Abstract
Al2O3–40% TiO2 coating is deposited on Superni 601 and Superco 605 superalloys by low-velocity oxy-fuel (LVOF) process. LVOF sprayed coating is characterized for surface roughness, microhardness, scanning electron microscopy and X-ray diffraction analysis. Hot corrosion of the coated and uncoated superalloys have been evaluated in an aggressive environment of Na2SO4–82% Fe2(SO4)3 under cyclic conditions at temperatures of 800 and 900°C. The microhardness and surface roughness values of the as-sprayed coatings are found to be in the range of 742–946 Hv and 14.40–14.80 μm, respectively. Al2O3–40% TiO2 coating on both the superalloys has indicated protective behaviour during hot corrosion studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hot corrosion of heat transfer pipes and other structural materials in coal-fired boilers is recognized as one of the principle engineering problems in such installations.[1–3]Maintenance costs for replacing hot corroded pipes are very high and the downtime associated with the unscheduled breakdowns caused by failure of tubes and structures is a cause of lost revenue.[4,5] The hot corrosion of alloys usually occurs in the environment where molten salts such as sulphates (Na2SO4), chlorides (NaCl) or oxides (V2O5) are deposited onto the surface. The Na2SO4–Fe2(SO4) environment is found usually in the coal-fired boilers where the coal ash corrosion is induced by the deposition of complex iron-alkali sulphates, (Na,K)3Fe(SO4)3.[6,7]
Protective coatings on superalloys have made a greater contribution towards increased temperatures and protection against the environmental degradation.[8–10]The demand for the protective coatings has been in recent times increased for almost all types of superalloys, since high-temperature corrosion problem has become much more significant with the increase in operating temperatures of boilers, turbines and heat engines. The necessities for higher performance and increased efficiency have resulted in the progressive increase in their operation temperatures.[11–15]
Ceramics are widely applied as protective coating because of their excellent wear, corrosion and high temperature oxidation resistance.[16–18]Surface coatings of aluminium and titanium oxides are used to improve the resistances of materials against wear, erosion, cavitations, fretting and corrosion. Al2O3–TiO2 coating with varying proportions of Al2O3 and TiO2 are used in combustion chambers of diesel engines, cutting tools and other industrial applications. Al2O3 powder particles are stable in α-form but after thermal spraying they soften and transform into γ-form that decreases the mechanical properties but increases the indentation fracture toughness of coating. The toughness and hardness of Al2O3–TiO2 coating are influenced by the amount of TiO2 addition. With the increase in the amount of TiO2, the hardness of the whole coating decreases, the toughness increases and porosity declines. The adhesive strength of the ceramic coating with the bond coat and the substrate improves significantly with the addition of TiO2.[19–22]The composites of Al2O3 with TiO2 have shown better performance under various conditions than those of the individual oxides.[23,24]However, ceramic coatings show poor adhesion with the metallic substrate due to the difference in coefficient of thermal expansion between the ceramic coating material and the metallic substrate material.[25,26]It is necessary to deposit an interlayer (bond coat) between the substrate and ceramic top coating as high anisotropy occurs between the metallic substrate and ceramic top coat because of variation in the coefficients of thermal expansions of the two. This anisotropy can cause excessive stresses on the boundary of the coating and substrate.[27]The bond coat minimizes the stress at the substrate–coating interface and increases the adhesion strength of the coating.[28]
The low-velocity oxy-fuel (LVOF) technique involves a particle velocity lower than high-velocity oxy-fuel (HVOF) spraying but higher than plasma spraying. A flame temperature of LVOF spraying is considerably lower than plasma spraying but slightly higher than HVOF spraying. LVOF spraying is a low power consuming and thus a cost-effective method as compared to other thermal spraying techniques. The flame temperature for LVOF spraying is in the range of 2300–2500°C which makes it suitable for depositing ceramic coatings as ceramics have high melting point.[29–31]In the present investigation, the LVOF technique is used to deposit Al2O3–40% TiO2 coating on Superni 601 and Superco 605 superalloys. A bond coat of Ni–20 wt% Cr is deposited between the substrate and the Al2O3–40% TiO2 top coat as it minimizes the stress at the substrate coating interface and increases the adhesion strength of coating. The hot corrosion of the coated and uncoated superalloys have been evaluated at 800 and 900°C under cyclic conditions for 50 cycles of 1-h duration.
