Abstract
Blends of poly(ρ-dioxanone) (PPDO) and poly(L-lactic acid) (PLLA) in different proportions were prepared by solution co-precipitation. The nonisothermal crystallization behaviour of pure PPDO and PPDO/PLLA blends was investigated by differential scanning calorimetry. The Avrami, Ozawa and Mo models were used to analyse the nonisothermal kinetics. The addition of PLLA significantly increases the crystallization peak temperature and crystallinity of PPDO, but has little effect on crystallization half-time. The activation energies of crystallization were calculated using the Kissinger equation. The results suggest that PLLA plays two roles in the nonisothermal crystallization of PPDO; PLLA both promotes the crystallization of PPDO as a nucleating agent and meanwhile restricts the motion of PPDO chains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Poly(ρ-dioxanone) (PPDO) is a useful biomaterial due to its biodegradability and good mechanical properties.[1–14]It is already being used in some biological and medical applications, especially surgery repair materials such as sutures, bone repair devices, and drug delivery systems.[15–17]Like other aliphatic polyesters, however, it has a low crystallization rate and low melt strength. These characteristics of PPDO make it difficult to use in the production of thin clips and sutures by injection molding or extrusion molding, which hinders the development of PPDO as a promising future biomedical material.
Among the methods employed to enhance crystallization rates of polymers, introducing additives as nucleating agents is the most convenient and most frequently chosen strategy.[8,9,18–20]Poly(L-lactic acid) (PLLA), another linear aliphatic polyester, is considered to be one of the most promising materials because it can be made from agricultural products and is readily biodegradable. However, PLLA has poor flexibility, limiting its application. The blending of polymers has proven to be an excellent strategy for developing new materials; the blended compounds often exhibit combinations of properties superior to either of the individual components.[21,22]Compared to chemical modification, blending polymers represents a more cost-effective way to modify polymer properties.[23]
Blending PPDO with PLLA has been identified as a conceivable approach to combine the merits of these two polymers. Pezzin et al[24] and Pezzin and Duek[25]prepared blends of PPDO and PLGA by fusion and studied their properties. The interfacial damage properties of plasma-treated biodegradable PPDO fibre/PLLA composites were nondestructively evaluated by Park et al[26] using micromechanical testing and surface wettability measurements. To the best of our knowledge, all existing reports on PPDO/PLLA blends are related to their thermal, mechanical, morphological, interfacial and degradation properties; they do not include investigations of crystallization behaviour, one of the most dominant factors[27–29]that affects biomaterial properties and determines their applicability.
In this work, we report for the first time the effect of PLLA on the nonisothermal crystallization behaviour of PPDO. Blends of PPDO and PLLA in different proportions were prepared by solution co-precipitation. Several kinetic models were applied to analyse the crystallization kinetics, and the effective activation energy was calculated by the Kissinger equation. The investigation of the nonisothermal crystallization kinetic properties of PPDO/PLLA blends in this work contributes to the understanding of their crystallization behaviours.
2 Experimental
2.1 Materials
PPDO (Chengdu Organic Chemistry Company) was prepared by molten state ring-opening copolymerization in using stannous octoate as an initiator under high vacuum. PLLA (Chengdu Organic Chemistry Company) was prepared according to the same protocol under high purity nitrogen. The measured intrinsic viscosity of PPDO in hexafluoroisopropanol (Sigma Aldrich) was 2.01 dl g −1. The measured intrinsic viscosity of PLLA in chloroform (Guangdong Guanghua Chemical Factory Co. Ltd) was 2.63 dl g −1. Ethanol (Guangdong Guanghua Chemical Factory Company) and hexafluoroisopropanol were analytical grade and used as-received.
2.2 Blend preparation
Prior to use, PPDO and PLLA were dried under vacuum for 24 h at 30 ∘C. The mass ratios of PPDO to PLLA were fixed at 100/0, 95/05, 90/10, 85/15, 70/30 and 60/40. PPDO/PLLA composites were prepared by solution co-precipitation; PPDO was dissolved in hexafluoroisopropanol and added to a solution of PLGA in chloroform. The mixture was subjected to ultrasonic agitation for 4 h to ensure complete mixing. Excess ethanol was then added to the mixture to separate the composites from the solvent. Upon removal of the solvent, the PPDO/PLLA composites were obtained and dried to constant weights under vacuum at 30 ∘C.
2.3 DSC characterization
Differential scanning calorimetry (DSC) measurements (Q2000; American TA Company Instruments) were obtained under nitrogen atmosphere. The temperature was calibrated with indium. Samples (4–5 mg) were enclosed in aluminum pans; an empty aluminum pan was used as a reference. For nonisothermal analysis, samples were first held at 140 ∘C for 5 min to erase thermal history. The samples were subsequently cooled to −10 ∘C at cooling rates of 5, 15, 30 and 50 ∘C min −1, and the exothermic curves were recorded.
