT, etc.) [16–18,34]was improved significantly. However, the lead-free (KNN) ceramic [19] exhibit very slight enhancement after the donor doping. This result indicate that the donor doping for lead-based and lead-free ceramics exhibit different doping effects, which should be studied further. For present ceramics sintered at 960∘C for 2 h, the Nb doping leads to lower piezoelectric and weaker ferroelectric properties. This result may be ascribed to the decreases in grain size. As is well known, the ferroelectricity has an approximately positive relationship with the grain size and the correlation of domain size and grain size can be defined as (domain size) \(\propto \) (grain size) m (m is related to the grain size) [35,36]. Therefore, the domain boundary increases as grain size and domain size decrease and thus weaker polarization is obtained in the ceramics with small grains than in those with large grains [37].
Figure 6a–i shows temperature dependences of relative dielectric constant ε r and loss tangent tan δ at 1 MHz for the Bi0.7Ba0.3(Fe0.7Ti0.3)1−x Nb0.66x O3 ceramics, while the variation in Curie temperature T C of the Bi0.7Ba0.3(Fe0.7Ti0.3)1−x Nb0.66x O3 ceramics are shown in figure 6j. All the ceramics exhibit a dielectric peak at T C. From figure 6a–i, the Bi0.7Ba0.3(Fe0.7Ti0.3)1−x Nb0.66x O3 ceramic with x = 0 exhibit a sharp dielectric peak related to the normal ferroelectric to paraelectric transition at T C. However, the dielectric peaks become boarder after the addition of Nb, indicating that a diffuse phase transition is induced in the ceramics with high Nb level, which may attribute to the cation disorder and the compositional fluctuate of the ceramics caused by Nb doping [38]. From figure 6j, the observed T C increases from 578 to 603∘C with x increasing from 0 to 0.0025 and then decreases greatly to 503∘C with x increasing to 0.015, finally increases slightly from 509 to 513∘C with x further increasing from 0.0175 to 0.02. Obviously, a small amount of Nb doping (x = 0.0025) can improve the T C of the ceramics.
4 Conclusions
Lead-free Bi0.7Ba0.3(Fe0.7Ti0.3)1−x Nb0.66x O3+1 mol% MnO 2 ceramics with x = 0, 0.0025, 0.05, 0.075, 0.01, 0.0125, 0.015, 0.0175 and 0.02 were synthesized by conventional solid-state reaction method and the effect of the Nb doping on microstructure, ferroelectric and piezoelectric properties of the ceramics were studied. The grain growth of the ceramics is inhibited after the addition of Nb doping. High electric insulation (R = 109–10 10 Ω⋅cm) and the poor piezoelectric performance and weak ferroelectricity are observed with Nb2O5 addition in the ceramics. The Bi0.7Ba0.3 (Fe0.7Ti0.3)1−x Nb0.66x O3+1 mol% MnO 2 ceramic with x = 0 exhibits the optimum piezoelectric properties with d 33 = 133 pN C −1 and k p = 0.29 and high Curie temperature (T C = 603∘C).