2 Experimental
2.1 Materials, coating formulation and characterization of coating
Nickel-based Superni 601 superalloy (Fe-13.20, Cr-22.95, Cu-0.01, Mn-0.81, Si-0.39, C-0.027, S-0.005, Ni–Bal) and cobalt-based Superco 605 superalloy (Fe-0.17, Ni-10.8, Cr-20.05, Mn-1.50, Si-0.07, C-0.85, Co–Bal) procured from Mishra Dhatu Nigam Limited, Hyderabad, India, have been selected as the substrate materials. The specimens measuring approximately 20 mm × 15 mm × 5 mm were cut, polished, and subsequently grit blasted with alumina particles of grit size 40 mesh just prior to deposition of the coating. Al2O3–40% TiO2 coating powder (H.C. Starck, Germany) of particle size 5–45 μm were deposited to approximately 250 μm thickness by LVOF process using CERAJET Spray nozzle. Ni–20 Cr powder was deposited to about 100 μm thickness before applying the top coating of 250 μm thickness of Al2O3–40% TiO2 coating. The process parameters for the LVOF coating and arc spray process employed for applying the coatings are summarized in table 1.
2.2 Characterization of coating
The as-sprayed Al2O3–40 TiO2 coating on Superni 601 and Superco 605 superalloys deposited by LVOF process has been characterized for surface roughness, microhardness, scanning electron microscopy (SEM) and X-ray diffraction (XRD) analysis. The surface morphology of the as-sprayed Al2O3–40 TiO2 coating was characterized by SEM 430 Model LEICA Electron Optix Unit, England). Digital Microhardness Tester (MMT-X7, Matsuzawa, Japan) was used to measure the microhardness values (average of ten indentations at each distance) of the coatings. Diamond point indenter with load of 500 g and dwell time of 10 s was used for the microhardness tests. Surtronic Taylor Hobson Precision Surface roughness tester (U.K.) was used to measure the surface roughness of the as-sprayed coatings. The average surface roughness (R a) value of the coating was measured using the centre line average method. XRD analysis was carried out using a Rigaku Desktop Miniflex-II X-Ray diffractrometer (Japan) employing Cu-K α radiation and Ni-filter.
2.3 Hot corrosion test
Hot corrosion tests were carried out at 800 and 900°C in the laboratory Kanthal wire tube furnace, which was calibrated using platinum–rhodium thermocouple with the variation of ± 5°C. The uncoated as well as coated samples were subjected to wheel cloth polishing, washed in acetone for removing any dirt and moisture content before hot corrosion tests. The samples were heated in an oven up to 250°C and the salt mixture of Na2SO4–82% Fe2(SO4)3 dissolved in distilled water was coated on the warm samples with the help of camel hair brush. The amount of the salt coating varies from 3.0 to 5.0 mg cm −2. The coated samples were then dried at 110°C for 3–4 h in the oven. For each experiment, the sample was kept in the alumina boat; weight of boat and sample was measured before keeping into the hot zone of the furnace at 800/900°C. All the alumina boats were pre-heated to 1000°C for 6 h with the assumption that their weight would remain constant during the cycles of study. The holding time in the furnace was 1 h and after 1 h the boat with sample was taken out and cooled at the ambient temperature for 20 min. Weight of the boat along with sample was measured and this constitutes one cycle. The hot corrosion studies were carried out for such 50 cycles.