3 Results and discussion
3.1 Nonisothermal crystallization behaviour of PPDO/PLLA blends
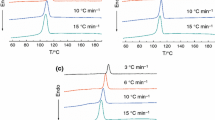
The nonisothermal crystallization exotherms of PPDO/ PLLA blends (100/0, 95/05, 90/10, 85/15, 70/30 and 60/40) at different cooling rates are presented in figure 1. The crystallization peak temperature (T p), onset temperature (T o), and crystallization enthalpy (ΔH C) of the blends are listed in table 1. A decrease in T o and T p along with the broadening of the exothermal peak is observed with increasing cooling rate, indicating that crystallization occurs at higher temperatures for lower cooling rates. This result can be explained by the fact that PPDO molecules are able to better arrange themselves at lower cooling rates; therefore, less supercooling is required to initiate the crystallization of PPDO at lower cooling rates.
Differential scanning calorimetry exothermal curves of nonisothermal crystallization with different cooling rates for PPDO / PLLA blends: (a) PPDO/PLLA100/0; (b) PPDO/PLLA95/05; (c) PPDO/PLLA90/10; (d) PPDO/PLLA85/15; (e) PPDO/PLLA70/30 and (f) PPDO/PLLA60/40, (1) cooling rate: 5 ∘C min −1; (2) cooling rate: 15 ∘C min −1; (3) cooling rate: 30 ∘C min −1; (4) cooling rate: 50 ∘C min −1.
The T o and T p of PDDO/PLLA blends are shifted to higher temperatures compared to those of pure PPDO, indicating that PLLA behaves as a nucleating agent for the crystallization of PPDO. Among the PPDO/PLLA blends with different proportions, the T p of the 85/15 blend is the highest. This can be attributed to two factors. First, the number of nucleation sites increases with increasing PLLA content, which facilitates the crystallization of PDDO. Second, increasing PLLA content introduces more steric hindrance, limiting the mobility of PPDO chains. On the other hand, the ΔH C of PPDO/PLLA blends are higher than that of pure PPDO for a given cooling rate, suggesting that PLLA enhances PPDO’s total crystallinity (X t):
where T 0 and \(T_{\mathrm {\infty }}\) represent the initial and end crystallization temperatures, respectively and dH/dT the heat flow rate. For nonisothermal crystallization, the time t and temperature T have the following relation:
where φ is the cooling rate and T the crystallization temperature at time t.
Figure 2 shows the changes in the relative crystallinity of PPDO/PLLA blends as a function of time. The crystallization half-time values t 1/2 of all blends are also listed in table 1. The addition of PLLA only slightly decreases the t 1/2 value of pure PPDO, which can be explained by the contradictory effects of the π– π interaction between PPDO and PLLA on polymer crystallization. On the one hand, the π– π interaction induces the orientation of PPDO chains along PLLA domains, prompting the crystallization of PPDO. On the other hand, the π– π interaction restricts the migration and diffusion of PPDO chains, suppressing PPDO crystallization. For solution crystallization processes, especially in dilute solutions, crystallization is dominated by nucleation processes because the diffusion of PPDO chains is weak. However, for melt crystallization processes, the contribution of nucleation to the total crystallization rate is greatly impaired by the restriction effect.
Plots of relative crystallization as a function of time (t) at different cooling rates for pure PPDO and PPDO/PLLA blends: (a) PPDO/PLLA100/0; (b) PPDO/PLLA95/05; (c) PPDO/PLLA90/10; (d) PPDO/PLLA85/15; (e) PPDO/PLLA70/30 and (f) PPDO/PLLA60/ 40, (1) cooling rate: 5 ∘C min −1; (2) cooling rate: 15 ∘C min −1; (3) cooling rate: 30 ∘C min −1; (4) cooling rate: 50 ∘C min −1.
3.2 Kinetic analysis by the Avrami model
The Avrami model for the analysis of the nonisothermal crystallization kinetics of PPDO/PLLA blends can be expressed by the following equations:[30,31]
where n is the Avrami exponent that depends on nucleation and growth during crystallization and K the temperature-dependent rate constant. Assuming a constant cooling rate φ in the nonisothermal process, Jeziorny[32] gave the corrected form of K as follows:
Figure 3 presents plots of ln[ −ln(1 −X t)] vs. ln t for PPDO/PLLA blends. The values of the Avrami exponent n and the rate constant K or K C are obtained from the slope and intercept of the linear region of these plots.[33] Because the middle portion of the curves represent 15–70% of the crystallization process, the n, K and K C values are adopted from these portions (table 1). The values of n range from 3.40 to 4.26 for pure PPDO and 2.47 to 5.48 for the blends. The n values are nonintegers that sometimes exceed 4, which suggests a complicated crystallization mechanism. The values of K C associated with nucleation and growth rates are comparable for pure PPDO and blends. They suggest that the incorporation of PLLA does not significantly change the nonisothermal crystallization rates of PDDO due to competition between the nucleation and restriction effects.