References
Qin H B, Zhang H L, Zhang B P and Xu L H 2011 J. Am. Ceram. Soc. 94 3671
Prasatkhetragarn A, Muangkonkad P, Aommongkol P, Jantaratana P, Vittayakorn N and Yimnirun R 2013 Ceram. Int. 39 S249
Wang Q Q, Wang Z, Liu X Q and Chen X M 2012 J. Am. Ceram. Soc. 95 670
Patil D R, Lokare S A, Devan R S, Chougule S S, Kolekar Y D and Chougule B K 2007 J. Phys. Chem. Solids 68 1522
Varshney D, Kumar A and Verma K 2011 J. Alloys Compd. 509 8421
Azough F, Freer R, Thrall M, Cernik R, Tuna F and Collison D 2010 J. Eur. Ceram. Soc. 30 727
Zhang Q, Zhu X H, Xu Y H, Gao H B, Xiao Y J, Liang D Y et al 2013, J. Alloys Compd. 546 57
Basu S, Hossain S K M, Chakravorty D and Pal M 2011 Curr. Appl. Phys. 11 976
Jianguo Zhao, Xianghui Zhang, Shijiang Liu, Weiying Zhang and Zhaojun Liu 2013, J. Alloys Compd. 557 120
Matsuo H, Noguchi Y, Miyayama M, Suzuki M and Watanabe A 2010 J. Appl. Phys. 108 104103
Liu X H, Xu Z, Wei X Y, Dai Z H and Yao X 2010 J. Am. Ceram. Soc. 93 2975
Pandey D and Singh A 2009 Bull. Mater. Sci. 32 361
Wei Y X, Wang X T, Zhu J T, Wang X L and Jia J J 2013 J. Am. Ceram. Soc. 96 3163
Leontsev S O and Eitel R E 2009 J. Am. Ceram. Soc. 92 2957
Yang H B, Zhou C R, Liu X Y, Zhou Q, Chen G H, Li W Z and Wang H 2013 J. Am. Ceram. Soc. 33 1177
Fan G F, Shi M B, Lu W Z, Wang Y Q and Liang F 2014 J. Eur. Ceram. Soc. 34 23
Chen B H, Huang C L and Wu L 2004 Solid-State Electron. 48 2293
Zhuang Z Q, Haun M J, Jang S J and Cross L E 1989 IEEE Trans. Ultrason. Ferroelectr. Freq. Control 36 413
Taub J, Ramajo L and Castro M S 2013 Ceram. Int. 39 3555
Lutterotti L 2011 MAUD—material analysis using diffraction, http://www. ing.unitn.it/~maud/index.html
Yang H B, Zhou C R, Liu X Y, Zhou Q, Chen G H, Wang H and Li W Z 2012 Mater. Res. Bull. 47 4233
Pereira M, Peixoto A G and Gomes M J M 2001 J. Eur. Ceram. Soc. 21 1353
Atkin R B and Fulrath R M 1971 J. Am. Ceram. Soc. 54 265
Yabuta H, Shimada M, Watanabe T, Hayashi J, Kubota M, Miura K et al 2012, Jpn. J. Appl. Phys. 51 09LD04
Yi J Y, Lee J K and Hong K S 2004 Jpn. J. Appl. Phys. 43 6188
Gao D J, Kwok K W, Lin D M and Chan H L W 2009 J. Phys. Appl. Phys. 42 035411
Pdungsap L, Udomkan N, Boonyuen S and Winotaia P 2005 Sens. Actuat. A 122 250
Wu J G, Kang G Q and Wang J 2009 Appl. Phys. Lett. 95 192901
Chauhana S, Kumara M, Chhokera S, Katyal S C, Singh H, Jewariya M and Yadav K L 2012 Solid State Commun. 152 525
Lin D, Zheng Q J, Li Y, Wan Y and Zhou Q L W 2013 J. Eur. Ceram. Soc. 33 3023
Chen L, Luo B C, Chan N Y, Dai J Y, Hoffman M, Li S and Wang D Y 2014 J. Alloys Compd. 587 339
Kayasu V and Ozenbas M 2009 J. Eur. Ceram. Soc. 29 1157
Tanasoiu C, Dimitriu E and Miclea C 1999 J. Eur. Ceram. Soc. 19 1187
Kalem V, Cam I and Timucin M 2011 Ceram. Int. 37 1265
Randall C A, Kim N, Kucera J P, Cao W and Shrout T R 1998 J. Am. Ceram. Soc. 81 677
Cao W and Randall C A 1996 J. Phys. Chem. Solids 57 1499
Yan Y, Cho K H and Priya S 2011 J. Am. Ceram. Soc. 94 3953
Petnoi N, Bomlai P, Jiansirisomboon S and Watcharapasorn A 2013 Ceram. Int. 39 S113
Acknowledgements
This work was supported by the projects of Education Department of Sichuan Province (11ZA104), Science and Technology Bureau of Sichuan Province (2010JQ0046) and the Open Project of State Key Laboratory of Electronic Thin Films and Integrated Devices of University of Electronic Science and Technology of China (KFJJ201108).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
WU, X., LUO, L., JIANG, N. et al. Effects of Nb doping on the microstructure, ferroelectric and piezoelectric properties of 0.7BiFeO3–0.3BaTiO3 lead-free ceramics. Bull Mater Sci 39, 737–742 (2016). https://doi.org/10.1007/s12034-016-1198-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-016-1198-7