3 Results and discussion
3.1 Characterization of coating
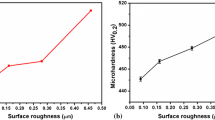
The surface roughness (R a) values of the as-sprayed coatings were found in the range of 14.40–14.80 μm. Surface morphologies for the LVOF sprayed Al2O3–40 TiO2 coating on Superni 601 and Superco 605 superalloy substrates are shown in figure 1. SEM micrographs as shown in figure 1a and b show that the coating consists of fully melted splats with some partially melted splats which is the typical characteristics of thermal sprayed ceramic coating. XRD analysis of the coating surfaces of the samples has indicated the formation of TiO2, Al2O3 and Al2Ti7O15 phases in the coating. The microhardness profile of Al2O3–40 TiO2 coating with bond coat of Ni–20 Cr coating is shown in figure 2. The microhardness value of the Al2O3–40 TiO2 coating is found to be in the range of 742–946 Hv. The microhardness value of Ni–20 Cr bond coat is found to be in the range of 552–616 Hv in this coating.
3.2 Hot corrosion of uncoated and Al 2 O 3 –40% TiO 2 -coated Superni 601 and Superco 605 superalloys at 800 and 900°C
Visual examination was made after each cycle to observe any change in colour, luster and spalling tendency. The colour of the scale for Superni 601 was light brown upto the fourth cycle and changed to dark brown with succeeding cycles during hot corrosion at 800°C. The colour of the scale for Superni 601 at 900°C was light brown upto 5th cycle and for rest of the cycles it was dark brown. The greyish scale with green spot appeared up to the 4th cycle in case of Superco 605 superalloy during hot corrosion at 800°C and later the colour of the whole surface changed to light brown. The colour of the scale for Superco 605 under hot corrosion environment at 900°C was greyish up to the 4th cycle and later converted to black colour.
The colour of Al2O3–40% TiO2-coated superni 601 hot corroded at 800°C was light green greyish, whereas the surface of Al2O3–40% TiO2-coated Superco 605 changed to brownish colour. The surface colour of Al2O3–40% TiO2-coated Superni 601 appeared brown when hot corroded at 900°C. A crack was observed in the scale of coated Superni 601 after 30th cycle. The surface of Al2O3–40% TiO2-coated Superco 605 appeared light green during the initial cycles at 900°C. During late cycles, black coloured scale appeared on the coated Superco 605 superalloy.
Figure 3 shows the weight gain per unit area against number of cycles plot for the uncoated and coated Superni 601 and Superco 605 superalloys subjected to hot corrosion at 800 and 900°C in Na2SO4–82% Fe2(SO4)3 environment for 50 cycles. The plots for all the uncoated superalloys at 800 and 900°C have shown negligible weight gain during the initial ten cycles followed by gradual weight gain during the subsequent cycles. Among the two superalloys, Superco 605 has shown slightly lower overall weight gain than Superni 601 at both the temperatures. Both the superalloys have shown higher weight gain at 900°C in comparison to that at 800°C. The weight gain for Al2O3–40% TiO2-coated Superni 601 superalloy during hot corrosion at 800°C increased sharply from 28th cycle onwards for the remaining cycles. Whereas the weight gain increase was gradual for the Al2O3–40% TiO2-coated Superni 601 superalloy during hot corrosion at 900°C. The weight gain for Al2O3–40% TiO2-coated Superco 605 superalloy was gradual from 11th to 40th cycle at 800 and 900°C and thereafter it increased sharply during the subsequent cycles. The weight gain is found unsteady during hot corrosion study of Al2O3–40 TiO2 coating. This may be due to the spallation/sputtering at various stages. The limitation of weight gain data is that it does not show the rate of material deterioration.