3.3 Kinetic analysis based on the Ozawa model
Considering the nonisothermal crystallization to be a cooling rate-dependent process, Ozawa[34] presented an extended Avrami model
where Z(T) is the crystallization rate constant and m the Ozawa exponent. Equation (6) can be rearranged into a logarithmic form
The plots of ln[ −ln(1 −X t)] vs. ln φ for pure PPDO and 95/05 PDDO/PLLA blends are shown in figure 4. A series of straight lines in figure 4 would indicate that the Ozawa model can correctly describe the nonisothermal crystallization kinetics, and Z(T) and m could be determined from the intercept and the slope, respectively. However, no such straight lines for pure PPDO and 95/05 blends are observed, meaning that the Ozawa model fails to effectively describe the nonisothermal crystallization of pure PPDO and polymer blends. The reason for this may be that secondary crystallization occurred as a melt crystallization processes, which is not considered in the Ozawa model.
3.4 Kinetic analysis based on the Mo model
Because both the Avrami and Ozawa models do not effectively describe the nonisothermal crystallization of polymer melts, the model of Liu et al,[35] which combines the Avrami and Ozawa models, was employed
where α is the ratio of the Avrami exponent n to the Ozawa exponent m and the parameter \(F\!\!\left (T \right )=\left [ {{Z\!\!\left (T \right )} / K} \right ]^{1 / m}\) indicates the cooling rate when the system reaches a certain degree of crystallinity in unit time. F(T) has a definite physical implication; higher values of F(T) correspond to slower crystallization rates. Plots of ln φ vs. ln t at different degrees of crystallinity are shown in figure 5. The linearity of these plots suggests that this improved model is valid for describing the nonisothermal crystallization of pure PPDO and PPDO/PLLA blends.
The parameter F(T) and α are listed in table 2; it can be seen that α values change little with crystallinity, while F(T) increases with increase in the relative crystallinity for all samples. In response to increasing PLLA content, the F(T) of blends first decreases for PLLA contents in PPDO ranging from 5 to 15%, but then increases upon further increase in the percentage of PLLA. This result suggests that a large PLLA content lowers the crystallization rate. As aforementioned, PLLA significantly suppresses the chain diffusion of PPDO due to the strong π– π interaction between PPDO and PLLA.
3.5 Crystallization activation energy
For nonisothermal crystallization, the activation energy can be derived from the crystallization peak temperature (T p) and cooling rate (φ) by the Kissinger[36] equation:
where R is the gas constant and ΔE the activation energy, which is composed of the transport activation energy ΔE* and the nucleation activation energy ΔF and related to the energy barrier to polymer segments moving toward the growing front of a crystal. Figure 6 shows plots of ln(φ/\(T_{\mathrm {p}}^{\mathrm {2}}\)) vs. 1/ T p for pure PPDO and PPDO/PLLA blends, and the activation energies are listed in table 3. The values of ΔE for all PPDO/PLLA blends are smaller than those obtained for pure PPDO, implying that PLLA content can accelerate PPDO crystallization. Additionally, ΔE is significantly decreased for the 95/05, 90/10 and 85/15 blends compared with pure PPDO, but is increased for the 70/30 and 60/40 blends compared with the above three blends. This suggests that the addition of 30% PLLA or above suppresses the motion of PPDO chains, and that the heterogeneous nucleation effect is not sufficient to offset the influence of the restriction effect; therefore, the ΔE of the 70/30 and 60/40 blends is higher than that of the 95/05, 90/10 and 85/15 blends. The 85/15 blend exhibits a relatively low ΔE due to the presence of more heterogeneous nucleation sites and a less-pronounced restriction effect.
4 Conclusions
PPDO/PLLA blends with different PLLA contents were prepared via solution co-precipitation. Three kinetic models were used to analyse the nonisothermal crystallization behaviour of pure PPDO and blends. The Avrami and Ozawa models failed to effectively describe the behaviour, while the combined Avrami–Ozawa model proposed by Mo and coworkers accurately describes the crystallization process.