The overall weight gains after 50 cycles for the coated and uncoated Superni 601 and Superco 605 superalloys at 800 and 900°C are reported in table 2. The overall weight gains of Al2O3–40% TiO2 coating on Superni 601 and Superco 605 superalloys during hot corrosion at 800 and 900°C are compared with the overall weight gains of the same coating during oxidation at 800 and 900°C.[22,31]The overall weight gains from hot corrosion under the present study are found approximately four times higher than the overall weight gains from oxidation at similar temperatures.[22,31] The weight gain of the Al2O3–40% TiO2 coating deposited by LVOF method on Superni 601 and Superco 605 superalloys in the present study is also compared with weight gain of Al2O3–40% TiO2 coating deposited by the detonation gun method on Superni 718 and AE 435 superalloys during hot corrosion study at 900°C in Na2SO4–82% Fe2(SO4)3 environment for 50 cycles.[15] The weight gain of Al2O3–40% TiO2 coating deposited by the LVOF method in present study is found slightly higher than the weight gain of Al2O3−40% TiO2 coating deposited by the detonation gun method. The weight gain during hot corrosion of the Al2O3–40% TiO2 coating is found higher than the weight gains during oxidation of the same coating due to the presence of aggressive Na2SO4–82% Fe2(SO4)3 environment. The hot corrosion behaviour of Al2O3–40 TiO2 coating is compared with hot corrosion and oxidation behaviour of enamel–Al2O3 composite and Al2O3–TiO2 coatings as reported in table 3. The higher overall weight gain from the hot corrosion behaviour of Al2O3–40 TiO2 coating in the present study from the literature[31,32] might be attributed to the variation of Al2O3 and TiO2 content in the coating and environmental conditions.
From the plot of weight gain square data against the number of hot corrosion cycles (figure 4), it can be seen that the Al2O3–40 TiO2 coated superalloys have followed nearly parabolic rate law. The calculated values of the parabolic rate constants (K p) for the two superalloys and coating on these superalloys are also added in table 2. The parabolic rate constant K p[2,33,34]was calculated by a linear least-square algorithm to a function in the form of
where W/A is the weight gain per unit surface area (mg cm −2); K p the parabolic rate constant and t the exposure time at different temperatures.
3.3 Surface morphologies of the oxidized coatings
The SEM micrographs of the Al2O3–40 TiO2 coating on Superni 601 and Superco 605 superalloys after exposure at 800°C are shown in figure 5. The surface morphology of the coated Superni 601 after exposure to Na2SO4–82% Fe2(SO4)3 environment indicates the presence of spheroidal grains and pits over the surface as evident from figure 5a. The Al2O3–40% TiO2 coating on Superco 605 after exposure to Na2SO4–82% Fe2(SO4)3 environment shows some pits on the surface, as can be seen from figure 5b. Figure 6 shows the surface morphology of Al2O3–40% TiO2-coated Superni 601 and Superco 605 superalloys after hot corrosion at 900°C. Al2O3–40% TiO2-coated Superni 601 superalloy after hot corrosion at 900°C indicates the formation of cracks and pits (figure 6a). The surface morphology of coated Superco 605 after hot corrosion also depicts the rough surface with pits may be due to spallation (figure 6b). The Al2O3–40% TiO2 coating on both the superalloys have indicated the protective behaviour under hot corrosion study in the molten salt environment at 800 and 900°C may be due to the presence of protective Al2O3 phase in the coating.
4 Conclusions
Al2O3–40% TiO2 coating is successfully deposited on Superni 601 and Superco 605 superalloys by LVOF process. The Al2O3–40 TiO2-coated superalloys during hot corrosion study in the molten salt environment showed lower weight gain than the uncoated superalloys at 800 and 900 oC. The weight gain during hot corrosion study of Al2O3–40 TiO2 coating is found unsteady may be due to spallation/sputtering at various stages. The parabolic rate constants of Al2O3−40% TiO2-coated superalloys are also found lower than the uncoated superalloys during cyclic hot corrosion in molten salt environment. The Al2O3–40% TiO2 coating on both the superalloys have indicated the protective behaviour under hot corrosion study in the molten salt environment at 800 and 900°C may be due to the presence of protective Al2O3 phase in the coating.
References
Chawla V, Chawla A, Puri D, Prakash S, Gurbuxani P G and Sidhu B S 2011 J. Miner. Mater. Charact. Eng. 10 367
Singh H, Puri D and Prakash S 2005 Surf. Coat. Technol. 192 27
Tao K, Zhou X, Cui H and Zhang 2009, J. Trans. Nonferrous Met. Soc. China 19 1151
Sidhu T S, Prakash S and Agrawal R D 2006 J. Scr. Mater. 55 179
Mishra N K, Rai A K, Mishra S B and Kumar R 2014 Int. J. Corros. Article ID 146391 4 doi: 10.1155/2014/146391
Seong B G, Hwang S Y and Kim K Y 2000 Surf. Coat. Technol. 126 256
Kaur M, Singh H and Prakash S 2012 Miner. Met. Mater. Soc. ASM Int. 43A 2979
Stringer J 1987 Mater. Sci. Technol. 3 482
Gurrappa I 2003 Mater. Sci. Technol. 19 178
Maledi N B, Potgieter J H, Sephton M, Cornish L A, Chown L and Süss R 2006 Southern Afr. Inst. Min. Met. 81
Yoshiba M 1993 Corros. Sci. 35 1115
Stott F H, De Wet D J and Taylor R 1994 Trans. Mater. Res. Soc. Jpn. 14A 135
Conner J A and Connor W B 1994 J. Miner. Met. Mater. Soc. 35
Mishra S B, Chandra K and Prakash S 2013 Surf. Coat. Technol. 216 23
Mishra N K, Kumar N and Mishra S B 2014 Indian J. Mater. Sci. Article ID 453607 1 doi: 10.1155/2014/453607
Evan H F 2011 Surf. Coat. Technol. 206 1512
Guo Q Q, Jiang B L and Li J P 2010 Trans. Nonferrous Met. Soc. China 20 2204
Wang D S, Tian Z J, Shen L D, Liu Z D and Hua Y H 2009 Appl. Surf. Sci. 255 4606
Hazar H and Ozturk U 2010 Renew. Energy 35 2211
Sure J, Shankar and Mudali U K 2013 Opt. Laser Technol. 48 366
Zhang J, He J, Dong Y, Li X and Yan D 2008 J. Mater. Process. Technol. 197 31
Mishra N K and Mishra S B 2014 Corros. Eng. Sci. Technol. 49 705
Gao X S, Tian Z J, Liu Z D and Shen L D 2012 Trans. Nonferrous Met. Soc. China 22 2498
Varacalle J. D J, Herman H, Bancke G A and Riggs I. W. 1992, Surf. Coat. Technol. 54/55 19
Bhatnagar H, Ghosh S and Walter M E 2010 Mech. Mater. 42 96
Han J C 2007 Acta Mater. 55 3573
Kasparova M, Houdkova S and Cubrova J 2012 Thermally sprayed coatings for high temperature application. Metal 2012 (Brno, Czech Republic) EU: 1–6
Hiemann R B 1996 Plasma sprayed coatings—principles and applications (New York: VCH Publishers Inc.) 2nd ed
Rana N, Mahapatra M M, Jayaganthan R and Prakash S 2014 J. Alloys Comp. 615 779
Mishra N. K., Mishra S. B. and Kumar R. 2014, Surf. Coat. Technol. 260 23
Mishra N K, Mishra S B and Kumar R 2015 Surf. Eng. 31 249
Zheng D, Zhu S and Wang F 2006 Surf. Coat. Technol. 200 5931
Mahesh R A, Jayaganthan R and Prakash S 2008 J. Alloys Compd. 460 220
Kamal S, Jayaganthan R and Prakash S 2010 Bull. Mater. Sci. 33 299
Acknowledgements
We wish to thank the Mishra Dhatu Nigam Limited, Hyderabad, India, for supplying the superalloys; Metallizing Equipment Company Private Ltd. (MECPL), Jodhpur, India, for providing the facility of the LVOF coating.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MISHRA, N.K., MISHRA, S.B. Hot corrosion performance of LVOF sprayed Al2O3–40% TiO2 coating on Superni 601 and Superco 605 superalloys at 800 and 900°C. Bull Mater Sci 38, 1679–1685 (2015). https://doi.org/10.1007/s12034-015-0986-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-015-0986-9