Compared with pure PPDO, the crystallization peak temperature and crystallinity of PPDO in blends is dramatically increased, although the change in crystallization half-time is slight. The activation energy determined by the Kissinger model first decreases and then increases with the increase in PLLA content, and ΔE for all PPDO/PLLA blends is smaller than in pure PPDO. This is attributed to the increase in heterogeneous nucleation sites brought about by PLLA, which reduces the activation energy of PPDO in blends. The restriction effect of PLLA on PPDO, however, is increased, reducing the mobility of polymer chains when the PLLA content in PPDO is further increased.
References
Yang K K, Wang X L and Wang Y Z 2002 J. Macromol. Sci. Polym. Rev. C42 373
Nishida H, Yamoshita M, Endo T and Tokiwa Y 2000 Macromolecules 33 6982
Huang H X, Yang K K, Wang Y Z, Wang X L and Jun L 2006 J. Polym. Sci. A: Polym. Chem. 44 1245
Yoon K R, Lee K B, Chi Y S, Yun W S, Joo S W and Choi I S 2003 Adv. Mater. 15 2063
Furuhashi Y, Nakayama A, Monno T, Kawahara Y, Yamane H, Kimura Y and Iwata T 2004 Macromol. Rapid Commun. 25 1943
Sabino M A, Albuerne J, Muller A J, Brisson J and Prudhomme R E 2004 Biomacromolecules 5 358
Yang K K, Wang X L, Wang Y Z and Huang H X 2004 Mater. Chem. Phys. 87 218
Sabino M A, Feijoo J L and Muller A J 2000a Macromol. Chem. Phys. 201 2687
Sabino M A, Ronca G and Muller A J 2000b J. Mater. Sci. 35 5071
Chen S C, Wang X L, Wang Y Z, Yang K K, Zhou Z X and Wu G 2007 J. Biomed. Mater. Res. A 80A 453
Wang X L, Yang K K, Wang Y Z, Wang D Y and Yang Z 2004 Acta Mater. 52 4899
Zheng L, Wang Y Z, Yang K K, Wang X L, Chen S C and Li J 2005 Eur. Polym. J. 41 1243
Bhattarai S R, Yi H K, Bhattarai N, Hwang P H and Kim H Y 2006 Acta Biomater. 2 207
Bhattarai S R, Bhattarai N, Yi H K, Hwang P H, Cha D I and Kim H Y 2004 Biomaterials 25 2595
Suzuki A, Terai H, Toyoda H, Namikawa T, Yokota Y, Tsunoda T and Takaoko K 2006 J. Orthopaedic Res. 24 327
Toyoda H, Terai H, Sasaoka R, Oda K and Takaoko K 2005 Bone 37 555
Yomeda M, Terai H, Imai Y, Okada T, Nozaki K, Inoue H, Miyamoto S and Takaoka K 2005 Biomaterials 26 5145
Dong T, He Y, Zhu B, Shin K M and Inoue Y 2005 Macromolecules 38 7736
Kai W H, He Y, Asakawa N and Inoue Y 2004 J. Appl. Polym. Sci. 94 2466
Liu W J, Yang H L, Wang Z, Dong L S and Liu J 2002 J. Appl. Polym. Sci. 86 2145
Pearce R and Marchessault R H 1994 Polymer 35 3990
Vazquez-Torres H and Cruz-Ramos C A 1994 J. Appl. Polym. Sci. 54 1141
Iannace S, Ambrosio L, Huang S J and Nicolais L 1994 J. Appl. Polym. Sci. 54 1525
Pezzin A P T, Alberda E G, Zavaglia C A C, Brinke G and Duek E A R 2003 J. Appl. Polym. Sci. 88 2744
Pezzin A P T and Duek E A R 2006 J. Appl. Polym. Sci. 101 1899
Park J M, Kim D S and Kim S R 2004 Comp. Sci. Tech. 64 847
Gilding D K and Reed A M 1979 Polymer 20 1459
Anderson J M and Shive M S 1997 Adv. Drug Deliv. Rev. 28 6
Eldsater C, Erlandsson B, Renstad R, Albertsson A and Karlsson S 2000 Polymer 41 1297
Avrami J M 1940 Chem. Phys. 8 212
Avrami J M 1941 Chem. Phys. 9 1771
Jeziorny A 1978 Polymer 19 1142
Weng W G, Chen G H and Wu D 2003 Polymer 44 8119
Ozawa T 1971 Polymer 12 150
Liu T X, Mo Z S and Zhang H F 1998 J. Appl. Polym. Sci. 67 815
Kissinger H E 1956 J. Res. Natl. Bur. Stand. 57 217
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (51103156) and the West Light Foundation of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ZHANG, X., BAI, W., CHEN, D. et al. Nonisothermal crystallization behaviour of poly(ρ-dioxanone) and poly(L-lactic acid) blends. Bull Mater Sci 38, 517–523 (2015). https://doi.org/10.1007/s12034-014-0833-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-014-0833-4